Geographic Atrophy Progression in Clinical Practice Before and After Pegcetacoplan Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results
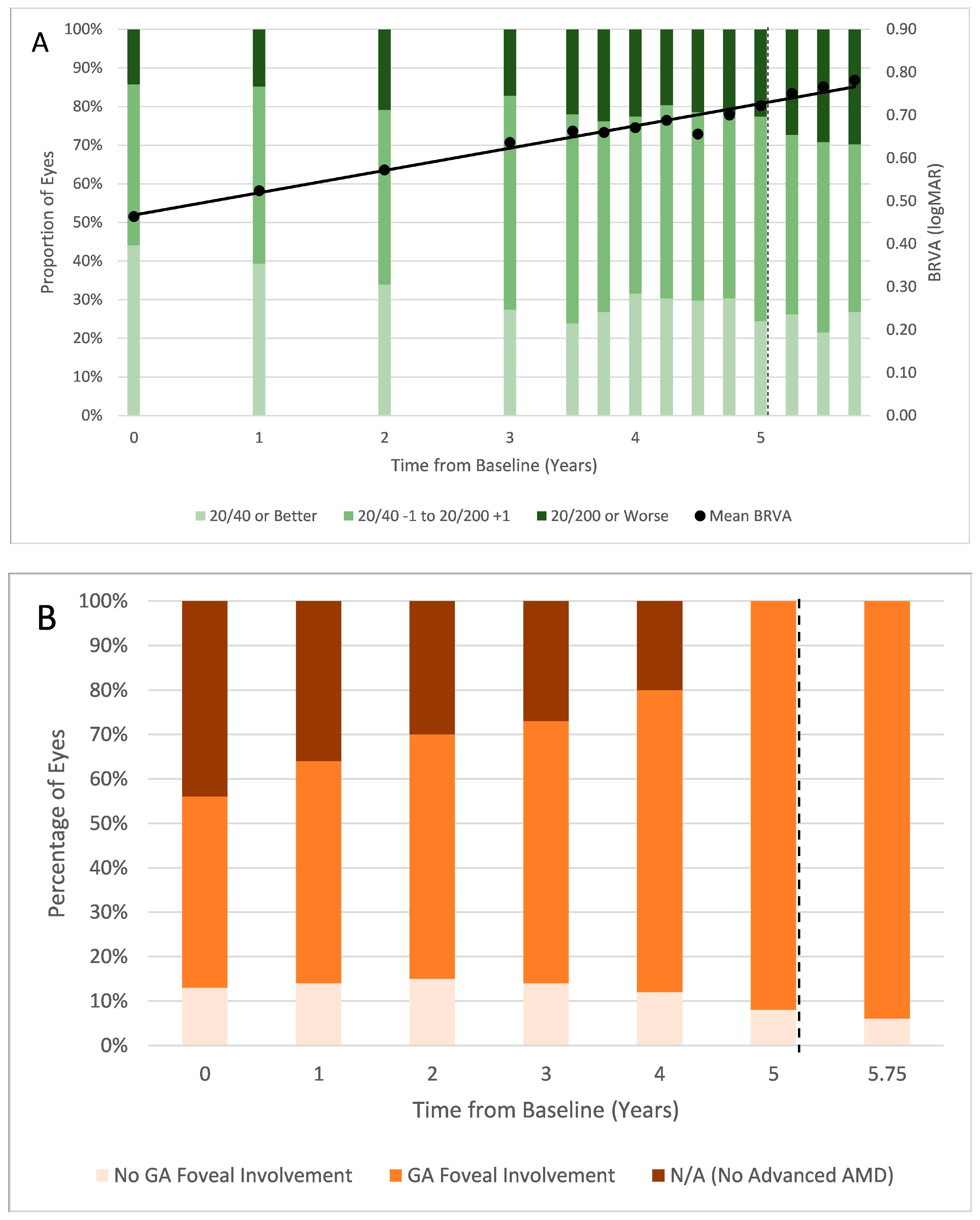
3.1. AMD Phenotype
3.2. BRVA and Foveal Involvement
3.3. Treatment Characteristics
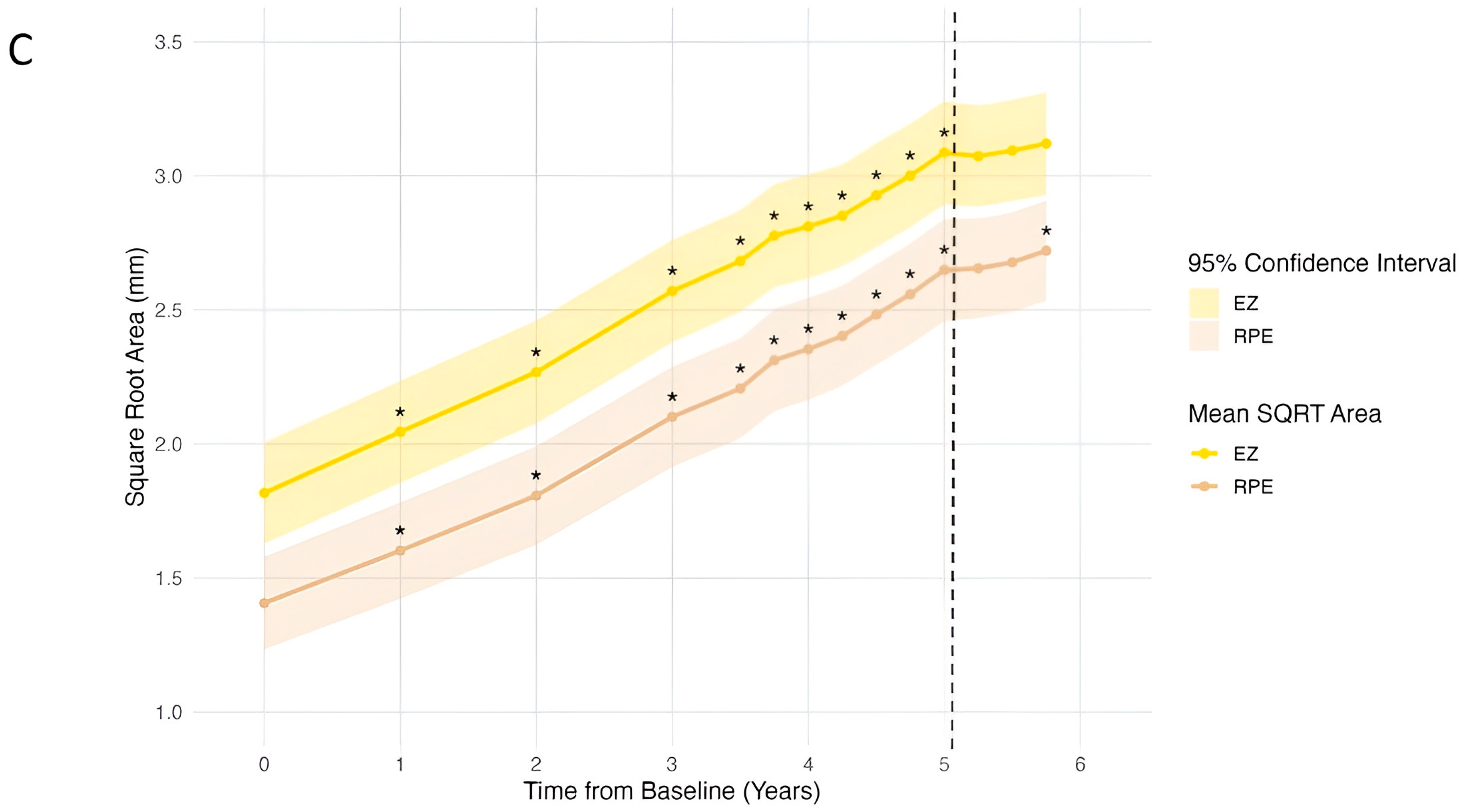
3.4. Progression of OCT Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| AMD | Age-related macular degeneration |
| BRVA | Best-recorded visual acuity |
| CI | Confidence interval |
| CNN | Convolutional Neural Network |
| cRORA | Complete retinal pigment epithelium and outer retinal atrophy |
| EZ | Ellipsoid zone |
| FA | Fundus autofluorescence |
| FDA | Food and Drug Administration |
| FP | Fundus photography |
| GA | Geographic atrophy |
| IOP | Intraocular pressure |
| IZ | Interdigitation zone |
| logMAR | Logarithm of the minimum angle of resolution |
| MZ | Myoid zone |
| n | Sample size |
| nAMD | Neovascular age-related macular degeneration |
| OCT | Optical coherence tomography |
| ONL | Outer nuclear layer |
| OPR | Outer photoreceptor segment |
| PR | Photoreceptor |
| RPE | Retinal pigment epithelium |
| SD | Standard deviation |
| SQRT | Square root |
| VA | Visual acuity |
| VEGF | Vascular endothelial growth factor-A |
| Y0 | Year zero (baseline) |
| Y5 | Year five (pegcetacoplan initiation) |
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Strauss, E.C.; Schmitz-Valckenberg, S.; van Lookeren Campagne, M. Geographic Atrophy: Clinical Features and Potential Therapeutic Approaches. Ophthalmology 2014, 121, 1079–1091. [Google Scholar] [CrossRef]
- Riley-Gillis, B.; Huh, H.; Shen, J.; den Hollander, A.I. Genetic and molecular biomarkers for geographic atrophy. Acta Ophthalmol. 2023, 101, 869–880. [Google Scholar] [CrossRef]
