Effect of Acetazolamide on Intraocular Pressure After Uneventful Phacoemulsification Using an Anterior Chamber Maintainer
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Selection and Study Design
2.2. Treatment Protocol and Measurements
2.3. Surgical Technique
2.4. Postoperative Treatment
2.5. Statistical Analysis
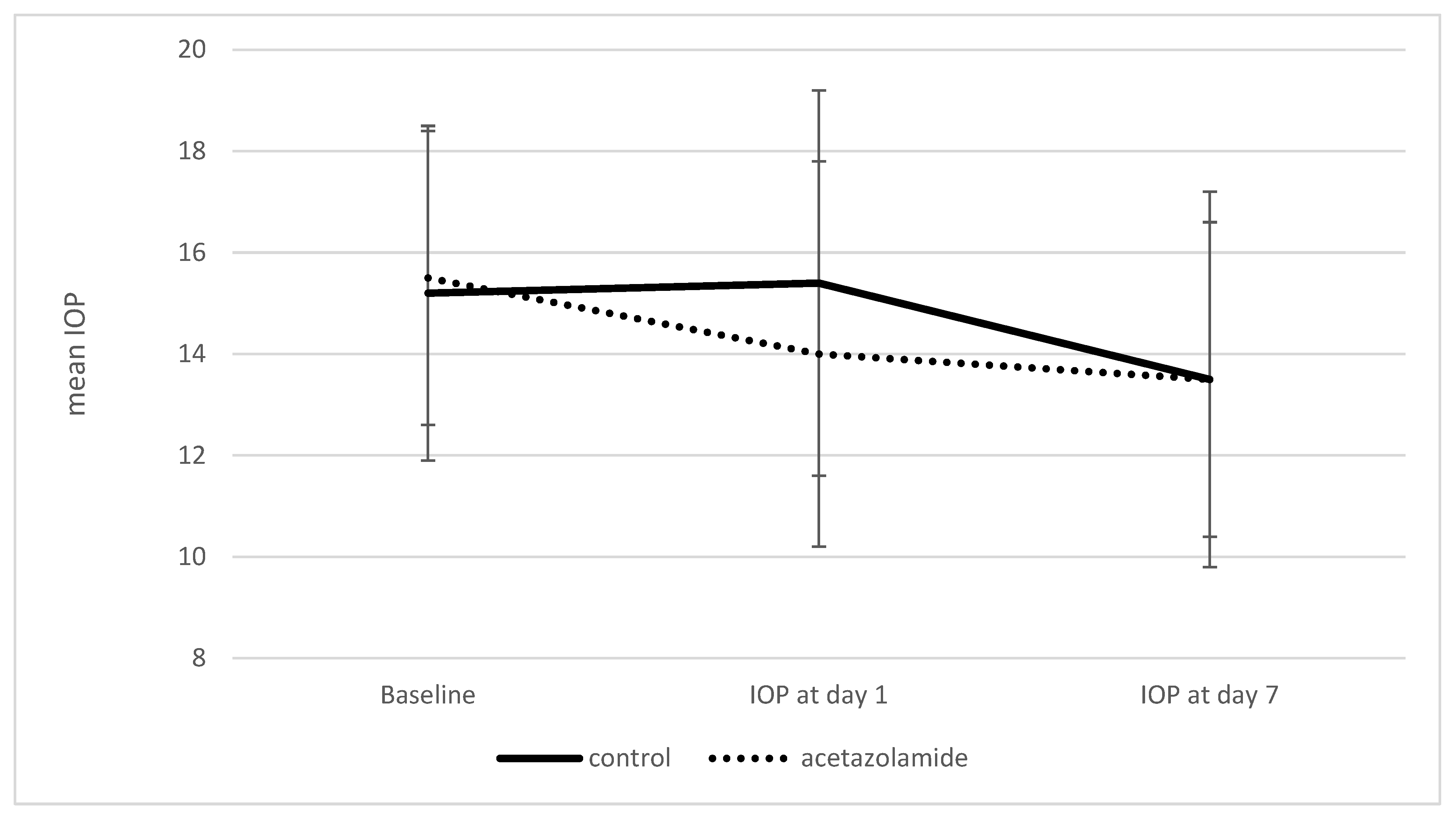
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lidder, A.K.; Vanner, E.A.; Chang, T.C.; Lum, F.; Rothman, A.L. Intraocular Pressure Spike Following Stand-Alone Phacoemulsification in the IRIS® Registry (Intelligent Research in Sight). Ophthalmology 2024, 131, 780–789. [Google Scholar] [CrossRef]
- Grzybowski, A.; Kanclerz, P. Early postoperative intraocular pressure elevation following cataract surgery. Curr. Opin. Ophthalmol. 2019, 30, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Slabaugh, M.A.; Bojikian, K.D.; Moore, D.B.; Chen, P.P. Risk factors for acute postoperative intraocular pressure elevation after phacoemulsification in glaucoma patients. J. Cataract Refract. Surg. 2014, 40, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Annam, K.; Chen, A.J.; Lee, I.M.; Paul, A.A.; Rivera, J.J.; Greenberg, P.B. Risk factors for early intraocular pressure elevation after cataract surgery in a cohort of United States veterans. Mil. Med. 2018, 183, 427–433. [Google Scholar] [CrossRef]
- Miller, K.M.; Oetting, T.A.; Tweeten, J.P.; Carter, K.; Lee, B.S.; Lin, S.; Nanji, A.A.; Shorstein, N.H.; Musch, D.C.; American Academy of Ophthalmology Preferred Practice Pattern Cataract/Anterior Segment Panel. Cataract in the adult eye preferred practice pattern. Ophthalmology 2022, 129, 1–126. [Google Scholar] [CrossRef]
- Shingleton, B.J.; Rosenberg, R.B.; Teixeira, R.; O’Donoghue, M.W. Evaluation of intraocular pressure in the immediate postoperative period after phacoemulsification. J. Cataract Refract. Surg. 2007, 33, 1953–1957. [Google Scholar] [CrossRef]
- O’Brien, P.D.; Ho, S.L.; Fitzpatrick, P.; Power, W. Risk factors for a postoperative intraocular pressure spike after phacoemulsification. Can. J. Ophthalmol. 2007, 42, 51–55. [Google Scholar] [CrossRef]
- Alwitry, A.; Rotchford, A.; Gardner, I. First day review after uncomplicated phacoemulsification: Is it necessary? Eur. J. Ophthalmol. 2006, 16, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Kasetti, S.R.; Desai, S.P.; Sivakumar, S.; Sunderraj, P. Preventing intraocular pressure increase after phacoemulsification and the role of perioperative apraclonidine. J. Cataract Refract. Surg. 2002, 28, 2177–2180. [Google Scholar] [CrossRef]
- Dayanir, V.; Özcura, F.; Kir, E.; Topaloğlu, A.; Ozkan, S.B.; Aktunç, T. Medical control of intraocular pressure after phacoemulsification. J. Cataract Refract. Surg. 2005, 31, 484. [Google Scholar] [CrossRef]
