Ocular and General Proprioception in Dyslexic Children: A Review of Their Diurnal and Nocturnal Dysfunctions and Their Repercussions
Abstract
1. Introduction
- (1)
- Integrative approach to theories: No theory that has been proposed to explain dyslexia, whether sensory (magnocellular, visual, etc.), motor (cerebellar) or cognitive (phonological, visual-attentional, etc.), is favored or discarded, as each is based on solid arguments,
- (2)
- Embodied cognition: Brain function is considered in interaction with the body and the environment, avoiding a strictly disconnected approach to both exteroceptive and interoceptive sensory data,
- (3)
- Reconsidering comorbidities: Rather than seeing them as independent disorders, they could be manifestations of the same neurodevelopmental dysfunction, suggesting a common origin,
- (4)
- Neurogenesis and the mechanisms of cerebral plasticity present particular temporal dynamics. It is therefore crucial not only to observe what dyslexics were like before learning to read, but also to follow them over the entire nycthemeris in order to analyze the evolution of these processes throughout the sleep-wake cycle,
- (5)
- Back to medical basics: Clinical observation and a holistic approach to the patient are essential. This approach naturally led to an interest in the work of the Portuguese physician Henrique Martins da Cunha, who associated dyslexia with a proprioceptive and visual dysfunction, responsible for disparate bodily and cognitive signs that appear unrelated to those unfamiliar with the physiology of proprioception [5].
2. Proprioceptive Dysfunction and Dyslexia
3. Postural Control and Dyslexia
4. Sleep Alterations in Dyslexic Children: Correlations with Neuroplasticity and Postural Control
5. Motor Imaging and Dyslexia
6. Spatial Attention and Dyslexia
- -
- In the lateral dimension, their response was influenced by the hand with which they started the exercise in proprioceptive condition.
- -
- In the radial dimension, they tended to project forward in the visuo-proprioceptive condition and to make more backward errors in the proprioceptive condition.
- -
- Dyslexic children showed a forward bias when exploring clockwise, and better accuracy when exploring anti-clockwise, particularly when starting on the left.
7. Interactions Between Postural Control, Proprioception and Visual Localization
8. Dyslexia, Proprioception and Multisensory Integration
- (1)
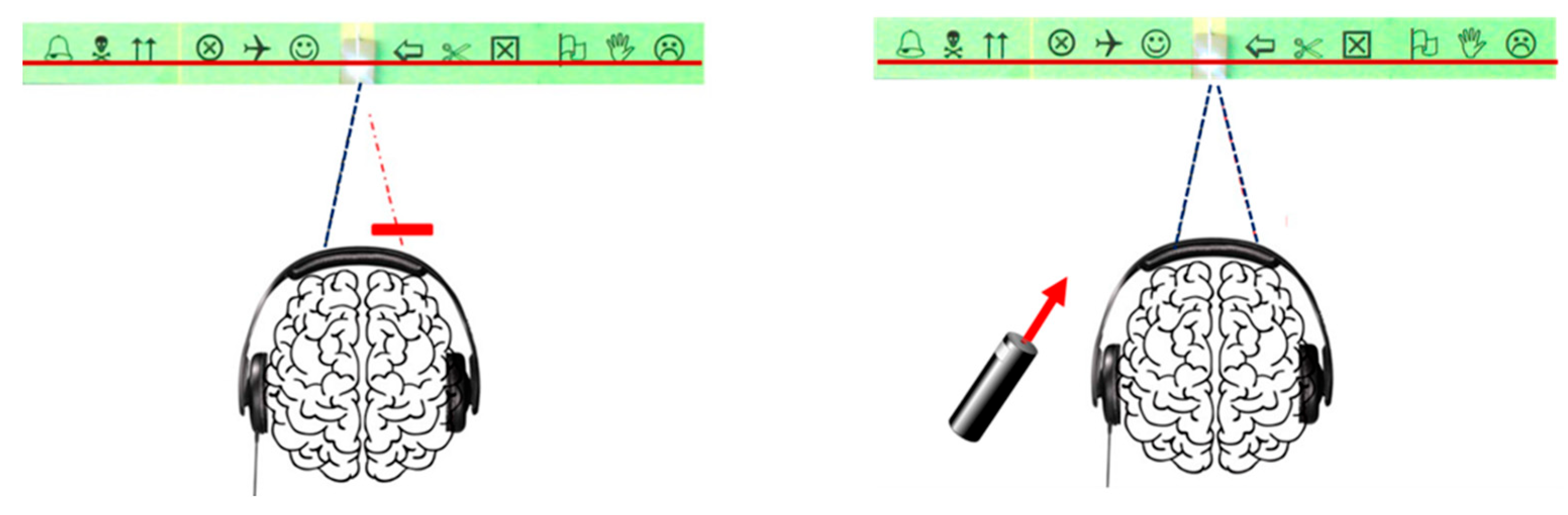
- Visual loss caused by listening to sounds:
- -
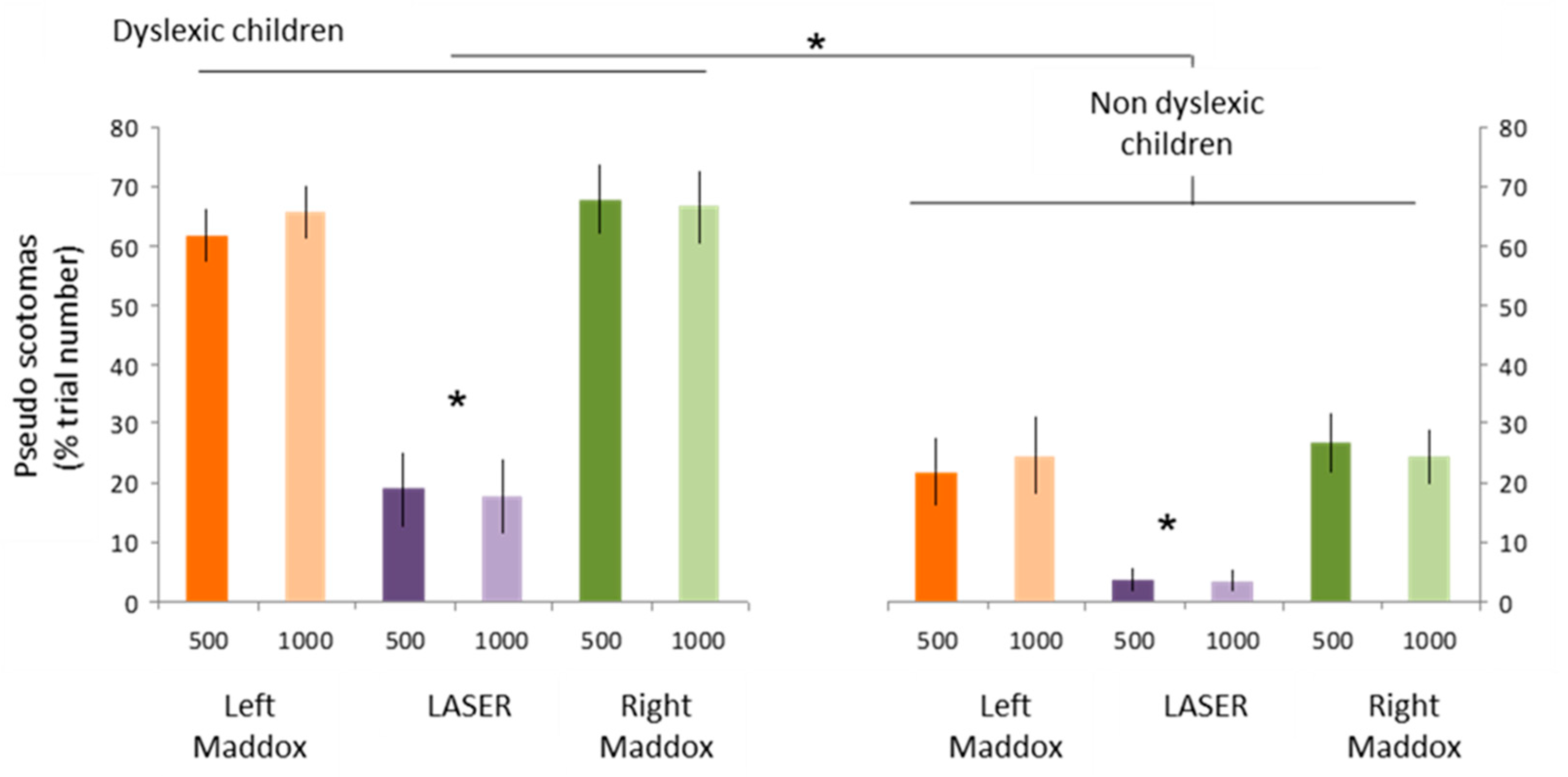
- Auditory stimulation leads to visual perception disturbances in the form of visual losses called pseudoscotomas, as they do not correspond to organic but rather functional scotomas,
- -
- This phenomenon is not specific to dyslexic children, but it is clearly more marked in this population, (Figure 8),
- -
- Pseudo-scotomas only occur when binocular vision is disrupted by the Maddox screen.
