The Effect of the Zonular Fiber Angle of Insertion on Accommodation
Abstract
1. Introduction
2. Methods
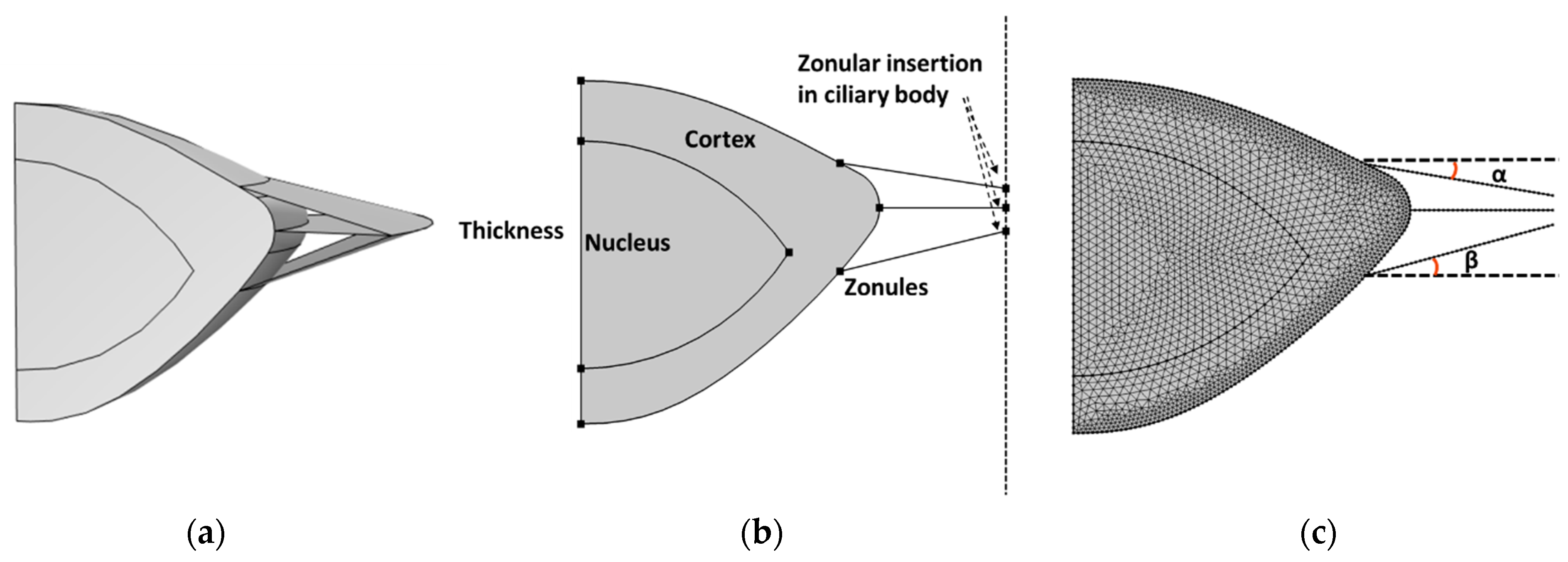
2.1. Geometry of Crystalline Lens and Zonular Fibers
2.2. Material Properties
2.3. Mechanical Modeling of Crystalline Lens Accommodation with Different Zonular Angles
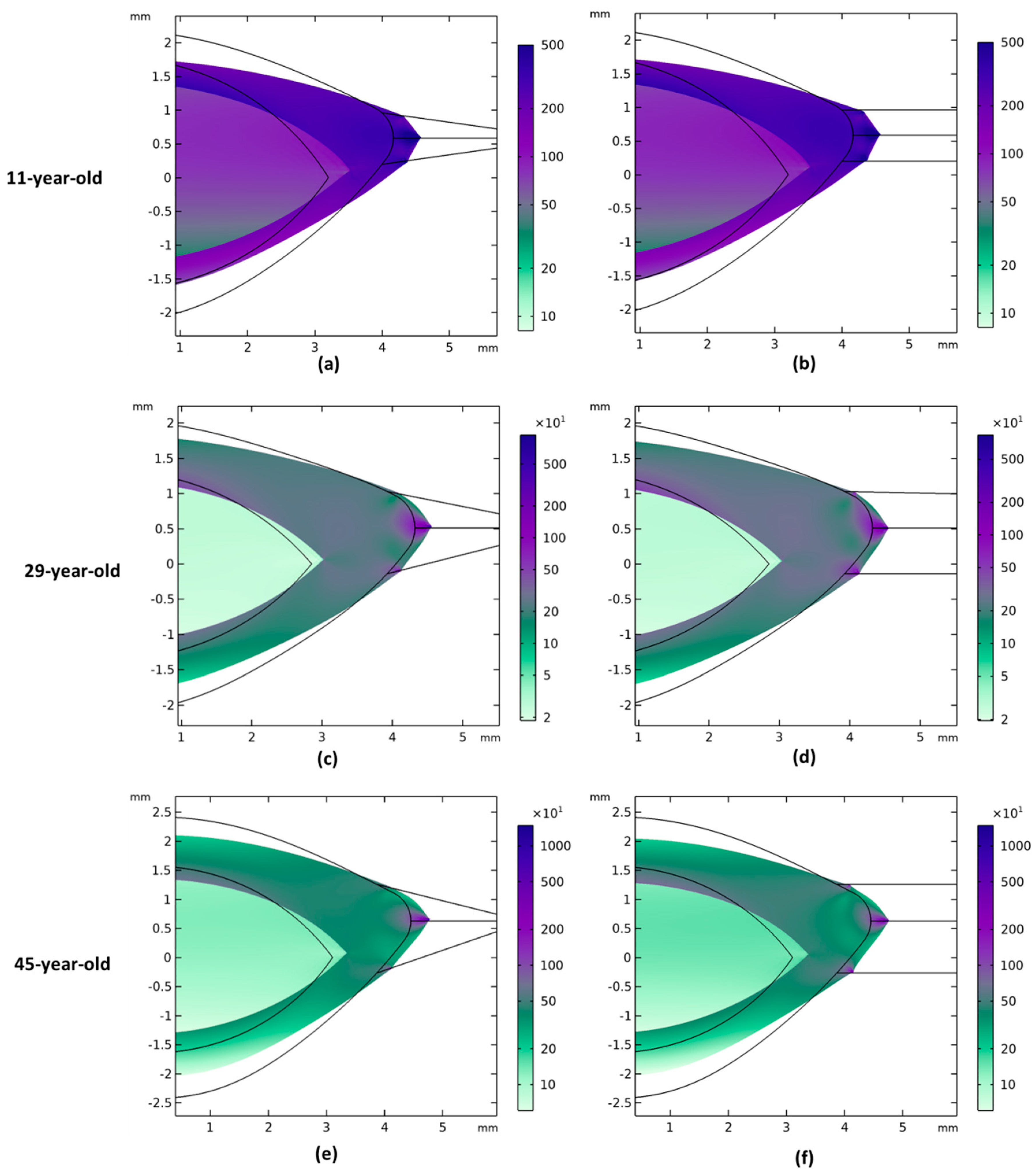
3. Results
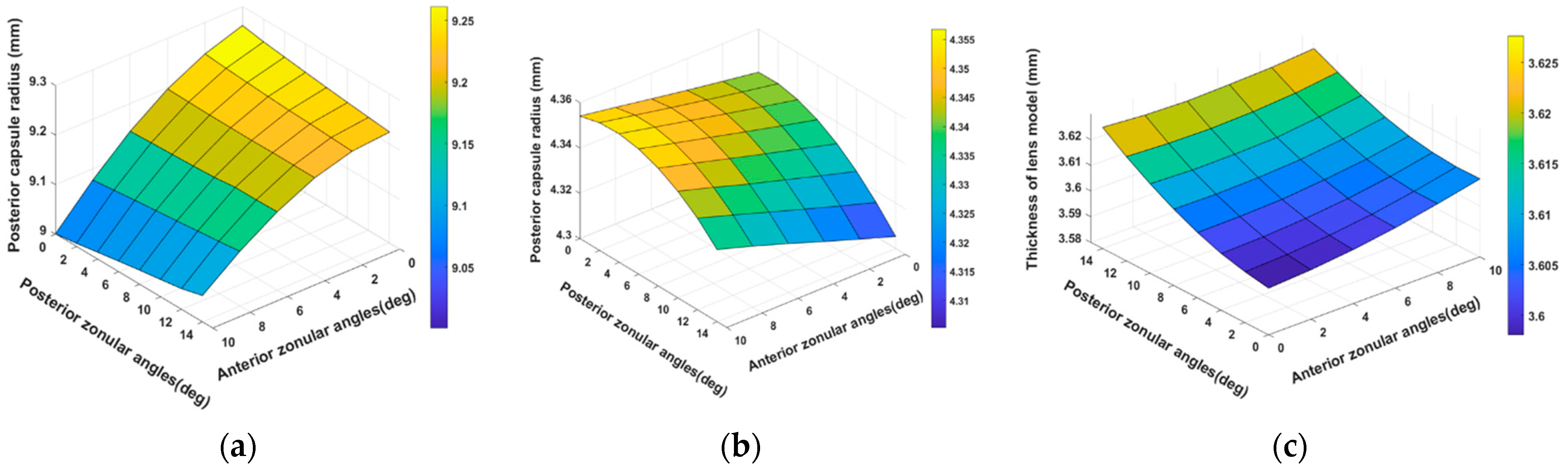
