Objective Refraction Status before and after Cycloplegia: From Childhood to Young Adulthood
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Analysis
2.3. Statistical Analysis
3. Results
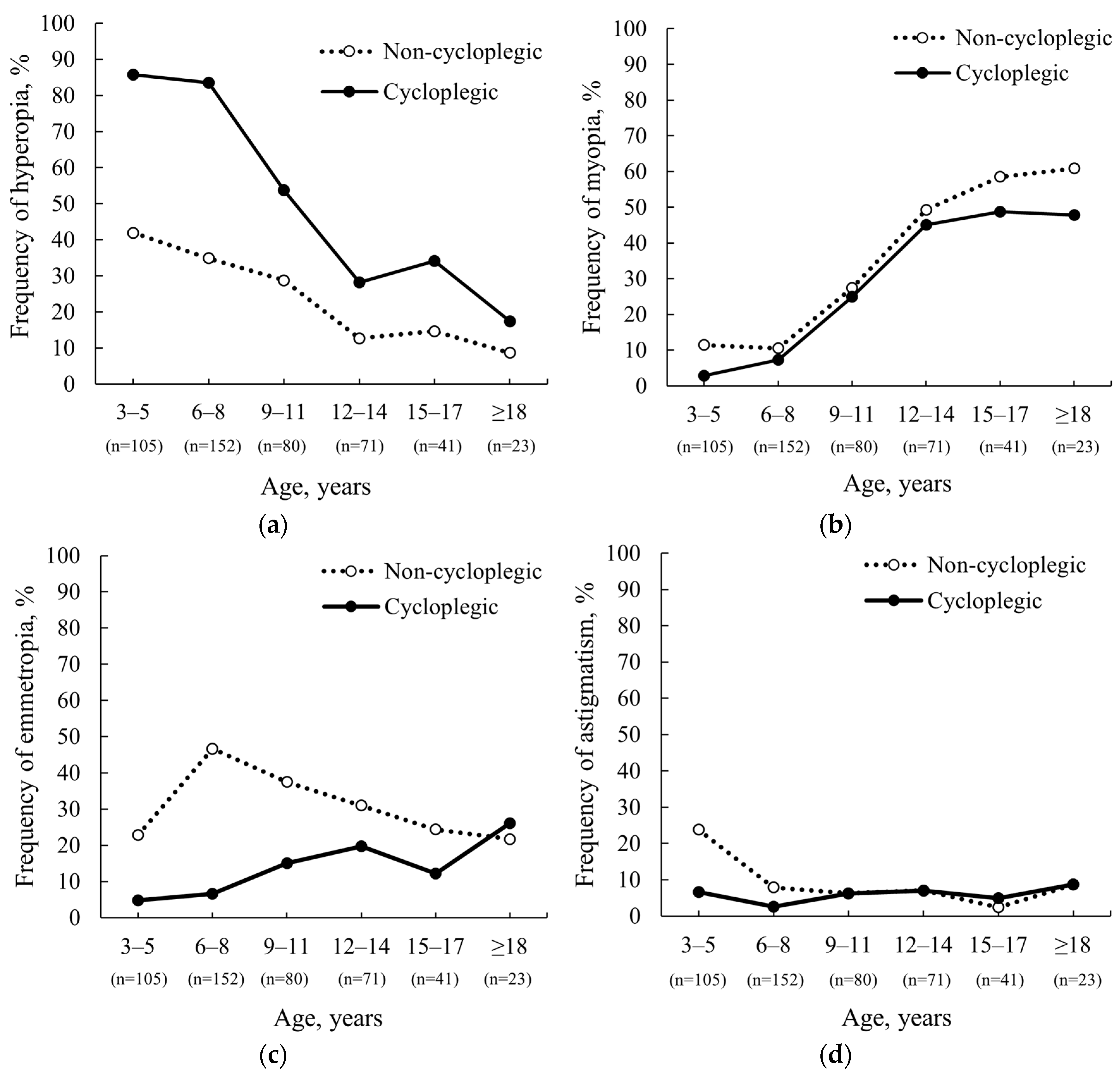
3.1. Frequency of Refractive Errors before and after Cycloplegia
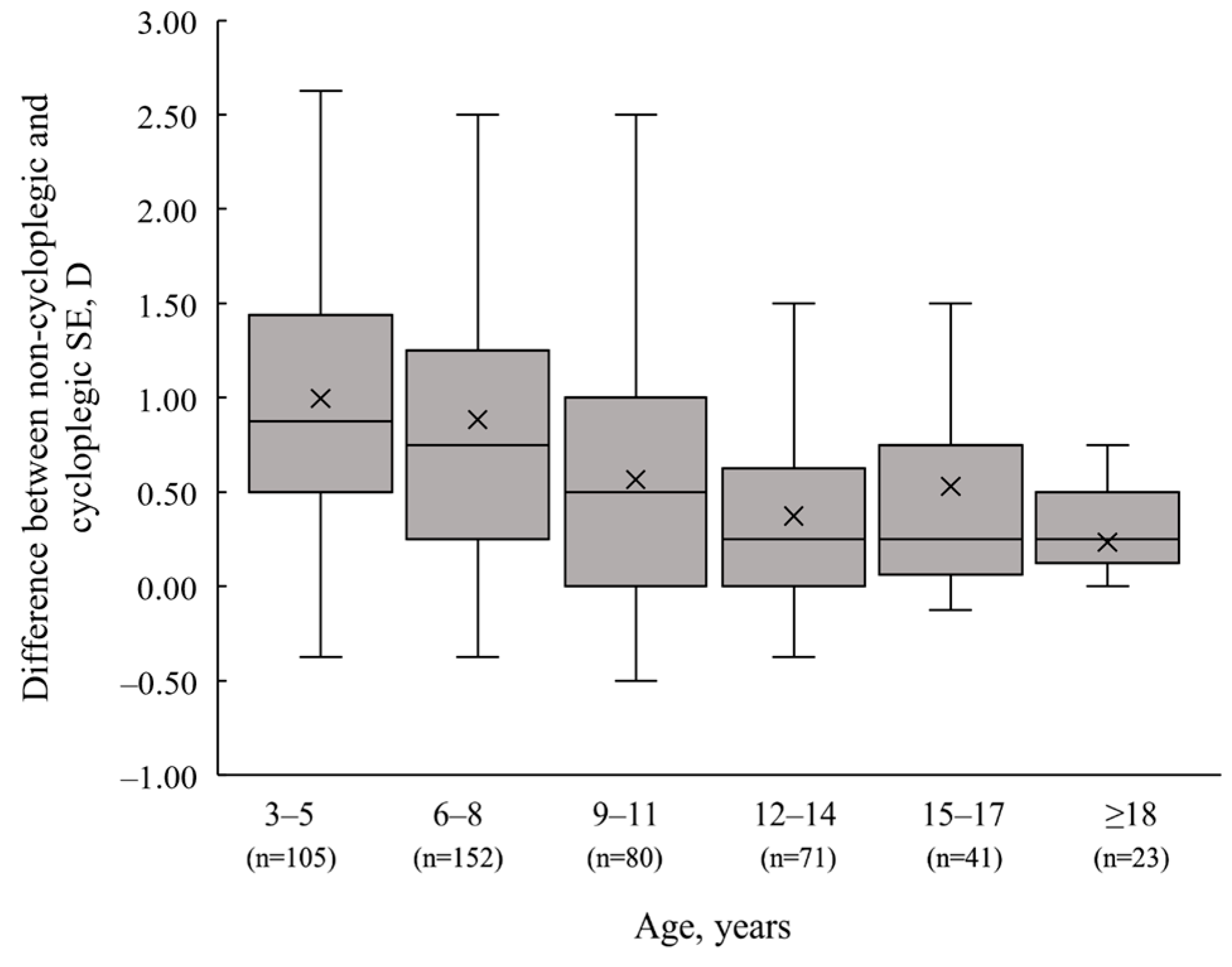
3.2. Spherical Equivalent (SE) Difference after Cycloplegia
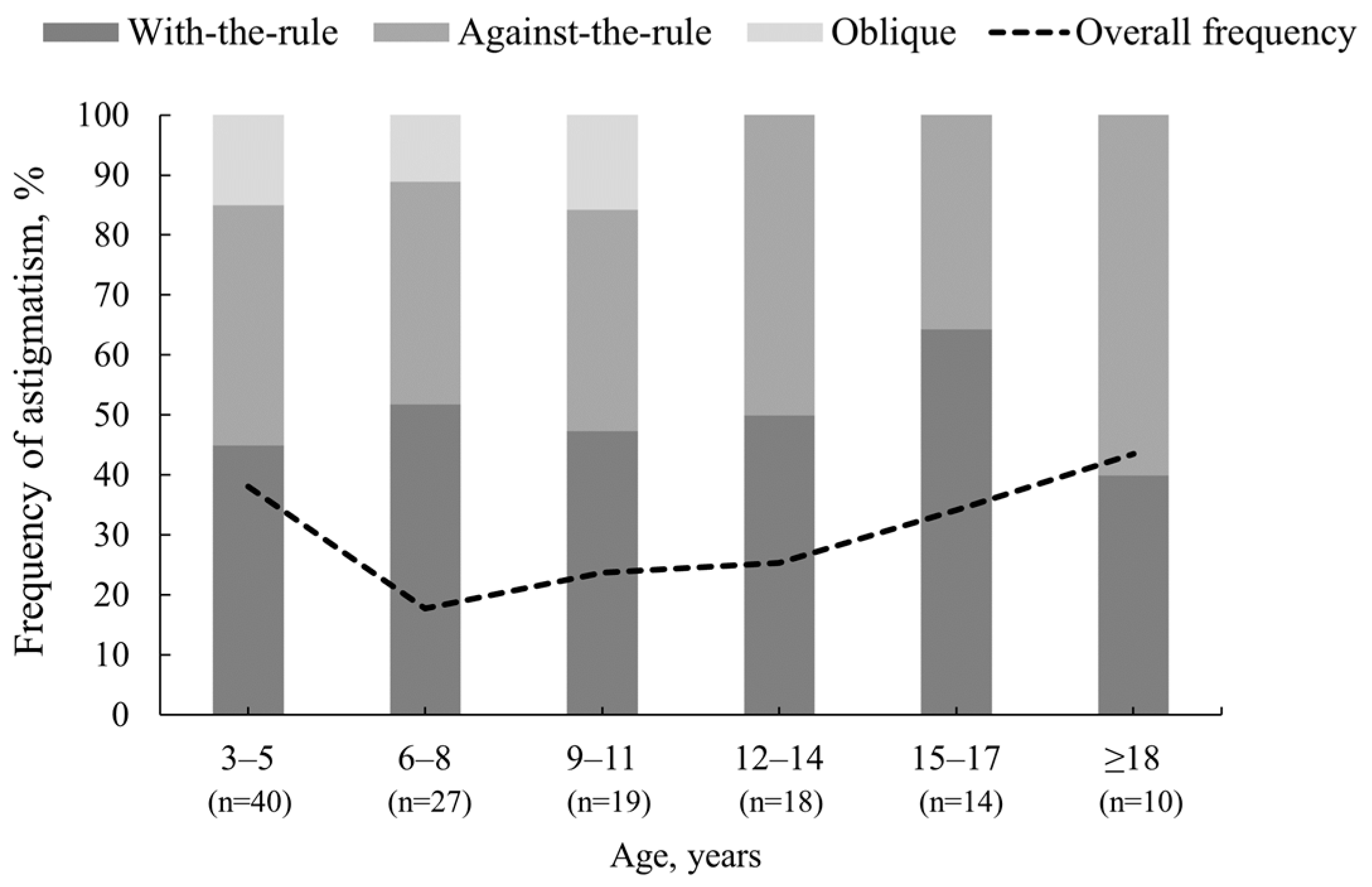
3.3. Changes in Astigmatism Power and Axis after Cycloplegia
4. Discussion
4.1. Study Limitations
4.2. Local Context
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, W.J. Borish’s Clinical Refraction, 2nd ed.; Butterworth-Heinemann: St. Louis, MI, USA, 2006; p. 795. [Google Scholar] [CrossRef]
- Hopkins, S.; Sampson, G.P.; Hendicott, P.; Lacherez, P.; Wood, J.M. Refraction in children: A comparison of two methods of accommodation control. Optom. Vis. Sci. 2012, 89, 1734–1739. [Google Scholar] [CrossRef]
- Zhao, J.; Mao, J.; Luo, R.; Li, F.; Pokharel, G.P.; Ellwein, L.B. Accuracy of noncycloplegic autorefraction in school-age children in China. Optom. Vis. Sci. 2004, 81, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Y.; Wu, J.F.; Lu, T.L.; Wu, H.; Sun, W.; Wang, X.R.; Bi, H.S.; Jonas, J.B. Effect of cycloplegia on the refractive status of children: The Shandong Children Eye Study. PLoS ONE 2015, 10, e0117482. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Y.; Yang, X.; Yang, D.; Guo, K.; Guo, Y.; Jing, X.; Pan, C.W. Pre- and postcycloplegic refractions in children and adolescents. PLoS ONE 2016, 11, e0167628. [Google Scholar] [CrossRef] [PubMed]