- (2)
- Temporal stability of pseudoscotomas: In the same subject, the same stimulation may or may not cause pseudoscotomas to appear.
- (3)
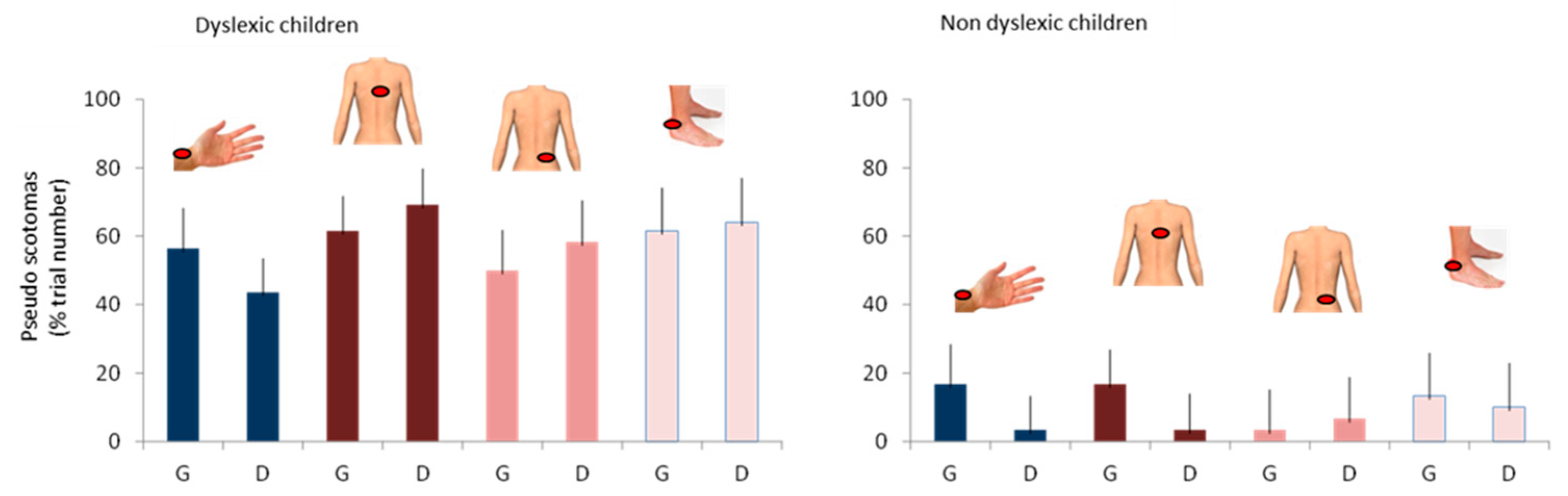
- Spatial stability of pseudo-scotomes:
- -
- The same sound stimulus can induce visual alterations in different spatial regions, in a random and unpredictable way. This stochastic nature suggests that the brain, which relies on predictive mechanisms, has difficulty adapting.
- (4)
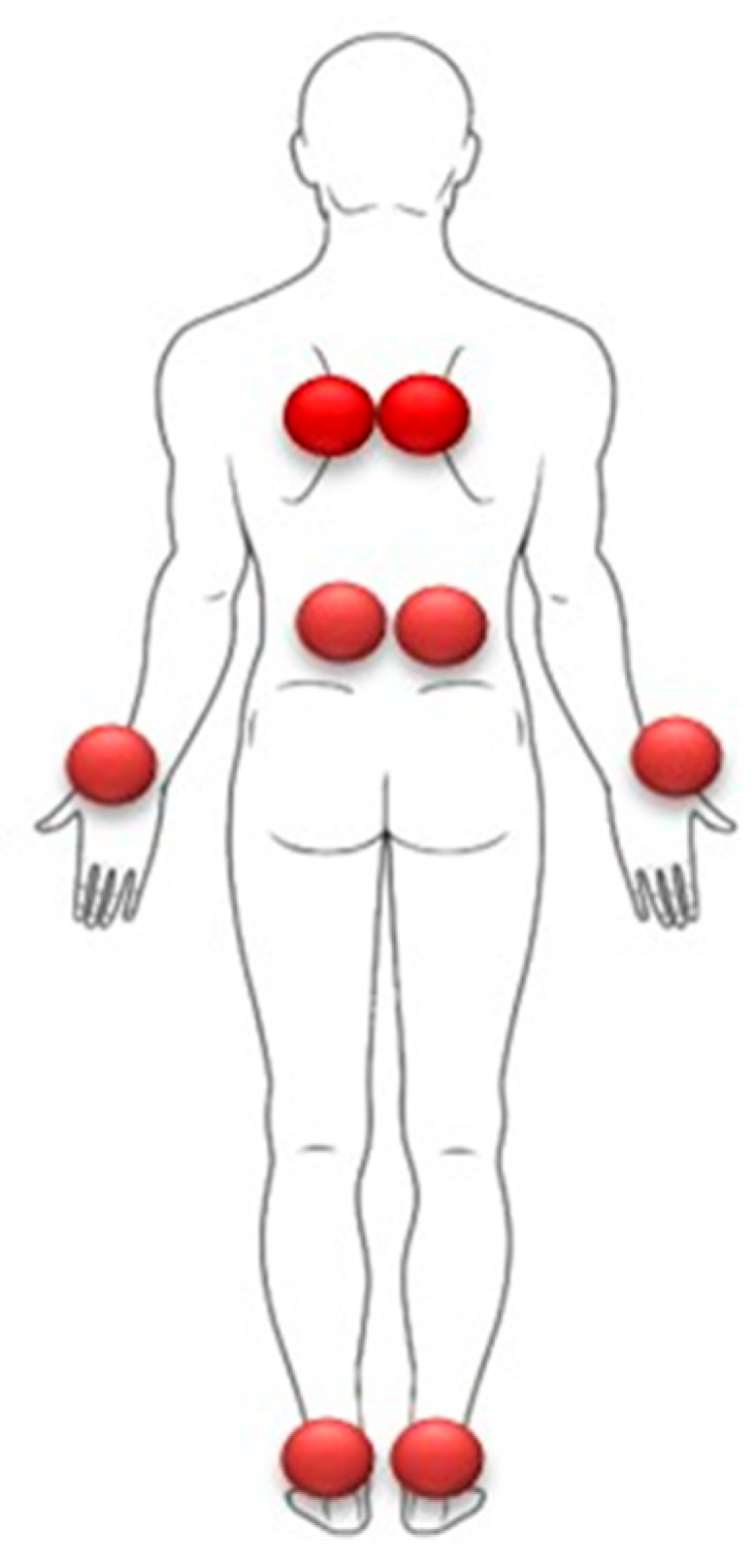
- Effects of proprioceptive stimulation:
- -
- The application of muscular vibrations at 70 Hz induces effects similar to auditory stimulation. These visual disturbances appear independently of the postural function of the targeted muscles, suggesting that the phenomenon is based on a dysfunction of global proprioceptive processes, rather than a simple alteration of proprioception involved in postural regulation (Figure 9).