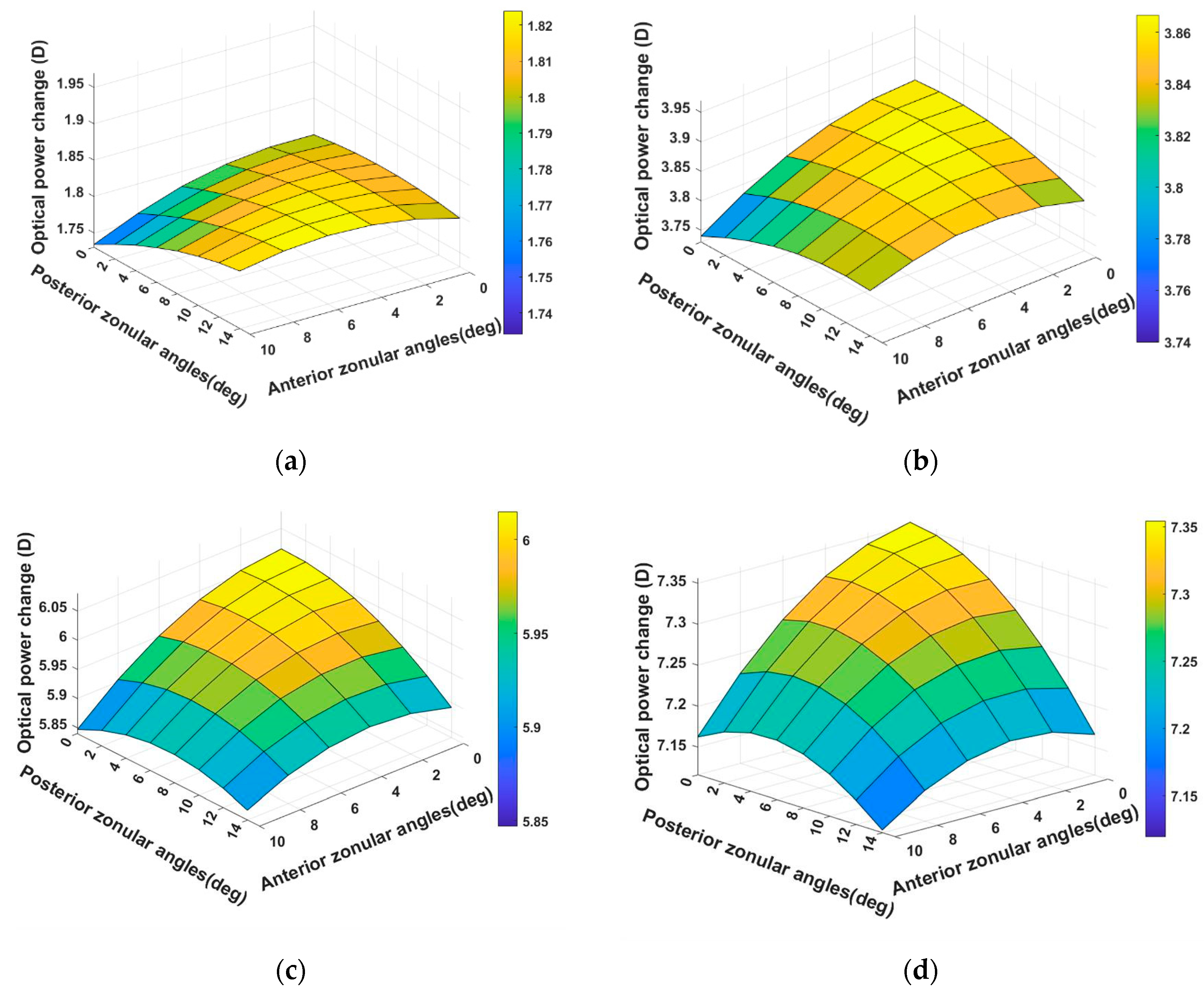
3.1. Modelling of 29-Year-Old Lens Accommodation with 48 Zonular Angle Combinations
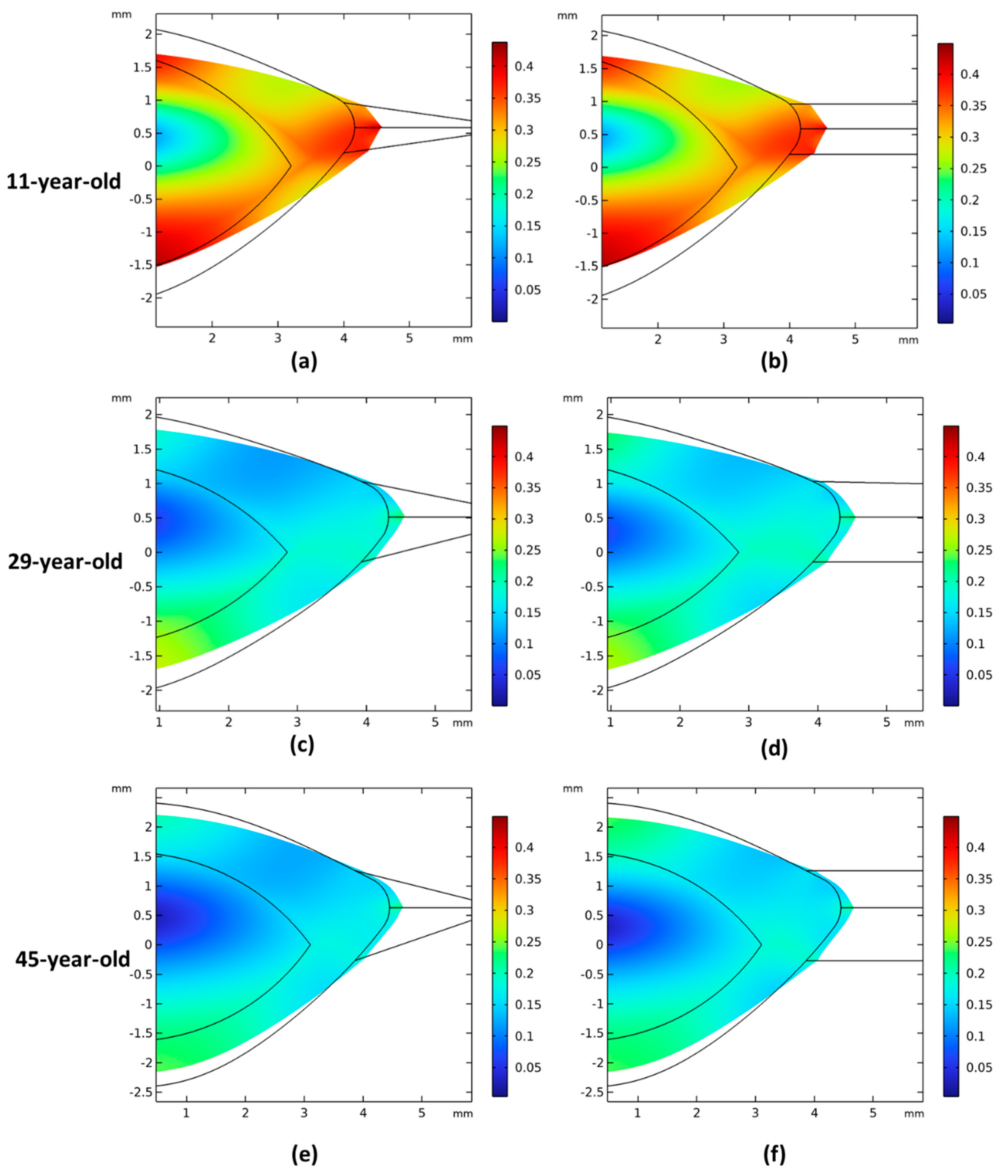
3.2. Comparison of Zonular Angles Effect on 11-, 29-, and 45-Year-Old Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rich, W.; Reilly, M.A. A Review of Lens Biomechanical Contributions to Presbyopia. Curr. Eye Res. 2023, 48, 182–194. [Google Scholar] [CrossRef]
- Borja, D.; Manns, F.; Ho, A.; Ziebarth, N.; Rosen, A.M.; Jain, R.; Amelinckx, A.; Arrieta, E.; Augusteyn, R.C.; Parel, J.M. Optical Power of the Isolated Human Crystalline Lens. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2541. [Google Scholar] [CrossRef]
- Beenett, A.G. A method of determining the equivalent powers of the eye and its crystalline lens without resort to phakometry. Ophthalmic Physiol. Opt. 1988, 8, 53–59. [Google Scholar] [CrossRef]
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. In Progress in Molecular Biology and Translational Science [Internet]; Elsevier: Amsterdam, The Netherlands, 2015; pp. 119–127. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1877117315000678 (accessed on 25 November 2023).