- Sujuan, J.L.; Handa, S.; Perera, C.; Chia, A. The psychological impact of eyedrops administration in children. J. AAPOS 2015, 19, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Salinas, H.; Orozco-Ceja, V.; Romero-López, M.S.; Barajas-Virgen, M.Y.; Baiza-Durán, L.M.; Rodríguez-Herrera, L.Y. Ocular Cyclopentolate: A Mini Review Concerning Its Benefits and Risks. Clin. Ophthalmol. 2022, 16, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.C.; Pei, R.X.; Du, B.; Liu, G.H.; Jin, N.; Liu, L.; Wei, R.H. Lag of accommodation predicts clinically significant change of spherical equivalents after cycloplegia. Int. J. Ophthalmol. 2021, 14, 1052–1058. [Google Scholar] [CrossRef]
- Abrams, D.; Duke-Elder, S. Duke-Elder’s Practice of Refraction, 10th ed.; Churchill Livingstone: Edinburgh, UK, 1993. [Google Scholar]
- Morgan, I.G.; Iribarren, R.; Fotouhi, A.; Grzybowski, A. Cycloplegic refraction is the gold standard for epidemiological studies. Acta Ophthalmol. 2015, 93, 581–585. [Google Scholar] [CrossRef]
- Yekta, A.A.; Hashemi, H.; Ostadimoghaddam, H.; Jafarzadehpur, E.; Salehabadi, S.; Sardari, S.; Norouzirad, R.; Khabazkhoob, M. Amplitude of accommodation and add power in an adult population of Tehran, Iran. Iran J. Ophthalmol. 2013, 25, 182–189. [Google Scholar]
- Fotouhi, A.; Morgan, I.G.; Iribarren, R.; Khabazkhoob, M.; Hashemi, H. Validity of noncycloplegic refraction in the assessment of refractive errors: The Tehran Eye Study. Acta Ophthalmol. 2012, 90, 380–386. [Google Scholar] [CrossRef]