- Chakravarthy, U.; Bailey, C.C.; Johnston, R.L.; McKibbin, M.; Khan, R.S.; Mahmood, S.; Downey, L.; Dhingra, N.; Brand, C.; Brittain, C.J.; et al. Characterizing Disease Burden and Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018, 125, 842–849. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Vujosevic, S.; Alovisi, C.; Chakravarthy, U. Epidemiology of geographic atrophy and its precursor features of intermediate age-related macular degeneration. Acta Ophthalmol. 2023, 101, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.L. Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmol. Sci. 2023, 3, 100306. [Google Scholar] [CrossRef]
- Patel, P.J.; Ziemssen, F.; Ng, E.; Muthutantri, A.; Silverman, D.; Tschosik, E.A.; Cantrell, R.A. Burden of Illness in Geographic Atrophy: A Study of Vision-Related Quality of Life and Health Care Resource Use. Clin. Ophthal. 2020, 14, 15–28. [Google Scholar] [CrossRef]
- Sunness, J.S.; Rubin, G.S.; Applegate, C.A.; Bressler, N.M.; Marsh, M.J.; Hawkins, B.S.; Haselwood, D. Visual Function Abnormalities and Prognosis in Eyes with Age-related Geographic Atrophy of the Macula and Good Visual Acuity. Ophthalmology 1997, 104, 1677–1691. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Mitchell, P.; Freund, K.B.; Sadda, S.; Holz, F.G.; Brittain, C.; Henry, E.C.; Ferrara, D. The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2018, 125, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Cleland, S.C.; Konda, S.M.; Danis, R.P.; Huang, Y.; Myers, D.J.; Blodi, B.A.; Domalpally, A. Quantification of Geographic Atrophy Using Spectral Domain OCT in Age-Related Macular Degeneration. Ophthalmol. Retina 2021, 5, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, R.G.; Simader, C.; Scheschy, U.; Montuoro, A.; Kiss, C.; Sacu, S.; Kreil, D.P.; Prünte, C.; Schmidt-Erfurth, U. A Systematic Comparison of Spectral-Domain Optical Coherence Tomography and Fundus Autofluorescence in Patients with Geographic Atrophy. Ophthalmology 2011, 118, 1844–1851. [Google Scholar] [CrossRef]
- Simader, C.; Sayegh, R.G.; Montuoro, A.; Azhary, M.; Koth, A.L.; Baratsits, M.; Sacu, S.; Prünte, C.; Kreil, D.P.; Schmidt-Erfurth, U. A Longitudinal Comparison of Spectral-Domain Optical Coherence Tomography and Fundus Autofluorescence in Geographic Atrophy. Am. J. Ophthalmol. 2014, 158, 557–566.e1. [Google Scholar] [CrossRef]