- Rainer, G.; Menapace, R.; Findl, O.; Georgopoulos, M.; Kiss, B.; Heinze, G. Randomised fellow eye comparison of the effectiveness of dorzolamide and apraclonidine on intraocular pressure following phacoemulsification cataract surgery. Eye 2000, 14, 757–760. [Google Scholar] [CrossRef][Green Version]
- Wedrich, A.; Menapace, R. Intraocular pressure following small-incision cataract surgery and polyHEMA posterior chamber lens implantation. A comparison between acetylcholine and carbachol. J. Cataract Refract. Surg. 1992, 18, 500–505. [Google Scholar] [CrossRef]
- Ermis, S.S.; Ozturk, F.; Inan, U.U. Comparing the effects of travoprost and brinzolamide on intraocular pressure after phacoemulsification. Eye 2005, 19, 303–307. [Google Scholar] [CrossRef]
- Rainer, G.; Menapace, R.; Schmetterer, K.; Findl, O.; Georgopoulos, M.; Vass, C. Effect of dorzolamide and latanoprost on intraocular pressure after small incision cataract surgery. J. Cataract Refract. Surg. 1999, 25, 1624–1629. [Google Scholar] [CrossRef]
- Çetinkaya, A.; Akman, A.; Akova, Y.A. Effect of topical brinzolamide 1% and brimonidine 0.2% on intraocular pressure after phacoemulsification. J. Cataract Refract. Surg. 2004, 30, 1736–1741. [Google Scholar] [CrossRef]
- Rainer, G.; Menapace, R.; Findl, O.; Petternel, V.; Kiss, B.; Georgopoulos, M. Intraindividual comparison of the effects of a fixed dorzolamide–timolol combination and latanoprost on intraocular pressure after small incision cataract surgery. J. Cataract Refract. Surg. 2001, 27, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Bömer, T.G.; Lagreze, W.D.; Funk, J. Intraocular pressure rise after phacoemulsification with posterior chamber lens implantation: Effect of prophylactic medication, wound closure, and surgeon’s experience. Br. J. Ophthalmol. 1995, 79, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Abbasoglu, E.; Tekeli, O.; Celikdogan, A.; Gürsel, E. A topical or oral carbonic anhydrase inhibitor to control ocular hypertension after cataract surgery. Eur. J. Ophthalmol. 2000, 10, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zohdy, G.A.; Rogers, Z.A.; Lukaris, A.; Sells, M.; Roberts-Harry, T.J. A comparison of the effectiveness of dorzolamide and acetazolamide in preventing post-operative intraocular pressure rise following phacoemulsification surgery. J. R. Coll. Surg. Edinb. 1998, 43, 344–346. [Google Scholar]
- Beidner, B.; Rothkoff, L.; Blumenthal, M. The effect of acetazolamide on early increased intraocular pressure after cataract extraction. Am. J. Ophthalmol. 1977, 83, 565–568. [Google Scholar] [CrossRef]