- Lovasik, J.V. Pharmacokinetics of topically applied cyclopentolate HCL and tropicamide. Optom. Vis. Sci. 1986, 63, 787–803. [Google Scholar] [CrossRef]
- Fan, D.S.P.; Rao, S.K.; Ng, J.S.K.; Yu, C.B.O.; Lam, D.S.C. Comparative study on the safety and efficacy of different cycloplegic agents in children with darkly pigmented irides. Clin. Exp. Ophthalmol. 2004, 32, 462–467. [Google Scholar] [CrossRef]
- Miranda, M.N. Residual accommodation: A comparison between cyclopentolate 1% and a combination of cyclopentolate 1% and tropicamide 1%. Arch Ophthalmol. 1972, 87, 515–517. [Google Scholar] [CrossRef]
- Li, T.; Zhou, X.; Zhu, J.; Tang, X.; Gu, X. Effect of cycloplegia on the measurement of refractive error in Chinese children. Clin. Exp. Optom. 2019, 102, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, P.G.; Chu, B.S.; Bigault, O.; Kearns, L.S.; Boon, M.Y.; Young, T.L.; Hammond, C.J.; Hewitt, A.W.; Mackey, D.A. What is the appropriate age cut-off for cycloplegia in refraction? Acta Ophthalmol. 2014, 92, e458–e462. [Google Scholar] [CrossRef] [PubMed]
- Manny, R.E.; Fern, K.D.; Zervas, H.J.; Cline, G.E.; Scott, S.K.; White, J.M.; Pass, A.F. 1% cyclopentolate hydrochloride: Another look at the time course of cycloplegia using an objective measure of the accommodative response. Optom. Vis. Sci. 1993, 70, 651–665. [Google Scholar] [CrossRef]