9. Statistical Analysis of Subjective Signs of Proprioceptive Dysfunction and Dyslexia
10. Management of Dysproprioception
- (1)
- Discreet deflection of light rays reaching the retina thanks to very low-power prisms (usually between 0.50 and 1.50Δ) designed to regulate the tone of the lower oblique muscles. The prisms are always bilateral, slightly asymmetrical in power, with a superior-external base, and are designed to correct vertical heterophoria and its lability. This optical modification leads to a global postural readjustment, affecting not only the ocular muscles, but also all proprioceptive chains down to the feet. Their very low power is necessary for their action on paravertebral muscle tone [80]. They do not induce habituation, and influence all proprioceptive chains, from the eye muscles to the lower limbs.
- (2)
- Postural adaptation with specific insoles incorporating thin overlays (0.3 to 2 mm) to modulate plantar pressure sensors and act on vertical heterophoria [72]. The location of stimuli is codified and determined according to their postural repercussions [81]. These insoles adjust the perception of support on the ground, inducing targeted proprioceptive adaptation.
- (3)
- Ergonomic optimization and respiratory reprogramming. Ergonomics during visual tasks are adapted to balance postural tone and minimize involvement of the upper oblique muscles. The use of a desk inclined at 30° promotes symmetrization of proprioceptive information. At the same time, specific daily breathing exercises aim to normalize diaphragmatic function, thereby improving sleep quality, particularly REM sleep. This optimization reduces micro-arousals, limiting their deleterious effects on attention and procedural memory [82]. Interaction between the ocular and oral proprioceptive systems via the trigeminal nerve can generate sensory interference. Additional management may be required in cases of persistent primary swallowing, oral ventilation, dental occlusion disorders or enlarged tonsils. These abnormalities can play a detrimental role in the severity of sleep disorders, by disrupting lingual and pharyngeal motricity and/or decreasing upper airway airflow [83].
11. Discussion
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peterson, R.L.; Pennington, B.F. Developmental dyslexia. Annu. Rev. Clin. Psychol. 2015, 11, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Körne, G.; Bruder, J. Clinical neurophysiology of visual and auditory processing in dyslexia: A review. Clin. Neurophysiol. 2010, 121, 1794–1809. [Google Scholar] [CrossRef]
- Galli, R.; Pozzoli, U.; Lorusso, M.L.; Facoetti, A.; Molteni, M. Wide and diffuse perceptual modes characterize dyslexics in vision and audition. Perception 2008, 37, 1745–1764. [Google Scholar] [CrossRef]
- Decarli, G.; Franchin, L.; Vitali, F. Motor skills and capacities in developmental dyslexia: A systematic review and meta-analysis. Acta Psychol. 2024, 246, 104269. [Google Scholar] [CrossRef]
- Da Cunhà, H.M. Syndrome de Déficience Posturale. In Actualités en Rééducation Fonctionnelle et en Réadaptation, 4th ed.; Masson: Paris, France, 1979; pp. 27–31. [Google Scholar]
- Laprevotte, J.; Papaxanthis, C.; Saltarelli, S.; Quercia, P.; Gaveau, J. Movement detection thresholds reveal proprioceptive impairments in developmental dyslexia. Sci. Rep. 2021, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Ramus, F.; Pidgeon, E.; Frith, U. The relationship between motor control and phonology in dyslexic children. J. Child Psychol. Psychiatry 2003, 44, 712–722. [Google Scholar] [CrossRef]
- Peterka, R.J. Sensory. integration for human balance control. Handb. Clin. Neurol. 2018, 159, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Bucci, M.P.; Villeneuve, P. Interaction between Feet and Gaze in Postural Control. Brain Sci. 2022, 12, 1459. [Google Scholar] [CrossRef]
- Frank, J.; Levinson, H. Dysmetric dyslexia and dyspraxia. Hypothesis and study. J. Am. Acad. Child Adolesc. Psychiatry 1973, 12, 690–701. [Google Scholar] [CrossRef]
- Nicolson, R.I.; Fawcett, A.J.; Dean, P. Developmental dyslexia: The cerebellar deficit hypothesis. Trends Neurosci. 2001, 24, 508–511. [Google Scholar] [CrossRef]
- Fawcett, A.J. Balance and reading are separate symptoms of dyslexia. Dev. Med. Child Neurol. 2011, 53, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, K.S.; Talcott, J.B. Impaired balance in developmental dyslexia? A meta-analysis of the contending evidence. J. Child Psychol. Psychiatry 2006, 47, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Walsh, V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997, 20, 147–152. [Google Scholar] [CrossRef]
- Cornelissen, P.L.; Hansen, P.C.; Hutton, J.L.; Evangelinou, V.; Stein, J.F. Magnocellular visual function and children’s single word reading. Vis. Res. 1998, 38, 471–482. [Google Scholar] [CrossRef]
- Nicolson, R.I.; Fawcett, A.J. Developmental dyslexia, learning and cerebellum. J. Neural Transm. Suppl. 2005, 69, 19–36. [Google Scholar] [CrossRef]
- Moon, K.M.; Kim, J.; Seong, Y.; Suh, B.C.; Kang, K.; Choe, H.K.; Kim, K. Proprioception, the regulator of motor function. BMB Rep. 2021, 54, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, T.; Vernet, P.; Creuzot-Garcher, C.; Robichon, F.; Bron, A.M.; Quercia, P. Static postural control in children with developmental dyslexia. Neurosci. Lett. 2006, 403, 211–215. [Google Scholar] [CrossRef]
- Quercia, P.; Seigneuric, A.; Chariot, S.; Vernet, P.; Pozzo, T.; Bron, A.M.; Creuzot-Garcher, C.; Robichon, F. Ocular proprioception and developmental dyslexia. Sixty clinical observations. J. Fr. Ophtalmol. 2005, 28, 713–723. [Google Scholar] [CrossRef]
- Assaraf, E.; Blecher, R.; Heinemann-Yerushalmi, L.; Krief, S.; Vinestock, R.C.; Biton, I.E.; Brumfeld, V.; Rotkopf, R.; Avisar, E.; Agar, G.; et al. Piezo2 expressed in proprioceptive neurons is essential for skeletal integrity. Nat. Commun. 2020, 11, 3168. [Google Scholar] [CrossRef]