- Zamvar, U.; Dhillon, B. Postoperative IOP prophylaxis practice following uncomplicated cataract surgery: A UK-wide consultant survey. BMC Ophthalmol. 2005, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.L.; Bach-Holm, D.; Holm, L.M.; Vestergaard, A.H. Prophylactic treatment of intraocular pressure elevation after uncomplicated cataract surgery in nonglaucomatous eyes—A systematic review. Acta Ophthalmol. 2019, 97, 545–557. [Google Scholar] [CrossRef]
- Hayashi, K.; Yoshida, M.; Sato, T.; Manabe, S.I. Effect of topical hypotensive medications for preventing intraocular pressure increase after cataract surgery in eyes with glaucoma. Am. J. Ophthalmol. 2019, 205, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yoshida, M.; Manabe, S.I.; Yoshimura, K. Prophylactic effect of oral acetazolamide against intraocular pressure elevation after cataract surgery in eyes with glaucoma. Ophthalmology 2017, 124, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yoshida, M.; Sato, T.; Manabe, S.I.; Yoshimura, K. Intraocular pressure elevation after cataract surgery and its prevention by oral acetazolamide in eyes with pseudoexfoliation syndrome. J. Cataract Refract. Surg. 2018, 44, 175–181. [Google Scholar] [CrossRef]
- Blumenthal, M.; Assia, E.I.; Chen, V.; Avni, I. Using an anterior chamber maintainer to control intraocular pressure during phacoemulsification. J. Cataract Refract. Surg. 1994, 20, 93–96. [Google Scholar] [CrossRef]
- Chawla, H.B.; Adams, A.D. Use of the anterior chamber maintainer in anterior segment surgery. J. Cataract Refract. Surg. 1996, 22, 172–177. [Google Scholar] [CrossRef]
- Grinbaum, A.; Blumenthal, M.; Assia, E. Comparison of intraocular pressure profiles during cataract surgery by phacoemulsification and extracapsular cataract extraction. Ophthalmic Surg. Lasers Imaging 2003, 34, 182–186. [Google Scholar] [CrossRef]
- Malik, K.P.S.; Goel, R. Nucleus management with Blumenthal technique: Anterior chamber maintainer. Indian J. Ophthalmol. 2009, 57, 23–25. [Google Scholar] [CrossRef]
- David, R.; Zangwill, L.; Stone, D.; Yassur, Y. Epidemiology of intraocular pressure in a population screened for glaucoma. Br. J. Ophthalmol. 1987, 71, 766–771. [Google Scholar] [CrossRef]
- Rubin, D.B. Estimating causal effects from large data sets using propensity scores. Ann. Intern. Med. 1997, 127, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Brízido, M.; Rodrigues, P.F.; Almeida, A.C.; Abegão Pinto, L. Cataract surgery and IOP: A systematic review of randomised controlled trials. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1257–1266. [Google Scholar] [CrossRef]