- Hashemi, H.; Fotouhi, A.; Yekta, A.; Pakzad, R.; Ostadimoghaddam, H.; Khabazkhoob, M. Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J. Curr. Ophthalmol. 2017, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, J.; Chen, W.; Meng, Z.; Sun, Y.; Su, H.; Yao, Y.; Dai, W. Difference of refractive status before and after cycloplegic refraction: The Lhasa Childhood Eye Study. Jpn. J. Ophthalmol. 2021, 65, 526–536. [Google Scholar] [CrossRef]
- Bagheri, A.; Feizi, M.; Shafii, A.; Faramarzi, A.; Tavakoli, M.; Yazdani, S. Effect of cycloplegia on corneal biometrics and refractive state. J. Ophthalmic Vis. Res. 2018, 13, 101–109. [Google Scholar] [CrossRef]
- Asharlous, A.; Hashemi, H.; Jafarzadehpur, E.; Mirzajani, A.; Yekta, A.; Nabovati, P.; Khabazkhoob, M. Does astigmatism alter with cycloplegia? J. Curr. Ophthalmol. 2016, 28, 131–136. [Google Scholar] [CrossRef]
- Grzybowski, A.; Kanclerz, P.; Tsubota, K.; Lanca, C.; Saw, S.M. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.; Suhr Thykjær, A.; Søgaard Hansen, R.; Vestergaard, A.H.; Jacobsen, N.; Goldschmidt, E.; Lima, R.A.; Peto, T.; Wedderkopp, N.; Grauslund, J. Physical activity and myopia in Danish children—The CHAMPS eye study. Acta Ophthalmol. 2018, 96, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, E.; Ingrand, P.; Pelen, F.; Bentaleb, Y.; Weber, M.; Korobelnik, J.F.; Souied, E.; Leveziel, N. Prevalence of myopia in France: A Cross-Sectional Analysis. Medicine 2015, 94, e1976. [Google Scholar] [CrossRef]
- Lee, K.E.; Sussberg, J.A.; Nelson, L.B.; Thuma, T. The economic downturn of pediatric ophthalmology and its impact on access to eye care. J. Pediatr. Ophthalmol. Strabismus 2023, 60, 18–24. [Google Scholar] [CrossRef] [PubMed]