- Bornstein, B.; Watkins, B.; Passini, F.S.; Blecher, R.; Assaraf, E.; Meng Sui, X.; Brumfeld, V.; Tsoory, M.; Kröger, S.; Zelzer, E. The mechanosensitive ion channel ASIC2 mediates both proprioceptive sensing and spinal alignment. Exp. Physiol. 2024, 109, 135–147. [Google Scholar] [CrossRef]
- Hodges, P.; Gandevia, S. Changes in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J. Appl. Physiol. 2000, 89, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.; Quercia, P.; Michel, C.; Pozzo, T.; Bonnetblanc, F. Cognitive demands impair postural control in developmental dyslexia: A negative effect that can be compensated. Neurosci. Lett. 2009, 462, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Quercia, P.; Demougeot, L.; Dos Santos, M.; Bonnetblanc, F. Integration of proprioceptive signals and attentional capacity during postural control are impaired but subject to improvement in dyslexic children. Exp. Brain Res. 2011, 209, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Quercia, P.; Seigneuric, A.; Chariot, S.; Bron, A.M.; Creuzot-Garcher, C.; Robichon, F. Proprioception changes induced by prismatic glasses wear in children suffering from developmental dyslexia. J. Fr. Ophtalmol. 2007, 30, 380–389. [Google Scholar] [CrossRef]
- Blumberg, M.S.; Dooley, J.C.; Tiriac, A. Sleep, plasticity, and sensory neurodevelopment. Neuron. 2022, 20, 3230–3242. [Google Scholar] [CrossRef]
- Kocjan, J.; Gzik-Zroska, B.; Nowakowska, K.; Burkacki, M.; Suchoń, S.; Michnik, R.; Czyżewski, D.; Adamek, M. Impact of diaphragm function parameters on balance maintenance. PLoS ONE 2018, 13, e0208697. [Google Scholar] [CrossRef]
- Lespert, Y.; Rivals, I.; Ing, R.K.; Clavel, L.; Similowski, T.; Sandoz, B.; Attali, V. Coupling Between Posture and Respiration Among the Postural Chain: Toward a Screening Tool for Respiratory-Related Balance Disorders. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 4338–4346. [Google Scholar] [CrossRef]
- Mercier, L.; Pivik, R.T.; Busby, K. Sleep patterns in reading disabled children. Sleep 1993, 16, 207–215. [Google Scholar] [CrossRef][Green Version]
- Bruni, O.; Ferri, R.; Novelli, L.; Terribili, M.; Troianiello, M.; Finotti, E.; Leuzzi, V.; Paolo Curatolo, P. Sleep spindle activity is correlated with reading abilities in developmental dyslexia. Sleep 2009, 32, 1333–1340. [Google Scholar] [CrossRef]
- Fostick, L.; Babkoff, H.; Zukerman, G. Effect of 24 hours of sleep deprivation on auditory and linguistic perception: A comparison among young controls, sleep-deprived participants, dyslexic readers, and aging adults. J. Speech Lang. Hear. Res. 2014, 57, 1078–1088. [Google Scholar] [CrossRef]
- Carotenuto, M.; Esposito, M.; Cortese, S.; Laino, D.; Verrotti, A. Children with developmental dyslexia showed greater sleep disturbances than controls, including problems initiating and maintaining sleep. Acta Paediatr. 2016, 105, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.R.H.; Gaskell, M.G.; Weighall, A.R.; Warmington, M.; Reid, A.M.; Henderson, L.M. Consolidation of vocabulary is associated with sleep in typically developing children, but not in children with dyslexia. Dev. Sci. 2018, 21, e12639. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Guilleminault, C. Obstructive sleep apnea and related disorders. Neurol. Clin. 1996, 14, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Respiration sur le dos. Available online: https://vimeo.com/950315095 (accessed on 15 March 2025).
- Position d’endormissement. Available online: https://vimeo.com/950314942 (accessed on 15 March 2025).
- Méthode Guillarme. Available online: https://www.methode-guillarme.com (accessed on 15 March 2025).
- Bruni, O.; Ottaviano, S.; Guidetti, V.; Romoli, M.; Innocenzi, M.; Cortesi, F.; Giannotti, F. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J. Sleep Res. 1996, 5, 251–261. [Google Scholar] [CrossRef]
- Heaton, S.C.; Reader, S.K.; Preston, A.S.; Fennell, E.B.; Puyana, O.E.; Gill, N.; Johnson, J.H. The Test of Everyday Attention for Children (TEA-Ch): Patterns of performance in children with ADHD and clinical controls. Child Neuropsychol. 2001, 7, 251–264. [Google Scholar] [CrossRef]
- Champagne, L.; Safi, D.; Gauthier, B. Alouette-R normative data for French-speaking school-aged children living in Quebec. Int. J. Lang. Commun. Disord. 2024, 59, 2071–2086. [Google Scholar] [CrossRef]
- Kershner, J.R. Network dynamics in dyslexia: Review and implications for remediation. Res. Dev. Disabil. 2016, 59, 24–34. [Google Scholar] [CrossRef]
- Lokhandwala, S.; Spencer, R.M.C. Relations between sleep patterns early in life and brain development: A review. Dev. Cogn. Neurosci. 2022, 56, 101130. [Google Scholar] [CrossRef]
- Ruffino, C.; Papaxanthis, C.; Lebon, F. Neural plasticity during motor learning with motor imagery practice: Review and perspectives. Neuroscience 2017, 341, 61–78. [Google Scholar] [CrossRef]
- Ruffino, C.; Gaveau, J.; Papaxanthis, C.; Lebon, F. An acute session of motor imagery training induces use-dependent plasticity. Sci. Rep. 2019, 9, 20002. [Google Scholar] [CrossRef]
- Badino, L.; D’Ausilio, A.; Fadiga, L.; Metta, G. Computational validation of the motor contribution to speech perception. Top. Cogn. Sci. 2014, 6, 461–475. [Google Scholar] [CrossRef] [PubMed]
- D’Ausilio, A.; Bufalari, I.; Salmas, P.; Fadiga, L. The role of the motor system in discriminating normal and degraded speech sounds. Cortex 2012, 48, 882–887. [Google Scholar] [CrossRef]
- Van de Walle de Ghelcke, A.; Skoura, X.; Edwards, M.G.; Quercia, P.; Papaxanthis, C. Action representation deficits in adolescents with developmental dyslexia. J. Neuropsychol. 2021, 15, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Fitts, P. The information capacity of the human motor system in controlling the amplitude of movement. J. Exp. Psychol. 1992, 121, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Valdois, S.; Bosse, M.L.; Tainturier, M.J. The cognitive deficits responsible for developmental dyslexia: Review of evidence for a selective visual attentional disorder. Dyslexia 2004, 10, 339–363. [Google Scholar] [CrossRef]
