Myopia Prediction Using Machine Learning: An External Validation Study
Abstract
1. Background
2. Methods
2.1. Eye Examinations and Ocular Biometry
2.2. Machine Learning Models
3. Results
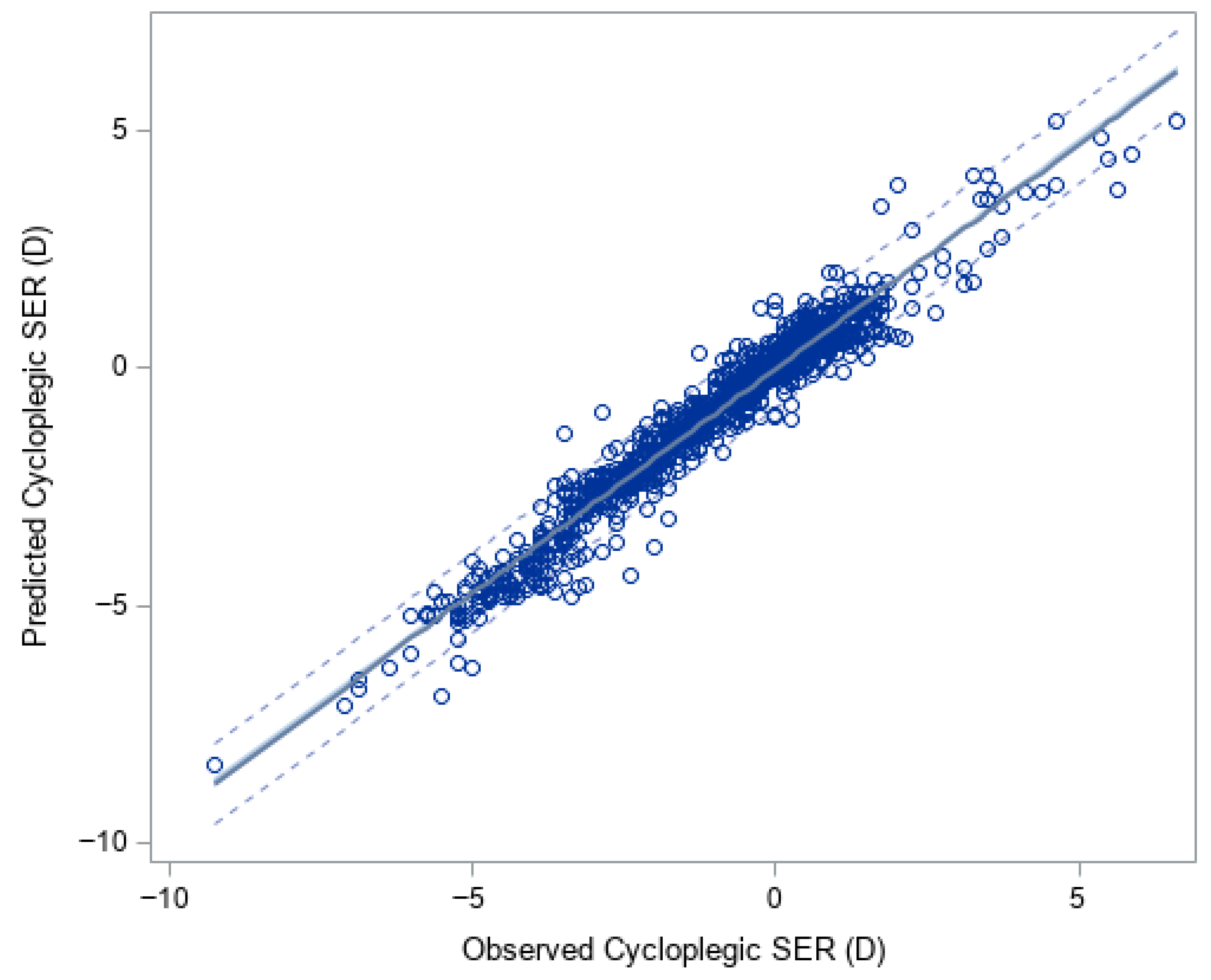
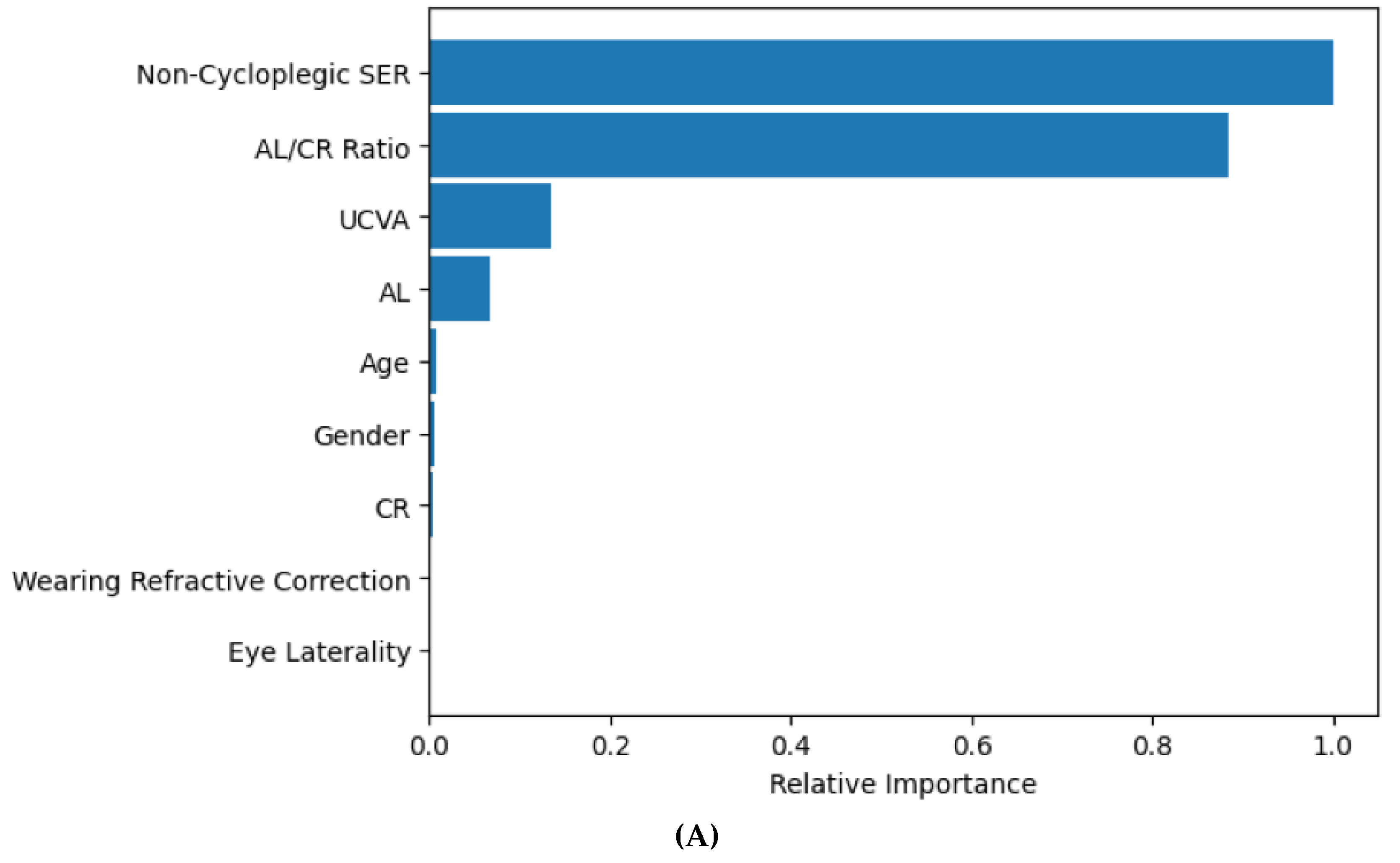
3.1. XGBoost for Predicting Cycloplegic SER and Myopia Status
3.2. XGBoost for Predicting Cycloplegic SER by Age Group and Refractive Error
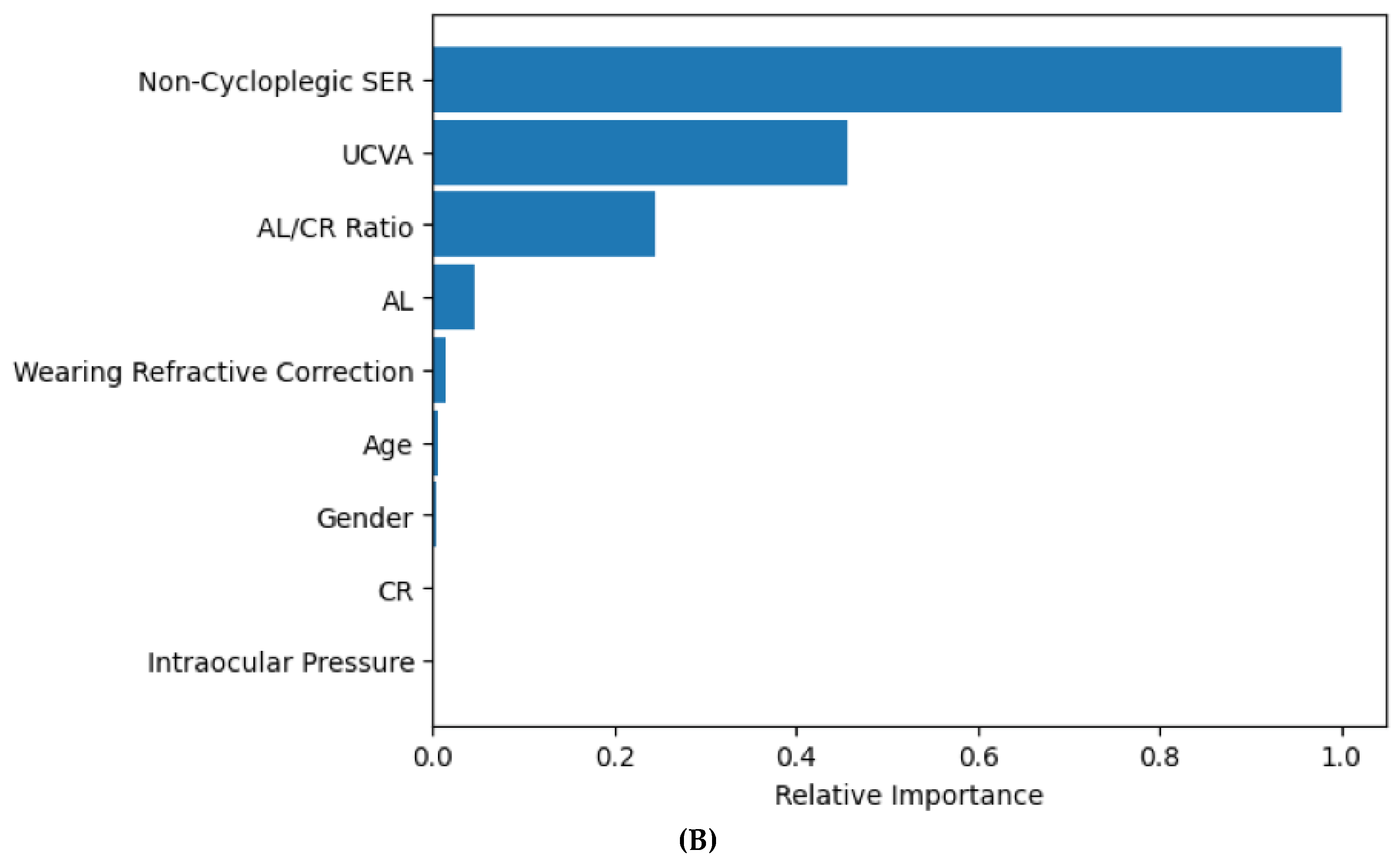
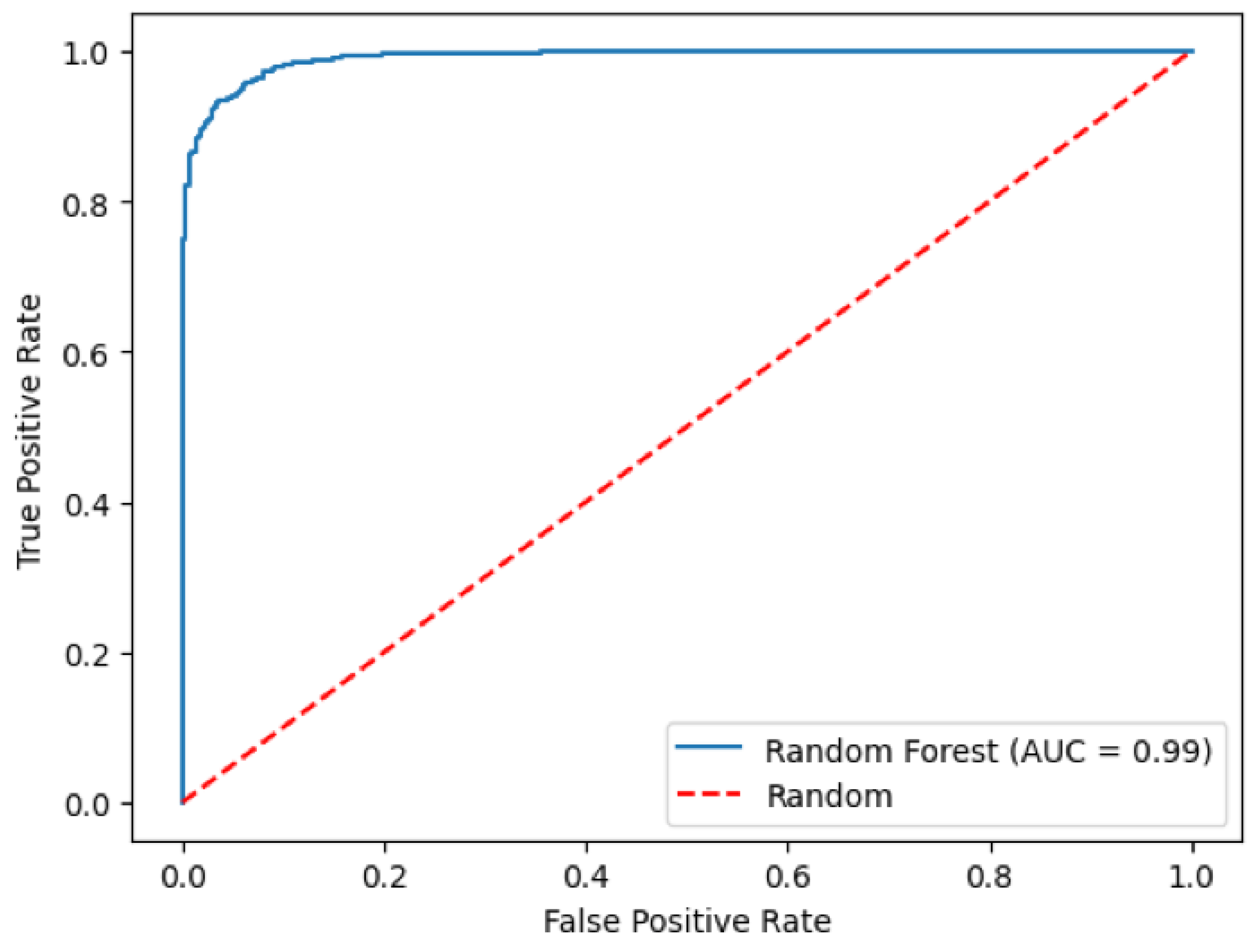
3.3. Random Forest Model for Predicting Myopia Status
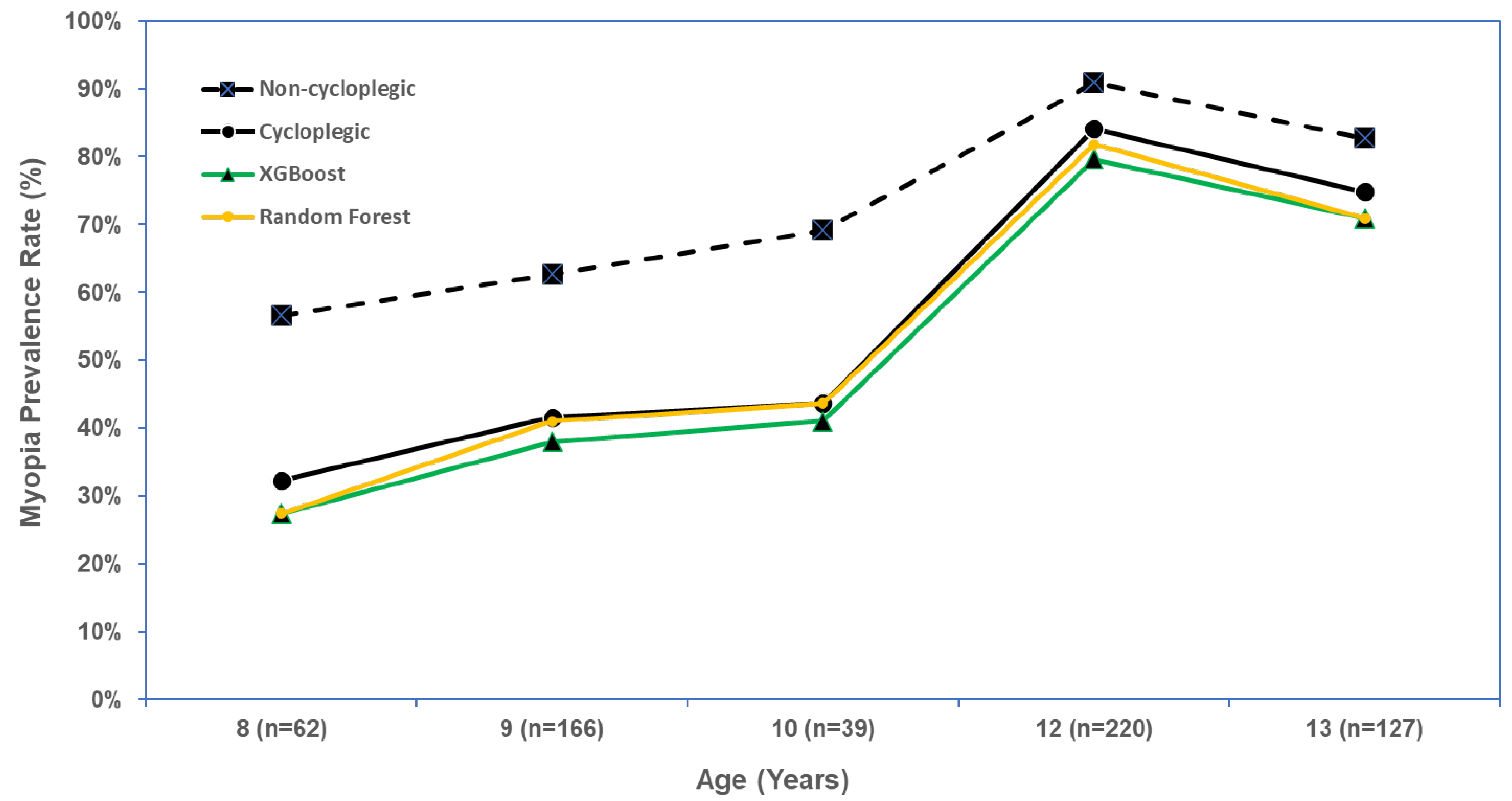
3.4. XGBoost and Random Forest for Predicting Myopia Prevalence Rate
3.5. Model Prediction Performance Stratified by Cycloplegic Agent and Ocular Biometer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sankaridurg, P.; Tahhan, N.; Kandel, H.; Naduvilath, T.; Zou, H.; Frick, K.D.; Marmamula, S.; Friedman, D.S.; Lamoureux, E.; Keeffe, J.; et al. IMI Impact of Myopia. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef] [PubMed]
- Koh, V.; Tan, C.; Nah, G.; Zhao, P.; Yang, A.; Lin, S.T.; Wong, T.Y.; Saw, S.M.; Chia, A. Correlation of structural and electrophysiological changes in the retina of young high myopes. Ophthalmic Physiol. Opt. 2014, 34, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Seet, B.; Yap, E.P.; Saw, S.M.; Lim, T.H.; Chia, K.S. Does education explain ethnic differences in myopia prevalence? A population-based study of young adult males in Singapore. Optom. Vis. Sci. 2001, 78, 234–239. [Google Scholar] [CrossRef]
- Yotsukura, E.; Torii, H.; Inokuchi, M.; Tokumura, M.; Uchino, M.; Nakamura, K.; Hyodo, M.; Mori, K.; Jiang, X.; Ikeda, S.I.; et al. Current Prevalence of Myopia and Association of Myopia with Environmental Factors Among Schoolchildren in Japan. JAMA Ophthalmol. 2019, 137, 1233–1239. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, H.; Zhao, L.; Wang, X.; Yang, X.; Ding, X.; Li, X.; Jiang, Z.; Zhang, X.; Zhang, Q.; et al. Green Space Morphology and School Myopia in China. JAMA Ophthalmol. 2024, 142, 115–122. [Google Scholar] [CrossRef]