- Mai, J.; Riedl, S.; Reiter, G.S.; Lachinov, D.; Vogl, W.D.; Bogunovic, H.; Schmidt-Erfurth, U. Comparison of Fundus Autofluorescence Versus Optical Coherence Tomography-based Evaluation of the Therapeutic Response to Pegcetacoplan in Geographic Atrophy. Am. J. Ophthalmol. 2022, 244, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef]
- Arslan, J.; Samarasinghe, G.; Benke, K.K.; Sowmya, A.; Wu, Z.; Guymer, R.H.; Baird, P.N. Artificial Intelligence Algorithms for Analysis of Geographic Atrophy: A Review and Evaluation. Transl. Vis. Sci. Technol. 2020, 9, 57. [Google Scholar] [CrossRef]
- Mares, V.; Nehemy, M.B.; Bogunovic, H.; Frank, S.; Reiter, G.S.; Schmidt-Erfurth, U. AI-based support for optical coherence tomography in age-related macular degeneration. Int. J. Retina Vitr. 2024, 10, 31. [Google Scholar] [CrossRef]
- Keenan, T.D.; Dharssi, S.; Peng, Y.; Chen, Q.; Agrón, E.; Wong, W.T.; Lu, Z.; Chew, E.Y. A Deep Learning Approach for Automated Detection of Geographic Atrophy from Color Fundus Photographs. Ophthalmology 2019, 126, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Sarao, V.; Veritti, D.; Nardin, A.D.; Misciagna, M.; Foresti, G.; Lanzetta, P. Explainable artificial intelligence model for the detection of geographic atrophy using colour retinal photographs. BMJ Open Ophthalmol. 2023, 8, e001411. [Google Scholar] [CrossRef]
- Peng, Y.; Dharssi, S.; Chen, Q.; Keenan, T.D.; Agrón, E.; Wong, W.T.; Chew, E.Y.; Lu, Z. DeepSeeNet: A Deep Learning Model for Automated Classification of Patient-based Age-related Macular Degeneration Severity from Color Fundus Photographs. Ophthalmology 2019, 126, 565–575. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, Y.; Keenan, T.; Dharssi, S.; Agro´n, E.; Wong, W.T.; Chew, E.Y.; Lu, Z. A multi-task deep learning model for the classification of Age-related Macular Degeneration. AMIA Summits Transl. Sci. Proc. 2019, 2019, 505–514. [Google Scholar] [PubMed]