- Zetterström, C.; Behndig, A.; Kugelberg, M.; Montan, P.; Lundström, M. Changes in intraocular pressure after cataract surgery: Analysis of the Swedish National Cataract Register data. J. Cataract Refract. Surg. 2015, 41, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Rothman, A.L.; Chang, T.C.; Lum, F.; Vanner, E.A. Intraocular pressure changes following stand-alone phacoemulsification: An IRIS Registry analysis. Am. J. Ophthalmol. 2023, 245, 25–36. [Google Scholar] [CrossRef] [PubMed]
| Control (No. of Eyes = 98) | Acetazolamide (No. of Eyes = 98) | p-Value | |
|---|---|---|---|
| Age (Mean ± SD) | 70.3 ± 10.1 | 71.2 ± 10.6 | 0.578 |
| Male | 51 (52.0%) | 38 (38.8%) | 0.06 |
| Female | 47 (48.0%) | 60 (61.2%) | |
| Mean preoperative IOP (mmHg, Mean ± SD) | 15.2 ± 3.3 | 15.5 ± 2.9 | 0.409 |
| Control (No. of Eyes = 98, Mean ± SD, mmHg) | Acetazolamide (No. of Eyes = 98, Mean ± SD, mmHg) | p-Value | |
|---|---|---|---|
| Preoperative IOP | 15.2 ± 3.3 | 15.5 ± 2.9 | 0.409 |
| IOP at day 1 | 15.4 ± 3.8 | 14.0 ± 3.8 | 0.01 |
| IOP at day 7 | 13.5 ± 3.7 | 13.5 ± 3.1 | 0.95 |
| Variable | Effect Estimate (95% CI) | p-Value |
|---|---|---|
| Pre-operative IOP (mmHg) | 0.57 (0.46 to 0.69) | <0.001 |
| Age | ||
| Under 65 | 1.6 (0.7 to 2.49) | 0.001 |
| 65–74 | 0.78 (−0.04 to 1.6) | 0.061 |
| 75 and older | 0 (reference) | |
| Female | 0.24 (−0.48 to 0.96) | 0.51 |
| Variable | Effect Estimate (95% CI) | p-Value |
|---|---|---|
| Acetazolamide prophylaxis | −0.07 (−0.01 to −0.12) | 0.01 |
| Pre-operative BCVA (LogMAR) | 0.18 (0.12 to 0.23) | <0.001 |
| Age (years) | 0.01 (0 to 0.01) | <0.001 |
| Male | 0.05 (0 to 0.1) | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kratz, A.; Kornhauser, T.; Walter, E.; Abuhasira, R.; Goldberg, I.; Hadad, A. Effect of Acetazolamide on Intraocular Pressure After Uneventful Phacoemulsification Using an Anterior Chamber Maintainer. Vision 2025, 9, 73. https://doi.org/10.3390/vision9030073
Kratz A, Kornhauser T, Walter E, Abuhasira R, Goldberg I, Hadad A. Effect of Acetazolamide on Intraocular Pressure After Uneventful Phacoemulsification Using an Anterior Chamber Maintainer. Vision. 2025; 9(3):73. https://doi.org/10.3390/vision9030073
Chicago/Turabian StyleKratz, Assaf, Tom Kornhauser, Eyal Walter, Ran Abuhasira, Ivan Goldberg, and Aviel Hadad. 2025. "Effect of Acetazolamide on Intraocular Pressure After Uneventful Phacoemulsification Using an Anterior Chamber Maintainer" Vision 9, no. 3: 73. https://doi.org/10.3390/vision9030073
APA StyleKratz, A., Kornhauser, T., Walter, E., Abuhasira, R., Goldberg, I., & Hadad, A. (2025). Effect of Acetazolamide on Intraocular Pressure After Uneventful Phacoemulsification Using an Anterior Chamber Maintainer. Vision, 9(3), 73. https://doi.org/10.3390/vision9030073