- Wilson, S.; Ctori, I.; Shah, R.; Suttle, C.; Conway, M.L. Systematic review and meta-analysis on the agreement of non-cycloplegic and cycloplegic refraction in children. Ophthalmic Physiol. Opt. 2022, 42, 1276–1288. [Google Scholar] [CrossRef]
- Gu, F.; Gao, H.M.; Zheng, X.; Gu, L.; Huang, J.Y.; Meng, J.; Li, J.J.; Gao, L.; Wang, J.Y.; Zhang, R.H.; et al. Effect of Cycloplegia on Refractive Error Measure in Chinese School Students. Ophthal. Epidemiol. 2022, 29, 629–639. [Google Scholar] [CrossRef]
- Foo, V.H.; Verkicharla, P.K.; Ikram, M.K.; Chua, S.Y.; Cai, S.; Tan, C.S.; Chong, Y.S.; Kwek, K.; Gluckman, P.; Wong, T.Y.; et al. Axial Length/Corneal Radius of Curvature Ratio and Myopia in 3-Year-Old Children. Transl. Vis. Sci. Technol. 2016, 5, 5. [Google Scholar] [CrossRef]
- He, X.; Zou, H.; Lu, L.; Zhao, R.; Zhao, H.; Li, Q.; Zhu, J. Axial length/corneal radius ratio: Association with refractive state and role on myopia detection combined with visual acuity in Chinese schoolchildren. PLoS ONE 2015, 10, e0111766. [Google Scholar] [CrossRef]
- Ip, J.M.; Huynh, S.C.; Kifley, A.; Rose, K.A.; Morgan, I.G.; Varma, R.; Mitchell, P. Variation of the contribution from axial length and other oculometric parameters to refraction by age and ethnicity. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4846–4853. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Hasebe, S.; Miyata, M.; Hamasaki, I.; Ohtsuki, H. Axial length measurement using partial coherence interferometry in myopic children: Repeatability of the measurement and comparison with refractive components. Jpn. J. Ophthalmol. 