- Jewell, G.; McCourt, M.E. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 2000, 38, 93–110. [Google Scholar] [CrossRef]
- Odoj, B.; Balslev, D. Role of Oculoproprioception in Coding the Locus of Attention. J. Cogn. Neurosci. 2016, 28, 517–528. [Google Scholar] [CrossRef]
- Balslev, D.; Odoj, B.; Karnath, H.O. Role of somatosensory cortex in visuospatial attention. J. Neurosci. 2013, 33, 18311–18318. [Google Scholar] [CrossRef]
- Rossetti, Y.; Rode, G.; Pisella, L.; Farné, A.; Li, L.; Boisson, D.; Pereninet, M.T. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 1998, 395, 166–169. [Google Scholar] [CrossRef]
- Karnath, H.O.; Christ, K.; Hartje, W. Decrease of contralateral neglect by neck muscle vibration and spatial orientation of trunk midline. Brain 1993, 116, 383–396. [Google Scholar] [CrossRef]
- Bisiach, E.; Bulgarelli, C.; Sterzi, R.; Vallar, G. Line bisection and cognitive plasticity of unilateral neglect of space. Brain Cogn. 1983, 2, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Michel, C. Simulating unilateral neglect in normals: Myth or reality? Restor. Neurol. Neurosci. 2006, 24, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Bidot, S.; Bonnetblanc, F.; Quercia, P. Left minineglect or inverse pseudoneglect in children with dyslexia? Neuroreport 2011, 22, 93–96. [Google Scholar] [CrossRef]
- Vieira, S.; Quercia, P.; Bonnetblanc, F.; Michel, C. Space representation in children with dyslexia and children without dyslexia: Contribution of line bisection and circle centering tasks. Res. Dev. Disabil. 2013, 34, 3997–4008. [Google Scholar] [CrossRef]
- Michel, C.; Quercia, P.; Joubert, L. Representational Bias in the Radial Axis in Children With Dyslexia: A Landmarks Alignment Study. J. Learn. Disabil. 2019, 52, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kavounoudias, A.; Gilhodes, J.C.; Roll, R.; Roll, J.P. From balance regulation to body orientation: Two goals for muscle proprioceptive information processing? Exp. Brain Res. 1999, 124, 80–88. [Google Scholar] [CrossRef]
- Roll, R.; Velay, J.L.; Roll, J.P. Eye and neck proprioceptive messages contribute to the spatial coding of retinal input in visually oriented activities. Exp. Brain Res. 1991, 85, 423–431. [Google Scholar] [CrossRef]
- Nuthmann, A.; Kliegl, R. An examination of binocular reading fixations based on sentence corpus data. J. Vis. 2009, 9, 31. [Google Scholar] [CrossRef]
- Blythe, H.I.; Liversedge, S.P.; Joseph, H.S.S.L.; White, S.J.; Findlay, J.M.; Rayner, K. The binocular coordination of eye movements during reading in children and adults. Vis. Res. 2006, 46, 3898–3908. [Google Scholar] [CrossRef]
- Kirkby, J.A.; Webster, L.A.D.; Blythe, H.I.; Liversedge, S.P. Binocular coordination during reading and non-reading tasks. Psychol. Bull. 2008, 134, 742–763. [Google Scholar] [CrossRef]
- Eden, G.F.; Stein, J.F.; Wood, H.M.; Wood, F.B. Differences in eye movements and reading problems in dyslexic and normal children. Vis. Res. 1994, 34, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, C.; Piñero, D.P. Clinical Characterization of Oculomotricity in Children with and without Specific Learning Disorders. Brain Sci. 2020, 10, 836. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, J.A.; Blythe, H.I.; Drieghe, D.; Liversedge, S.P. Reading text increases binocular disparity in dyslexic children. PLoS ONE 2011, 6, e27105. [Google Scholar] [CrossRef]
- Matheron, E.; Kapoula, Z. Vertical phoria and postural control in upright stance in healthy young subjects. Clin. Neurophysiol. 2008, 119, 2314–2320. [Google Scholar] [CrossRef]
- Matheron, E.; Kapoula, Z. Incidence of vertical phoria on postural control during binocular vision: What perspective for prevention to nonspecific chronic pain management? Med. Hypothesis Discov. Innov. Ophthalmol. 2015, 4, 27–30. [Google Scholar] [PubMed]
- Quercia, P.; Quercia, M.; Feiss, L.J.; Allaert, F. The distinctive vertical heterophoria of dyslexics. Clin. Ophthalmol. 2015, 9, 1785–1797. [Google Scholar] [CrossRef]
- Mettey, A.; Bouvier, A.M.; Jooste, V.; Boucher, Y.; Quercia, P. Are changes in the stomatognatic system able to modify the eye balance in dyslexia? J. Oral. Biol. Craniofac. Res. 2019, 9, 166–171. [Google Scholar] [CrossRef]
- Loureau, S.; Poulain, R.; Cappe, C.; Janin, M. Les variations tactiles plantaires influencent-elles les Hétérophories Verticales? Mov. Sport Sci.-Sci. Mot. 2023, 4, 53–61. [Google Scholar] [CrossRef]
- Scott, A.B. Ocular mobility. In Physiology of the Human Eye; Harper and Row: Hagerstown, MD, USA, 1979; pp. 577–642. [Google Scholar]
- Quercia, P. L’hétérophorie verticale du dyslexique au test de Maddox: Hétérophorie ou localisation spatiale erronée ? Etude en vidéo-oculographie de 14 cas. J. Français D’orthoptique 2008, 40, 25–45. [Google Scholar]
- Quercia, P.; Pozzo, T.; Marino, A.; Guillemant, A.L.; Cappe, C.; Gueugneau, N. Alteration in binocular fusion modifies audiovisual integration in children. Clin. Ophthalmol. 2019, 13, 1137–1145. [Google Scholar] [CrossRef]
- Quercia, P.; Pozzo, T.; Marino, A.; Guillemant, A.L.; Cappe, C.; Gueugneau, N. with Dyslexia Have Altered Cross-Modal Processing Linked to Binocular Fusion. A Pilot Study. Clin. Ophthalmol. 2020, 14, 437–448. [Google Scholar] [CrossRef]
- Available online: https://www.dysproprioception.fr/dossiers/VALIDATION-QUESTIONNAIRE-QUERCIA.pdf (accessed on 15 April 2025).
- Available online: https://www.dysproprioception.fr/dossiers/QUERCIA-QUESTIONNAIRE-proprioception-DYSL.pdf (accessed on 15 April 2025).
- Enjeux Sanitaires-p15. Available online: https://www.santepubliquefrance.fr (accessed on 15 March 2025).