| Participants, n (%) | Age, Years (Mean (Median) ± SD (IQR)) | |
|---|---|---|
| Age groups (years): | ||
| 3–5 years (n = 105) | 105 (22%) | 3.8 (3.7) ± 0.7 (1.0) |
| 6–8 years (n = 152) | 152 (32%) | 7.0 (7.0) ± 0.6 (0.7) |
| 9–11 years (n = 80) | 80 (17%) | 9.9 (9.9) ± 0.9 (1.6) |
| 12–14 years (n = 71) | 71 (15%) | 13.0 (12.8) ± 0.8 (1.4) |
| 15–17 years (n = 41) | 41 (9%) | 15.9 (16.0) ± 0.9 (1.7) |
| ≥18 years (n = 23) | 23 (5%) | 20.2 (19.2) ± 3.0 (3.9) |
| Gender: | ||
| Female | 260 (55%) | 9.4 (7.9) ± 4.7 (6.4) |
| Male | 212 (45%) | 8.8 (7.3) ± 4.5 (5.3) |
| Total: | 472 (100%) | 9.1 (7.7) ± 4.6 (6.1) |
| Frequency, n (%), Non-Cycloplegic | Frequency, n (%), Cycloplegic | Change in Frequency ∆, n (%) | Clinically Significant Changes in SE, n (%) | |
|---|---|---|---|---|
| 3–5 years | 105 (100) | 84 (80.0) | ||
| Hyperopia (n = 44) | 44 (41.9) | 90 (85.7) | 46 (43.8) | 32 (72.8) |
| Myopia (n = 12) | 12 (11.4) | 3 (2.9) | 9 (8.5) | 12 (100) |
| Emmetropia (n = 24) | 24 (22.9) | 5 (4.8) | 19 (18.1) | 20 (83.3) |
| Astigmatism (n = 25) | 25 (23.8) | 7 (6.7) | 18 (17.1) | 20 (80.0) |
| 6–8 years | 152 (100) | 106 (69.7) | ||
| Hyperopia (n = 53) | 53 (34.9) | 127 (83.6) | 75 (48.7) | 32 (60.4) |
| Myopia (n = 16) | 16 (10.5) | 11 (7.2) | 5 (3.3) | 6 (37.5) |
| Emmetropia (n = 71) | 71 (46.7) | 10 (6.6) | 61 (40.1) | 60 (84.5) |
| Astigmatism (n = 12) | 12 (7.9) | 4 (2.6) | 8 (5.3) | 8 (66.7) |
| 9–11 years | 80 (100) | 44 (55.0) | ||
| Hyperopia (n = 23) | 23 (28.7) | 43 (53.8) | 20 (25.1) | 18 (78.3) |