2007, 51, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Magome, K.; Morishige, N.; Ueno, A.; Matsui, T.A.; Uchio, E. Prediction of cycloplegic refraction for noninvasive screening of children for refractive error. PLoS ONE 2021, 16, e0248494. [Google Scholar] [CrossRef] [PubMed]
- Ojaimi, E.; Rose, K.A.; Morgan, I.G.; Smith, W.; Martin, F.J.; Kifley, A.; Robaei, D.; Mitchell, P. Distribution of ocular biometric parameters and refraction in a population-based study of Australian children. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2748–2754. [Google Scholar] [CrossRef]
- Sankaridurg, P.; He, X.; Naduvilath, T.; Lv, M.; Ho, A.; Smith, E., 3rd; Erickson, P.; Zhu, J.; Zou, H.; Xu, X. Comparison of noncycloplegic and cycloplegic autorefraction in categorizing refractive error data in children. Acta Ophthalmol. 2017, 95, e633–e640. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Gao, H.M.; Zhang, H.; Yang, Y.; Gu, F.; Zheng, X.; Gu, L.; Huang, J.; Meng, J.; et al. Prediction for Cycloplegic Refractive Error in Chinese School Students: Model Development and Validation. Transl. Vis. Sci. Technol. 2022, 11, 15. [Google Scholar] [CrossRef]
- Du, B.; Wang, Q.; Luo, Y.; Jin, N.; Rong, H.; Wang, X.; Nian, H.; Guo, L.; Liang, M.; Wei, R. Prediction of spherical equivalent difference before and after cycloplegia in school-age children with machine learning algorithms. Front. Public Health 2023, 11, 1096330. [Google Scholar] [CrossRef]
- Ying, B.; Chandra, R.S.; Wang, J.; Cui, H.; Oatts, J. Machine Learning Models for Predicting Cycloplegic Refractive Error and Myopia Status Based on Noncycloplegic Data in Chinese Students. Transl. Vis. Sci. Technol. 2024, 13, 16. [Google Scholar] [CrossRef]