- Baron, J.B.; Fowler, E. Prismatic lenses for vertigo and some experimental background of the role of the extrinsic ocular muscles in desequilibrium. Trans. Am. Acad. Ophthalm. Otolaryngol. 1952, 56, 916–926. [Google Scholar]
- Kavounoudias, A.; Roll, R.; Roll, J.P. The plantar sole is a ‘dynamometric map’ for human balance control. Neuroreport 1998, 9, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Pelayo, R. Sleep-disordered breathing in children. Ann. Med. 1998, 30, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.A.; Blumenthal, D.L.; Almasri, M.M.; Zalzal, H.; Riley, C.A.; Lawlor, C.M. Management of Obstructive Sleep Apnea in the Infant: A Systematic Review and Meta-analysis. Otolaryngol. Head. Neck Surg. 2025, 172, 759–773. [Google Scholar] [CrossRef]
- Scheveig, F.; Bucci, M.P. Postural and Proprioceptive Deficits Clinically Assessed in Children with Reading Disabilities: A Case-Control Study. Vision 2023, 7, 37. [Google Scholar] [CrossRef]
- Reynolds, D.; Nicolson, R.I. Follow-up of an exercise-based treatment for children with reading difficulties. Dyslexia 2007, 13, 78–96. [Google Scholar] [CrossRef]
- Ben Dhia, A.; Bucci, M.P.; Naffeti, C.; Ben Saad, H.; Hammouda, O.; Driss, T. Combined Cognitive and Motor Training Improves Reading, Writing and Motor Coordination in Dyslexic Children. Pediatr. Rep. 2025, 17, 46. [Google Scholar] [CrossRef]
- Virlet, L.; Sparrow, L.; Barela, J.; Berquin, P.; Bonnet, C. Proprioceptive intervention improves reading performance in developmental dyslexia: An eye-tracking study. Res. Dev. Disabil. 2024, 153, 104813. [Google Scholar] [CrossRef]
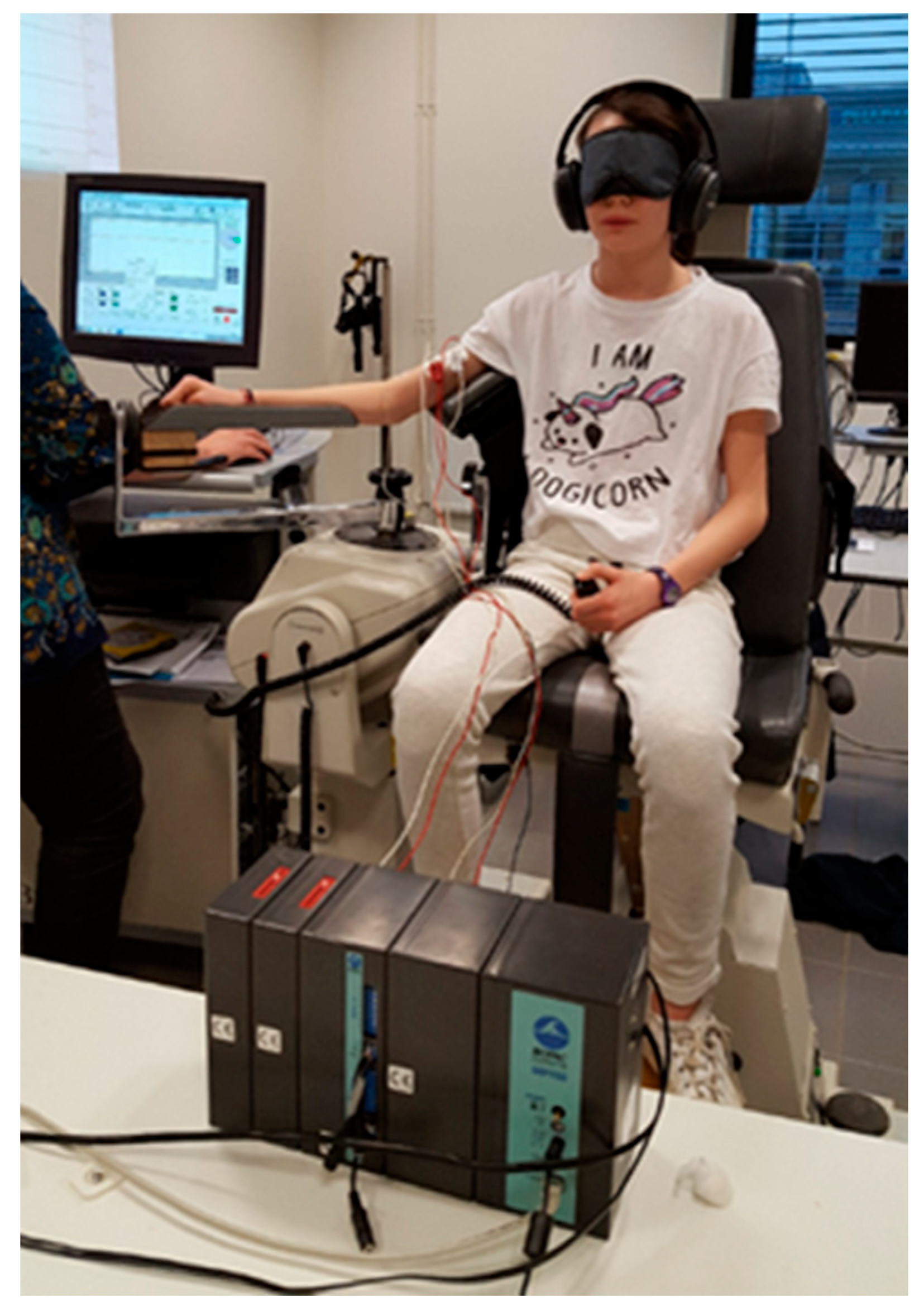
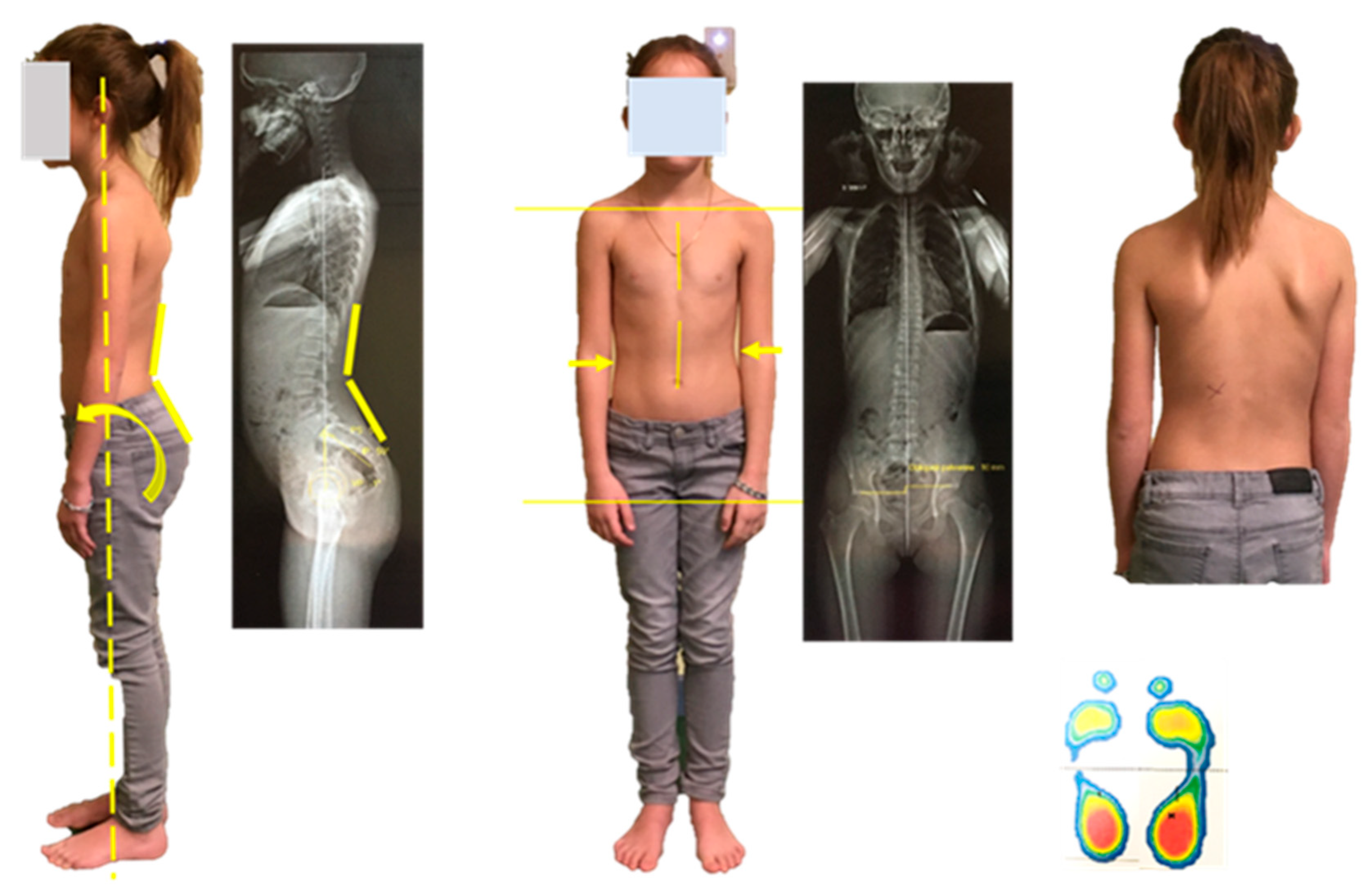
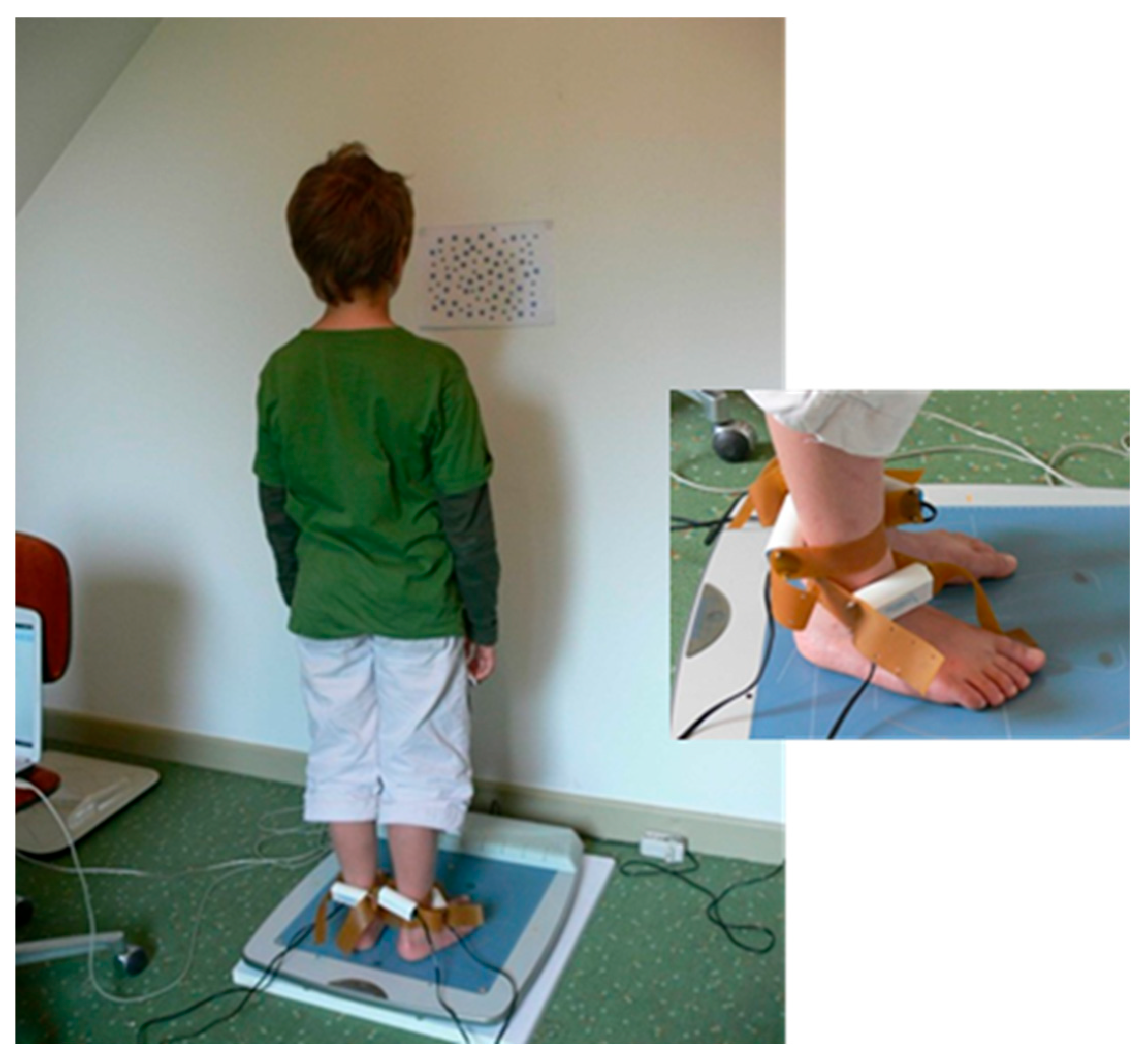

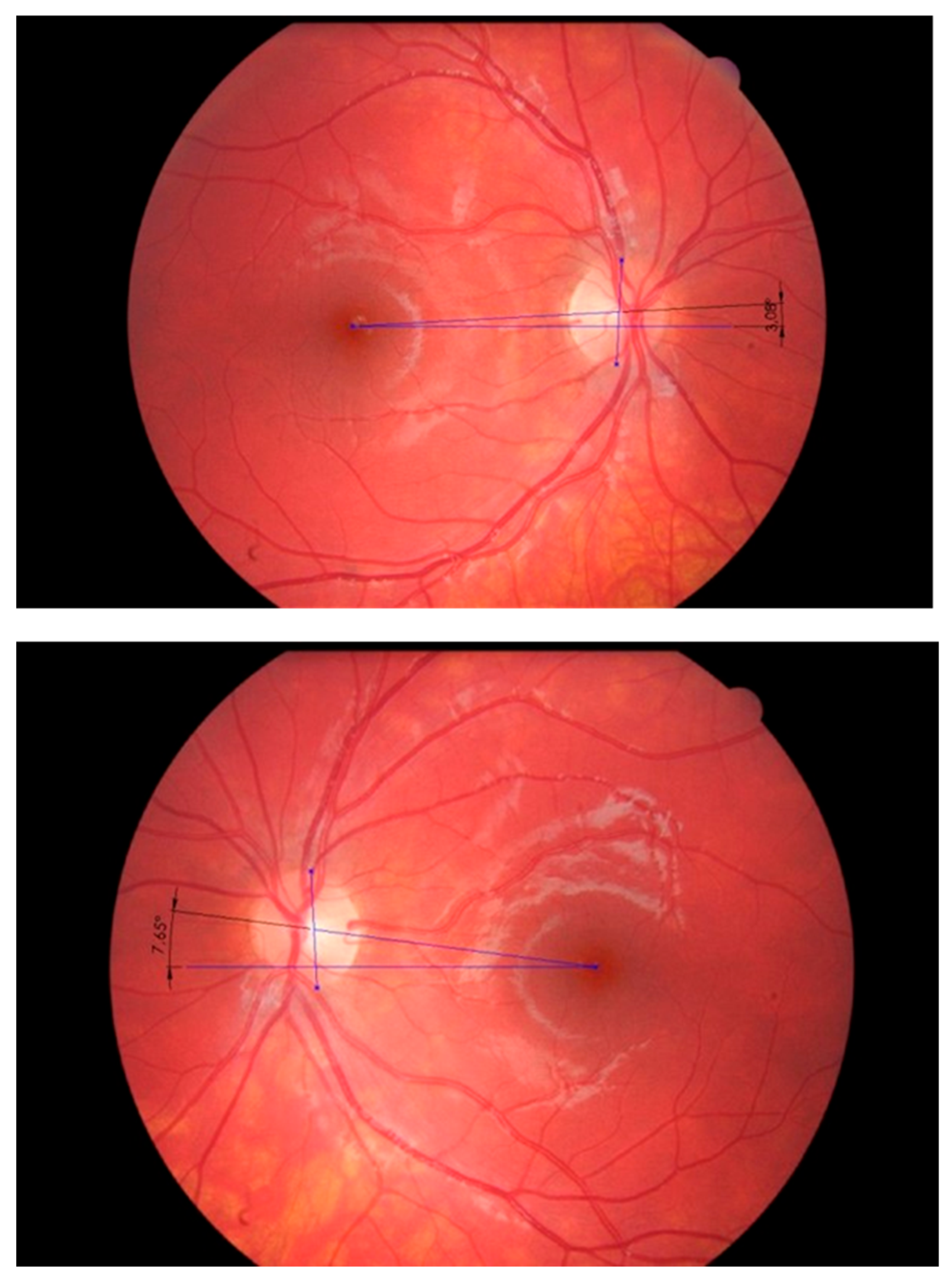
| M-3/M0 p-Value | M0/M+3 p-Value | |
|---|---|---|
| 0.16 | 0.08 |
| 0.84 | 0.03 |
| 1 | 0.09 |
| 0.81 | 0.04 |
| 0.81 | 0.07 |
| 0.38 | <0.001 |
| 0.13 | 0.13 |
| 0.01 | <0.001 |
| 0.35 | 0.003 |
| 0.46 | 0.20 |
| 0.07 | 0.20 |
| 0.84 | <0.001 |
| 0.26 | 0.04 |
| 0.16 | 0.31 |
| 1 | 0.28 |
| 0.50 | 0.003 |
| 1 | 0.03 |
| 0.41 | 0.01 |
| 1 | 0.02 |
| 0.38 | 0.002 |
| 0.86 | 0.09 |
| 0.08 | 0.12 |
| 0.46 | 0.008 |
| 1 | 0.04 |
| 0.08 | 1 |
| 0.65 | 0.02 |
| 0.02 | 0.001 |
| 0.23 | <0.001 |
| 0.72 | 0.03 |
| 0.87 | <0.001 |
| 0.42 | 0.003 |
| 0.815 | <0.001 |
| 0.107 | 0.14 |
| p-value of score differences (counting only signs with a frequency ≥ 3 | 0.69 | <0.001 |
| M-3/M0 | M0/M+3 | M-3/M+3 | p M-3/M0 | p M0/M+3 | p M-3/M+3 | |
|---|---|---|---|---|---|---|
| Number of words correctly read | 10.14 | 14.31 | 25.90 | 0.036 | 0.003 | <0.001 |