| Myopia (n = 22) | 22 (27.5) | 20 (25.0) | 2 (2.5) | 4 (18.2) |
| Emmetropia (n = 30) | 30 (37.5) | 12 (15.0) | 18 (22.5) | 19 (63.3) |
| Astigmatism (n = 5) | 5 (6.3) | 5 (6.3) | 0 (0) | 3 (60.0) |
| ≥12 years | 135 (100) | 55 (40.7) | ||
| Hyperopia (n = 17) | 17 (12.6) | 38 (28.1) | 21 (15.5) | 12 (70.6) |
| Myopia (n = 73) | 73 (54.1) | 63 (46.7) | 10 (7.4) | 18 (24.7) |
| Emmetropia (n = 37) | 37 (27.4) | 25 (18.5) | 12 (8.9) | 20 (54.1) |
| Astigmatism (n = 8) | 8 (5.9) | 9 (6.7) | 1 (0.8) | 5 (62.5) |
| Total | 472 (100) | 289 (61.2) | ||
| Hyperopia (n = 137) | 137 (29.0) | 298 (63.1) | 161 (34.1) | 94 (68.6) |
| Myopia (n = 123) | 123 (26.1) | 97 (20.6) | 26 (5.5) | 40 (32.5) |
| Emmetropia (n = 162) | 162 (34.3) | 52 (11.0) | 110 (23.3) | 119 (73.5) |
| Astigmatism (n = 50) | 50 (10.6) | 25 (5.3) | 25 (5.0) | 36 (72.0) |
| Mean SE ± SD, D | Median (IQR), D | 95% CI | |
|---|---|---|---|
| 3–5 years (n = 105) | |||
| Non-cycloplegic | 0.37 ± 0.82 | 0.50 (1.06) | 0.21 to 0.53 |
| Cycloplegic | 1.37 ± 0.81 | 1.50 (0.75) | 1.21 to 1.52 |
| Δ | 1.00 ± 0.73 | 0.88 (0.94) | 0.86 to 1.14 |
| 6–8 years (n = 152) | |||
| Non-cycloplegic | 0.42 ± 1.23 | 0.44 (0.75) | 0.22 to 0.61 |
| Cycloplegic | 1.30 ± 1.43 | 1.25 (1.00) | 1.07 to 1.53 |
| Δ | 0.88 ± 0.74 | 0.75 (1.00) | 0.77 to 1.00 |
| 9–11 years (n = 80) | |||
| Non-cycloplegic | 0.14 ± 2.01 | 0.25 (1.47) | −0.30 to 0.59 |
| Cycloplegic | 0.71 ± 2.27 | 0.75 (2.09) | 0.21 to 1.22 |
| Δ | 0.57 ± 0.63 | 0.50 (1.00) | 0.43 to 0.71 |
| 12–14 years (n = 71) | |||