- Chen, B.; Tian, L.; Tian, F.; Yang, Q.; Ruan, Y.; Li, Y.; Cao, M.; Wu, C.; Yang, M.; Xu, S.; et al. Machine learning-driven prediction of cycloplegic refractive error in Chinese children. Front. Cell Dev. Biol. 2025, 13, 1608494. [Google Scholar] [CrossRef]
- Feng, Q.; Wu, X.; Liu, Q.; Xiao, Y.; Zhang, X.; Chen, Y. Interpretable machine learning models for predicting childhood myopia from school-based screening data. Sci. Rep. 2025, 15, 19811. [Google Scholar] [CrossRef]
- Breiman, L. Random Forest. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Ying, G.-S.; Maguire, M.G.; Glynn, R.J.; Rosner, B. Calculating Sensitivity, Specificity, and Predictive Values for Correlated Eye Data. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Wang, X.; Zhang, H.; Zhao, E.; Wang, J.; Yang, Y.; Gu, F.; Gu, L.; Huang, J.; Zhang, R.; et al. Ocular biometrics and uncorrected visual acuity for detecting myopia in Chinese school students. Sci. Rep. 2022, 12, 18644. [Google Scholar] [CrossRef] [PubMed]
- Major, E.; Dutson, T.; Moshirfar, M. Cycloplegia in Children: An Optometrist’s Perspective. Clin. Optom. 2020, 12, 129–133. [Google Scholar] [CrossRef]
- Yazdani, N.; Sadeghi, R.; Momeni-Moghaddam, H.; Zarifmahmoudi, L.; Ehsaei, A. Comparison of cyclopentolate versus tropicamide cycloplegia: A systematic review and meta-analysis. J. Optom. 2018, 11, 135–143. [Google Scholar] [CrossRef]
- Barraza-Bernal, M.J.; Ohlendorf, A.; Sanz Diez, P.; Feng, X.; Yang, L.H.; Lu, M.X.; Wahl, S.; Kratzer, T. Prediction of refractive error and its progression: A machine learning-based algorithm. BMJ Open Ophthalmol. 2023, 8, e001298. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, Y.; Wang, L.; Chen, X.; Tang, J.; Liu, L.; Ma, T.; Ju, L.; Chen, Y.; Ge, Z.; et al. Effect of vault on predicting postoperative refractive error for posterior chamber phakic intraocular lens based on a machine learning model. J. Cataract. Refract. Surg. 2024, 50, 319–327. [Google Scholar] [CrossRef]