- Mai, J.; Lachinov, D.; Riedl, S.; Reiter, G.S.; Vogl, W.D.; Bogunovic, H.; Schmidt-Erfurth, U. Clinical validation for automated geographic atrophy monitoring on OCT under complement inhibitory treatment. Sci Rep. 2023, 13, 7028. [Google Scholar] [CrossRef]
- Montesel, A.; Gigon, A.; Mosinska, A.; Apostolopoulos, S.; Ciller, C.; De Zanet, S.; Mantel, I. Automated foveal location detection on spectral-domain optical coherence tomography in geographic atrophy patients. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 2261–2270. [Google Scholar] [CrossRef]
- Martin-Pinardel, R.; Izquierdo-Serra, J.; De Zanet, S.; Parrado-Carrillo, A.; Garay-Aramburu, G.; Puzo, M.; Arruabarrena, C.; Sararols, L.; Abraldes, M.; Broc, L.; et al. Artificial intelligence-based fluid quantification and associated visual outcomes in a real-world, multicentre neovascular age-related macular degeneration national database. Br. J. Ophthalmol. 2024, 108, 253–262. [Google Scholar] [CrossRef]
- Camacho, P.; Dutra-Medeiros, M.; Salgueiro, L.; Sadio, S.; Rosa, P.C. Manual Segmentation of 12 Layers of the Retina and Choroid through SD-OCT in Intermediate AMD: Repeatability and Reproducibility. J. Ophthalmic Vis. Res. 2021, 16, 384–392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Desai, D.; Dugel, P.U. Complement cascade inhibition in geographic atrophy: A review. Eye 2022, 36, 294–302. [Google Scholar] [CrossRef]
- Boyer, D.S.; Schmidt-Erfurth, U.; van Lookeren Campagne, M.; Henry, E.C.; Brittain, C. The Pathophysiology of Geographic Atrophy Secondary to Age-Related Macular Degeneration and the Complement Pathway as a Therapeutic Target. Retina 2017, 37, 819–835. [Google Scholar] [CrossRef]
- About SYFOVRE® (Pegcetacoplan Injection). Available online: https://syfovreecp.com/how-syfovre-works (accessed on 22 April 2024).
- FDA Approves SYFOVRETM (Pegcetacoplan Injection) as the First and Only Treatment for Geographic Atrophy (GA), a Leading Cause of Blindness—Apellis Pharmaceuticals, Inc. Available online: https://investors.apellis.com/news-releases/news-release-details/fda-approves-syfovretm-pegcetacoplan-injection-first-and-only (accessed on 9 September 2024).