| Accuracy | 1.23 | 4.11 | 5.39 | 0.763 | 0.014 | 0.002 |
| Speed | 13.29 | 14.45 | 29.66 | 0.025 | 0.004 | <0.001 |
| Efficiency | 134.80 | 122.99 | 107.87 | 0.103 | 0.016 | <0.001 |
| Maddox in Front of Right Eye | Maddox in Front of Left Eye | Changes in the Lability Index | ||
|---|---|---|---|---|
| 1 | Seated in natural position without plantar support. | OV | ↓ | |
| 2 | Sitting upright without plantar support (modification of spinal proprioception) | OV | OV | +1 |
| 3 | Bielchowski test on the right shoulder | more ↓ | +1 | |
| 4 | Bielchowski test on the left shoulder | more ↑ | +1 | |
| 5 | Tip of tongue pressed against retro incisor papillae (V2 stimulation) | OV | ↓ | +1 |
| 6 | Tip of tongue pressed against lower incisors (stimulation of V3) | ↓ | ↓ | +1 |
| 7 | Tight lips (VII stimulation) | OV | OV | +1 |
| 8 | Standing upright with foot support on hard ground | OV | ↓ | +1 |
| 9 | Upright standing with plantar support on foam insoles (modification of plantar exteroception) | OV | ↓ | 0 |
| Lability Index | Σ = 7 | |||
| FOR EACH SYMPTOM, INDICATE THE NUMBER CORRESPONDING TO THE FREQUENCY 1 = Never, 2 = Occasionally (1 or 2 times/month), 3 = Sometimes (1 to 2 times/week), 4 = Often (3 to 5 times a week), 5 = Every day. | |
| 15 Questions for PARENTS (after examining the child’s sleep for at least 3–4 h of continuous sleep) | SCORE |
| The child jerks or moves body parts when falling asleep. | |
| The child has agitated daydream scenes when falling asleep. | |
| The child moves his legs a lot when he sleeps or often changes position during the night or kicks the bed covers. | |
| You have observed that your child sleepwalks | |
| Your child has nightmares (terrors) that he can’t remember the next morning. | |
| He has great difficulty waking up in the morning | |
| The child feels unable to move around, feels very tired when waking up in the morning. | |
| The child is sleepy during the day (falls asleep easily in the car, quiet, …) | |
| The child salivates a lot at night or there are traces of drool on the pillow in the morning. | |
| Child complains of a headache in the morning | |
| The child breathes with its mouth open while sleeping | |
| The child still pees or often gets up at night to go to the toilet. | |
| The child has trouble remembering lessons learned the night before (even though he knew them the night before). | |
| The child tends to be a little sleepy at times at school | |
| The child has an abnormal head position while sleeping (head tilted back and in extension). | |
| 19 Questions to be answered by the CHILD | |