| Demographic Characteristics | (N = 614 Participants) |
| Age (Years) | n (%) |
| 8 | 62 (10.1%) |
| 9 | 166 (27.0%) |
| 10 | 39 (6.4%) |
| 12 | 220 (35.8%) |
| 13 | 127 (20.7%) |
| Mean (SD) | 11.3 (1.8) |
| Sex: Female (%) | 276 (45.0%) |
| Wearing refractive correction (%) | 221 (36.0%) |
| Cycloplegic agent | |
| 0.5% Tropicamide | 505 (82.2%) |
| 1% Cyclopentolate Hydrochloride | 109 (17.8%) |
| Ocular Biometer | |
| IOLMaster 700 | 207 (33.7%) |
| Optical Biometer SW-9000 | 407 (66.3%) |
| Ocular characteristics | (N = 1221 eyes) |
| Cycloplegic SER (diopter) | Number of eyes (%) |
| ≤−6.0 | 7 (0.6%) |
| >−6.0 to ≤−3.0 | 186 (15.2%) |
| >−3.0 to ≤−0.5 | 472 (38.7%) |
| >−0.5 to ≤0.5 | 279 (22.9%) |
| >0.5 to ≤3.0 | 257 (21.1%) |
| >3.0 | 20 (1.6%) |
| Mean (SD) | −0.93 (1.92) |
| Non-cycloplegic SER (diopter) | |
| ≤−6.0 | 12 (1.0%) |
| >−6.0 to ≤−3.0 | 226 (18.5%) |
| > −3.0 to ≤−0.5 | 583 (47.8%) |
| > −0.5 to ≤0.5 | 321 (26.3%) |
| >0.5 to ≤3.0 | 70 (5.7%) |
| >3.0 | 9 (0.7%) |
| Mean (SD) | −1.45 (1.79) |
| Uncorrected visual acuity | |
| 20/200 or worse | 68 (5.6%) |
| >20/200–20/100 | 203 (16.6%) |
| >20/100–20/50 | 220 (18.0%) |
| 20/40 | 80 (6.6%) |
| 20/33 | 103 (8.4%) |
| 20/25 | 101 (8.3%) |
| 20/20 or better | 446 (36.5%) |
| Intraocular pressure (mmHg): Mean (SD) | 16.9 (2.4) |
| Ocular Biometric Values | Mean (SD) |
| Axial length (mm) | 24.2 (1.1) |
| Corneal curvature radius (mm) | 7.90 (0.28) |
| Axial length/corneal curvature radius ratio | 3.07 (0.13) |
| Anterior chamber depth (mm) | 3.31 (0.34) |
| Central corneal thickness (μm) | 550 (31) |
| SER = Spherical equivalent refraction; SD = standard deviation | |