- Heier, J.S.; Lad, E.M.; Holz, F.G.; Rosenfeld, P.J.; Guymer, R.H.; Boyer, D.; Grossi, F.; Baumal, C.R.; Korobelnik, J.F.; Slakter, J.S.; et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): Two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet 2023, 402, 1434–1448. [Google Scholar] [CrossRef]
- Liao, D.S.; Grossi, F.V.; Mehdi, D.E.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Sikorav, A.; Semoun, O.; Zweifel, S.; Jung, C.; Srour, M.; Querques, G.; Souied, E.H. Prevalence and quantification of geographic atrophy associated with newly diagnosed and treatment-naïve exudative age-related macular degeneration. Br. J. Ophthalmol. 2017, 101, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.A.; Boucher, N.; Aggarwal, N.; Harris, A. Geographic Atrophy is Associated with Meaningful Disease Burden: Visual Acuity Changes and Conversion to Neovascular AMD over 3 Years in 18,712 Patient Eyes. Investig. Ophthalmol. Vis. Sci. 2023, 64, 4327. [Google Scholar]
- Kleerekooper, I.; Verschueren, D.V.; Trip, S.A.; Plant, G.T.; Petzold, A. Ellipsoid Zone Reflectivity: Exploring its Potential as a Novel Non-Invasive Biomarker for Assessing Mitochondrial Function. Neuro-Ophthalmology 2024, 48, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Saßmannshausen, M.; Behning, C.; Isselmann, B.; Schmid, M.; Finger, R.P.; Holz, F.G.; Schmitz-Valckenberg, S.; Pfau, M.; Consortium, M.; Thiele, S. Relative ellipsoid zone reflectivity and its association with disease severity in age-related macular degeneration: A MACUSTAR study report. Sci. Rep. 2022, 12, 14933. [Google Scholar] [CrossRef] [PubMed]
- Newswire, P.R. Stealth BioTherapeutics Announces Positive End-of-Phase 2 Meeting with FDA on the Development of Elamipretide in Patients with Dry Age-related Macular Degeneration. Available online: https://www.prnewswire.com/news-releases/stealth-biotherapeutics-announces-positive-end-of-phase-2-meeting-with-fda-on-the-development-of-elamipretide-in-patients-with-dry-age-related-macular-degeneration-301848690.html (accessed on 30 August 2024).
- Derradji, Y.; Mosinska, A.; Apostolopoulos, S.; Ciller, C.; De Zanet, S.; Mantel, I. Fully-automated atrophy segmentation in dry age-related macular degeneration in optical coherence tomography. Sci. Rep. 2021, 11, 21893. [Google Scholar] [CrossRef]
- Kurmann, T.; Yu, S.; Márquez-Neila, P.; Ebneter, A.; Zinkernagel, M.; Munk, M.R.; Wolf, S.; Sznitman, R. Expert-level Automated Biomarker Identification in Optical Coherence Tomography Scans. Sci. Rep. 2019, 9, 13605. [Google Scholar] [CrossRef]
- European Commission. (n.d.). UDI-DI: 07640486840856. EUDAMED—European Database on Medical Devices. Available online: https://ec.europa.eu/tools/eudamed/#/screen/search-device/e466e4d2-5e59-45dc-bdcf-0be3a914f17b (accessed on 29 October 2025).
- RetinAI Discovery. RetinAI. (n.d.). Available online: https://www.retinai.com/products/discovery (accessed on 29 October 2025).