| Muscular dimension | |
| Do you feel tired even if you haven’t exerted yourself physically or mentally? | |
| Is it hard for you to stand by and do nothing? | |
| Do you get a headache after school? | |
| Do you have recurring lower or upper back pain? | |
| Do your legs ever hurt? | |
| Is it hard for you to stare at a text (or a person) up close? | |
| Do you ever see double when you’re tired, after reading a text? | |
| Do you get out of breath quickly when you exert yourself (for example, as soon as you run)? | |
| Can you see blurred up close after reading a few lines (with your glasses, if you have them)? | |
| Spatial dimension | |
| Is it difficult for you to walk on something narrow (a beam, for example)? | |
| Is it difficult for you to catch an object on the first try—a ball, for example? | |
| Do you fall easily or twist your ankles? | |
| You bite your tongue or cheeks when you eat. | |
| Do you bump into simple obstacles (doorframes for example, …) as if you didn’t perceive the space around you properly? | |
| Perceptive dimension | |
| Do you feel like you’re reading without understanding what you’re reading? | |
| Do you find it hard to concentrate for long? | |
| When someone speaks to you, do you feel like you don’t really understand what you’re hearing? | |
| When you read, you have the impression that you can’t see properly: you skip words, you miss line breaks, … | |
| It’s hard for you to express an idea when you’re talking, and you have trouble constructing your sentences properly? | |
| TOTAL SCORE—Enter the SUM of the scores for all 34 questions | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quercia, P.; Chavet, K.; Gaveau, J. Ocular and General Proprioception in Dyslexic Children: A Review of Their Diurnal and Nocturnal Dysfunctions and Their Repercussions. Vision 2025, 9, 44. https://doi.org/10.3390/vision9020044
Quercia P, Chavet K, Gaveau J. Ocular and General Proprioception in Dyslexic Children: A Review of Their Diurnal and Nocturnal Dysfunctions and Their Repercussions. Vision. 2025; 9(2):44. https://doi.org/10.3390/vision9020044
Chicago/Turabian StyleQuercia, Patrick, Kalvin Chavet, and Jérémie Gaveau. 2025. "Ocular and General Proprioception in Dyslexic Children: A Review of Their Diurnal and Nocturnal Dysfunctions and Their Repercussions" Vision 9, no. 2: 44. https://doi.org/10.3390/vision9020044
APA StyleQuercia, P., Chavet, K., & Gaveau, J. (2025). Ocular and General Proprioception in Dyslexic Children: A Review of Their Diurnal and Nocturnal Dysfunctions and Their Repercussions. Vision, 9(2), 44. https://doi.org/10.3390/vision9020044