| Eyes | Observed Mean (SD) | Predicted Mean (SD) | Mean Difference (Predicted–Observed) (95% Confidence Interval) (Predicted–Observed) | Absolute Difference (95% Confidence Interval) (Predicted–Observed) | R2 | |
|---|---|---|---|---|---|---|
| Overall | 1221 | −0.93 (1.92) | −0.89 (1.86) | 0.05 (0.02, 0.07) | 0.32 (0.31, 0.34) | 0.95 |
| By age (Years) | ||||||
| 8 | 120 | 0.16 (1.43) | 0.19 (1.28) | 0.03 (−0.05, 0.11) | 0.34 (0.29, 0.40) | 0.90 |
| 9 | 330 | −0.14 (1.39) | −0.10 (1.28) | 0.04 (−0.01, 0.08) | 0.32 (0.29, 0.36) | 0.90 |
| 10 | 78 | −0.11 (1.52) | −0.13 (1.50) | −0.02 (−0.10, 0.06) | 0.30 (0.25, 0.34) | 0.94 |
| 12 | 440 | −1.61 (1.89) | −1.55 (1.89) | 0.06 (0.02, 0.11) | 0.32 (0.29, 0.35) | 0.95 |
| 13 | 253 | −1.57 (2.14) | −1.50 (2.08) | 0.07 (0.01, 0.12) | 0.33 (0.29, 0.37) | 0.96 |
| By cycloplegic spherical equivalent refraction (Diopter) | ||||||
| ≤−3.0 | 193 | −4.12 (0.94) | −4.03 (1.06) | 0.09 (0.02, 0.17) | 0.39 (0.34, 0.43) | 0.88 |
| >−3.0 to ≤−0.5 | 472 | −1.55 (0.72) | −1.40 (0.81) | 0.14 (0.11, 0.18) | 0.30 (0.27, 0.32) | 0.89 |
| >−0.5 to ≤0.5 | 279 | 0.11 (0.30) | 0.22 (0.47) | 0.11 (0.07, 0.15) | 0.29 (0.26, 0.32) | 0.61 |
| >0.5 | 277 | 1.28 (0.96) | 1.07 (0.89) | −0.21 (−0.26, −0.15) | 0.37 (0.32, 0.41) | 0.88 |
| Observed Cycloplegic Spherical Equivalent (Diopter) | |||||||
|---|---|---|---|---|---|---|---|
| Predicted Cycloplegic Spherical Equivalent from XGBoost (Diopter) | ≤−6.0 | >−6.0 to ≤−3.0 | >−3.0 to ≤−0.5 | >−0.5 to ≤0.5 | >0.5 to ≤3.0 | >3.0 | Total |
| ≤−6.0 | 6 (0.5%) | 3 (0.3%) | 0 | 0 | 0 | 0 | 9 (0.7%) |
| >−6.0 to ≤−3.0 | 1 (0.1%) | 144 (11.8%) | 7 (0.6%) | 0 | 0 | 0 | 152 (12.5%) |
| >−3.0 to ≤−0.5 | 0 | 39 (3.2%) | 399 (32.7%) | 18 (1.5%) | 0 | 0 | 456 (37.4%) |
| >−0.5 to ≤0.5 | 0 | 0 | 66 (5.4%) | 181 (14.8%) | 38 (3.1%) | 0 | 285 (23.3%) |