- Mishchuk, A.; Blair, J.; Munk, M.R.; Mantel, I.; Ciller, C.; Apostolopoulos, S.; De Zanet, S. Correlations between GA lesions in FAF and morphological outer retinal changes in OCT. Investig. Ophthalmol. Vis. Sci. 2024, 65, 2277. [Google Scholar]
- Tiew, S.; Lim, C.; Sivagnanasithiyar, T. Using an excel spreadsheet to convert Snellen visual acuity to LogMAR visual acuity. Eye 2020, 34, 2148–2149. [Google Scholar] [CrossRef]
- Yehoshua, Z.; Rosenfeld, P.J.; Gregori, G.; Feuer, W.J.; Falcão, M.; Lujan, B.J.; Puliafito, C. Progression of geographic atrophy in age-related macular degeneration imaged with spectral domain optical coherence tomography. Ophthalmology 2011, 118, 679–686. [Google Scholar] [CrossRef]
- Riedl, S.; Vogl, W.D.; Mai, J.; Reiter, G.S.; Lachinov, D.; Grechenig, C.; McKeown, A.; Scheibler, L.; Bogunović, H.; Schmidt-Erfurth, U. The Effect of Pegcetacoplan Treatment on Photoreceptor Maintenance in Geographic Atrophy Monitored by Artificial Intelligence–Based OCT Analysis. Ophthalmol. Retina 2022, 6, 1009–1018. [Google Scholar] [CrossRef]
- Pfau, M.; Schmitz-Valckenberg, S.; Ribeiro, R.; Safaei, R.; McKeown, A.; Fleckenstein, M.; Holz, F.G. Association of complement C3 inhibitor pegcetacoplan with reduced photoreceptor degeneration beyond areas of geographic atrophy. Sci. Rep. 2022, 12, 17870. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Mai, J.; Reiter, G.S.; Riedl, S.; Vogl, W.D.; Sadeghipour, A.; McKeown, A.; Foos, E.; Scheibler, L.; Bogunovic, H. Disease Activity and Therapeutic Response to Pegcetacoplan for Geographic Atrophy Identified by Deep Learning-Based Analysis of OCT. Ophthalmology 2025, 132, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Holz, F.G.; Chiang, A.; Boyer, D.; Dhoot, D.S.; Loewenstein, A.; Mones, J.; Heier, J.; Abbey, A.M.; Singerman, L.J.; et al. Pegcetacoplan Treatment for Geographic Atrophy in Age-Related Macular Degeneration over 36 Months: Data from OAKS, DERBY, and GALE. Am. J. Ophthalmol. 2025, 276, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Sunness, J.S. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol. Vis. 1999, 5, 25. [Google Scholar] [PubMed]
- Lindner, M.; Böker, A.; Mauschitz, M.M.; Göbel, A.P.; Fimmers, R.; Brinkmann, C.K.; Schmitz-Valckenberg, S.; Schmid, M.; Holz, F.G.; Fleckenstein, M. Directional Kinetics of Geographic Atrophy Progression in Age-Related Macular Degeneration with Foveal Sparing. Ophthalmology 2015, 122, 1356–1365. [Google Scholar] [CrossRef]
- Wu, Z.; Ayton, L.N.; Luu, C.D.; Guymer, R.H. Longitudinal Changes in Microperimetry and Low Luminance Visual Acuity in Age-Related Macular Degeneration. JAMA Ophthalmol. 2015, 133, 442–448. [Google Scholar] [CrossRef]
- Chang, D.S.; Callaway, N.F.; Steffen, V.; Csaky, K.; Guymer, R.H.; Birch, D.G.; Patel, P.J.; Ip, M.; Gao, S.S.; Briggs, J.; et al. Macular Sensitivity Endpoints in Geographic Atrophy: Exploratory Analysis of Chroma and Spectri Clinical Trials. Ophthalmol. Sci. 2023, 4, 100351. [Google Scholar] [CrossRef]
- Hoffmann, L.; Rossouw, P.; Guichard, M.M.; Hatz, K. Strongest Correlation Between Contrast Sensitivity and Morphological Characteristics in Bilateral nAMD. Front. Med. 2021, 7, 622877. [Google Scholar] [CrossRef]
- Heier, J.S.; Pieramici, D.; Chakravarthy, U.; Patel, S.S.; Gupta, S.; Lotery, A.; Lad, E.M.; Silverman, D.; Henry, E.C.; Anderesi, M.; et al. Visual Function Decline Resulting from Geographic Atrophy: Results from the Chroma and Spectri Phase 3 Trials. Ophthalmol. Retina 2020, 4, 673–688. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Shapiro, H.; Tuomi, L.; Webster, M.; Elledge, J.; Blodi, B. Characteristics of Patients Losing Vision after 2 Years of Monthly Dosing in the Phase III Ranibizumab Clinical Trials. Ophthalmology 2011, 118, 523–530. [Google Scholar] [CrossRef]