| Non-cycloplegic | −0.77 ± 1.80 | −0.50 (1.63) | −1.20 to −0.35 |
| Cycloplegic | −0.40 ± 1.97 | −0.12 (2.13) | −0.87 to 0.07 |
| Δ | 0.37 ± 0.57 | 0.25 (0.63) | 0.24 to 0.51 |
| 15–17 years (n = 41) | |||
| Non-cycloplegic | −0.99 ± 2.59 | −0.62 (3.00) | −1.80 to −0.17 |
| Cycloplegic | −0.46 ± 3.23 | −0.50 (3.63) | −1.47 to 0.56 |
| Δ | 0.53 ± 0.87 | 0.25 (0.69) | 0.26 to 0.81 |
| ≥18 years (n = 23) | |||
| Non-cycloplegic | −1.03 ± 2.73 | −0.75 (3.38) | −2.21 to 0.15 |
| Cycloplegic | −0.80 ± 2.89 | −0.25 (3.25) | −2.05 to 0.45 |
| Δ | 0.23 ± 0.31 | 0.25 (0.38) | 0.10 to 0.37 |
| Total (n = 472) | |||
| Non-cycloplegic | −0.01 ± 1.74 | 0.25 (1.25) | −0.17 to 0.15 |
| Cycloplegic | 0.71 ± 2.03 | 1.00 (1.59) | 0.52 to 0.89 |
| Δ | 0.72 ± 0.73 | 0.50 (1.00) | 0.65 to 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panke, K.; Jorova, M. Objective Refraction Status before and after Cycloplegia: From Childhood to Young Adulthood. Vision 2024, 8, 51. https://doi.org/10.3390/vision8030051
Panke K, Jorova M. Objective Refraction Status before and after Cycloplegia: From Childhood to Young Adulthood. Vision. 2024; 8(3):51. https://doi.org/10.3390/vision8030051
Chicago/Turabian StylePanke, Karola, and Megija Jorova. 2024. "Objective Refraction Status before and after Cycloplegia: From Childhood to Young Adulthood" Vision 8, no. 3: 51. https://doi.org/10.3390/vision8030051
APA StylePanke, K., & Jorova, M. (2024). Objective Refraction Status before and after Cycloplegia: From Childhood to Young Adulthood. Vision, 8(3), 51. https://doi.org/10.3390/vision8030051