| >0.5 to ≤3.0 | 0 | 0 | 0 | 80 (6.6%) | 217 (17.8%) | 5 (0.4%) | 302 (24.7%) |
| >3.0 | 0 | 0 | 0 | 0 | 2 (0.2%) | 15 (1.2%) | 17 (1.4%) |
| Total | 7 (0.6%) | 186 (15.2%) | 472 (38.7%) | 279 (22.9%) | 257 (21.1%) | 20 (1.6%) | 1221 (100%) |
| Percent agreement = 78.8%, weight Kappa = 0.84 (0.82, 0.86) | |||||||
| Per-Eye Analysis | Per-Person Analysis | |||
|---|---|---|---|---|
| Model | Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) |
| Using predicted cycloplegic SER ≤ −0.5 D from XGBoost | 90.1% (87.3%, 92.3%) | 96.8% (94.8%, 98.0%) | 90.7% (87.8%, 93.6%) | 95.2% (92.4%, 98.0%) |
| Using predicted myopia yes/no from Random Forest | 93.4% (91.1%, 95.1%) | 96.4% (94.4%, 97.7%) | 93.3% (90.3%, 95.6%) | 94.7% (91.8%, 97.6%) |
| Myopia Rate (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Number of Students | Observed (Using Non-Cycloplegic SER ≤ −0.5 D in Either Eye) | Observed (Using Cycloplegic SER ≤ −0.5 D in Either Eye) | XGBoost (Using Predicted Cycloplegic SER ≤ −0.5 D in Either Eye) | Random Forest (Using the Predicted Presence of Myopia in Either Eye) | |
| Overall | 614 | 471 (76.7%) (73.4–80.1%) | 386 (62.9%) (59.0–66.7%) | 361 (58.8%) (54.9–62.7%) | 372 (60.6%) (56.7–64.5%) |
| By age (Years) | |||||
| 8 | 62 | 35 (56.5%) (44.1–68.8%) | 20 (32.3%) (20.6–43.9%) | 17 (27.4%) (16.3–40.2%) | 17 (27.4%) (16.3–38.5%) |
| 9 | 166 | 104 (62.7%) (55.3–70.0%) | 69 (41.6%) (34.1–49.1%) | 63 (38.0%) (30.6–45.3%) | 68 (41.0%) (33.5–48.4%) |
| 10 | 39 | 27 (69.2%) (54.8–83.7%) | 17 (43.6%) (28.0–59.2%) | 16 (41.0%) (25.6–56.5%) | 17 (43.6%) (28.0–59.2%) |
| 12 | 220 | 200 (90.9%) (87.1–94.7%) | 185 (84.1%) (79.3–88.9%) | 175 (79.6%) (73.6–84.7%) | 180 (81.8%) (76.7–86.9%) |
| 13 | 127 | 105 (82.7%) (76.1–89.3%) | 95 (74.8%) (67.3–82.4%) | 90 (70.9%) (63.0–78.8%) | 90 (70.9%) (63.0–78.8%) |