- Armendariz, B.G.; Chakravarthy, U. Fibrosis in age-related neovascular macular degeneration in the anti-VEGF era. Eye 2024, 38, 3243–3251. [Google Scholar] [CrossRef]
- Witkin, A.J.; Jaffe, G.J.; Srivastava, S.K.; Davis, J.L.; Kim, J.E. Retinal Vasculitis After Intravitreal Pegcetacoplan: Report From the ASRS Research and Safety in Therapeutics (ReST) Committee. J. Vitreoretin. Dis. 2024, 8, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Douros, S.; Mostafavi, D.; Danias, M. Retinal vasculitis following intravitreal pegcetacoplan administration. Am. J. Ophthalmol. Case Rep. 2024, 33, 101999. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Distribution | |
|---|---|---|
| Total number of patients | 110 | |
| Total number of eyes | 168 | |
| Age, mean (SD) | 77.9 (10.6) | |
| Sex | Male | 28 (25.5%) |
| Female | 82 (74.5%) | |
| Race | White | 38 (34.5%) |
| Black | 0 (0.0%) | |
| Asian | 1 (0.9%) | |
| Unknown/Other | 71 (64.5%) | |
| Ethnicity | Hispanic or Latino | 0 (0.0%) |
| Not Hispanic or Latino | 74 (67.3%) | |
| Ethnicity not specified | 36 (32.7%) | |
| Smoking Status | Never | 60 (54.5%) |
| Current | 6 (5.5%) | |
| Former | 44 (40.0%) | |
| Diabetes | Yes | 18 (16.4%) |
| No | 91 (82.7%) | |
| Pre-diabetic | 1 (0.9%) | |
| Hypertension | Yes | 74 (67.3%) |
| No | 36 (32.7%) | |
| Characteristic | Distribution | |
|---|---|---|
| BRVA, logMAR | Mean (SD) [Snellen approximate] | 0.49 (0.47) [20/63] |
| 20/40 or better | 74 (44.0%) | |
| 20/40 to 20/200 | 77 (45.8%) | |
| 20/200 or worse | 17 (10.1%) | |
| IOP, mean (SD) mmHg | 15.3 (3.3) | |
| Phakic status | Phakic | 43 (25.6%) |
| Pseudophakic | 125 (74.4%) | |
| Glaucoma | Yes | 18 (10.7%) |
| No | 150 (89.3%) | |
| GA + nAMD | 76 (45.2%) | |
| GA only | 18 (10.7%) | |
| nAMD only | 56 (33.3%) | |
| Intermediate AMD only | 18 (10.7%) | |
| Characteristic | Count | |
|---|---|---|
| Pegcetacoplan injections, mean (SD) | 6.1 (1.4) | |
| Pegcetacoplan injection interval, mean (SD) weeks | 7.4 (0.9) | |
| Adverse events after pegcetacoplan initiation | nAMD | 2 (11.7%) |
| Endophthalmitis | 1 (0.6%) | |
| Intraocular inflammation | 2 (1.2%) | |
| Anti-VEGF treatment interval 6 months before pegcetacoplan initiation, mean (SD) weeks | 7.6 (2.7) | |
| Anti-VEGF treatment interval 9 months after pegcetacoplan initiation, mean (SD) weeks | 6.7 (1.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.A.; Zhou, A.W.; Teagle, G.M.; Baumann, L.M.; Sahraravand, R.A.; Wong, C.W.; De Zanet, S.; Jovic, N.; Steiner, P.; Patel, S.B.; et al. Geographic Atrophy Progression in Clinical Practice Before and After Pegcetacoplan Treatment. Vision 2025, 9, 95. https://doi.org/10.3390/vision9040095
Cao JA, Zhou AW, Teagle GM, Baumann LM, Sahraravand RA, Wong CW, De Zanet S, Jovic N, Steiner P, Patel SB, et al. Geographic Atrophy Progression in Clinical Practice Before and After Pegcetacoplan Treatment. Vision. 2025; 9(4):95. https://doi.org/10.3390/vision9040095
Chicago/Turabian StyleCao, Jessica A., Avery W. Zhou, Gail M. Teagle, Liisa M. Baumann, Ryan A. Sahraravand, Calvin W. Wong, Sandro De Zanet, Natasa Jovic, Patrick Steiner, Sagar B. Patel, and et al. 2025. "Geographic Atrophy Progression in Clinical Practice Before and After Pegcetacoplan Treatment" Vision 9, no. 4: 95. https://doi.org/10.3390/vision9040095
APA StyleCao, J. A., Zhou, A. W., Teagle, G. M., Baumann, L. M., Sahraravand, R. A., Wong, C. W., De Zanet, S., Jovic, N., Steiner, P., Patel, S. B., Minaker, S. A., MacCumber, M. W., Brown, D. M., Al-khersan, H., & Wykoff, C. C. (2025). Geographic Atrophy Progression in Clinical Practice Before and After Pegcetacoplan Treatment. Vision, 9(4), 95. https://doi.org/10.3390/vision9040095