| XGBoost for Predicting Cycloplegic SER | Random Forest for Predicting Myopia Status | ||||||
|---|---|---|---|---|---|---|---|
| Subgroups | Number of eyes | Observed Cycloplegic SER (SD) | Predicted SER (SD) | Mean Difference (Predicted– Observed) (SD) | Mean Absolute Difference (SD) | R2 | AUC (95% CI) |
| Overall | 1221 | −0.93 (1.92) | −0.89 (1.86) | 0.05 (0.44) | 0.32 (0.30) | 0.95 | 0.991 (0.988, 0.995) |
| By Cycloplegic agent | |||||||
| Cyclopentolate | 212 | 0.22 (1.29) | 0.22 (1.16) | 0.004 (0.49) | 0.35 (0.34) | 0.86 | 0.991 (0.983, 0.999) |
| Tropicamide | 1009 | −1.18 (1.94) | −1.12 (1.90) | 0.06 (0.43) | 0.32 (0.29) | 0.95 | 0.990 (0.987, 0.994) |
| By Ocular Biometer | |||||||
| IOLMaster 700 | 407 | −0.32 (1.70) | −0.30 (1.61) | 0.02 (0.43) | 0.31 (0.29) | 0.95 | 0.992 (0.986, 0.997) |
| Optical Biometer SW-9000 | 814 | −1.24 (1.95) | −1.18 (1.91) | 0.06 (0.45) | 0.33 (0.31) | 0.94 | 0.991 (0.986, 0.995) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, R.S.; Ying, B.; Wang, J.; Cui, H.; Ying, G.; Oatts, J.T. Myopia Prediction Using Machine Learning: An External Validation Study. Vision 2025, 9, 84. https://doi.org/10.3390/vision9040084
Chandra RS, Ying B, Wang J, Cui H, Ying G, Oatts JT. Myopia Prediction Using Machine Learning: An External Validation Study. Vision. 2025; 9(4):84. https://doi.org/10.3390/vision9040084
Chicago/Turabian StyleChandra, Rajat S., Bole Ying, Jianyong Wang, Hongguang Cui, Guishuang Ying, and Julius T. Oatts. 2025. "Myopia Prediction Using Machine Learning: An External Validation Study" Vision 9, no. 4: 84. https://doi.org/10.3390/vision9040084
APA StyleChandra, R. S., Ying, B., Wang, J., Cui, H., Ying, G., & Oatts, J. T. (2025). Myopia Prediction Using Machine Learning: An External Validation Study. Vision, 9(4), 84. https://doi.org/10.3390/vision9040084