- Bassnett, S.; Šikić, H. The lens growth process. Prog. Retin. Eye Res. 2017, 60, 181–200. [Google Scholar] [CrossRef]
- Kasthurirangan, S.; Markwell, E.L.; Atchison, D.A.; Pope, J.M. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J. Vis. 2011, 11, 19. [Google Scholar] [CrossRef]
- Richdale, K.; Sinnott, L.T.; Bullimore, M.A.; Wassenaar, P.A.; Schmalbrock, P.; Kao, C.Y.; Patz, S.; Mutti, D.O.; Glasser, A.; Zadnik, K. Quantification of Age-Related and per Diopter Accommodative Changes of the Lens and Ciliary Muscle in the Emmetropic Human Eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1095. [Google Scholar] [CrossRef]
- Richdale, K.; Bullimore, M.A.; Sinnott, L.T.; Zadnik, K. The Effect of Age, Accommodation, and Refractive Error on the Adult Human Eye. Optom. Vis. Sci. 2016, 93, 3–11. [Google Scholar] [CrossRef]
- Wilde, G.S.; Burd, H.J.; Judge, S.J. Shear modulus data for the human lens determined from a spinning lens test. Exp. Eye Res. 2012, 97, 36–48. [Google Scholar] [CrossRef]
- Weeber, H.A.; Eckert, G.; Pechhold, W.; Van Der Heijde, R.G.L. Stiffness gradient in the crystalline lens. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1357–1366. [Google Scholar] [CrossRef]
- Wang, K.; Pierscionek, B.K. Biomechanics of the human lens and accommodative system: Functional relevance to physiological states. Prog. Retin. Eye Res. 2019, 71, 114–131. [Google Scholar] [CrossRef]
- Fisher, R.F. The elastic constants of the human lens. J. Physiol. 1971, 212, 147–180. [Google Scholar] [CrossRef]
- Strenk, S.A.; Semmlow, J.L.; Strenk, L.M.; Munoz, P.; Gronlund-Jacob, J.; DeMarco, J.K. Age-related changes in human ciliary muscle and lens: A magnetic resonance imaging study. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1162–1169. [Google Scholar]
- Farnsworth, P.N.; Shyne, S.E. Anterior Zonular Shifts with Age. Exp. Eye Res. 1979, 28, 291–297. [Google Scholar] [CrossRef]
- Besner, S.; Scarcelli, G.; Pineda, R.; Yun, S.H. In Vivo Brillouin Analysis of the Aging Crystalline Lens. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5093–5100. [Google Scholar] [CrossRef]
- Pierscionek, B.; Bahrami, M.; Hoshino, M.; Uesugi, K.; Regini, J.; Yagi, N. The eye lens: Age-related trends and individual variations in refractive index and shape parameters. Oncotarget 2015, 6, 30532–30544. [Google Scholar] [CrossRef]
- Stachs, O.; Martin, H.; Behrend, D.; Schmitz, K.P.; Guthoff, R. Three-dimensional ultrasound biomicroscopy, environmental and conventional scanning electron microscopy investigations of the human zonula ciliaris for numerical modelling of accommodation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 836–844. [Google Scholar] [CrossRef]
- Ghaderi, H.; Ní Dhubhghaill, S.; Tassignon, M.J.; Van Os, L.; Koppen, C.; Rozema, J.J. The potential influence of the ligament of Wieger on the crystalline lens shape. Sci. Rep. 2024, 14, 4004. [Google Scholar] [CrossRef]
- Pu, Y.; Liu, Z.; Ye, L.; Xia, Y.; Chen, X.; Wang, K.; Pierscionek, B.K. The major influence of anterior and equatorial zonular fibres on the far-to-near accommodation revealed by a 3D pre-stressed model of the anterior eye. Comput. Methods Programs Biomed. 2023, 242, 107815. [Google Scholar] [CrossRef]
- Knaus, K.R.; Hipsley, A.; Blemker, S.S. The action of ciliary muscle contraction on accommodation of the lens explored with a 3D model. Biomech. Model. Mechanobiol. 2021, 20, 879–894. [Google Scholar] [CrossRef]
- Pour, H.M.; Kanapathipillai, S.; Zarrabi, K.; Manns, F.; Ho, A. Stretch-dependent changes in surface profiles of the human crystalline lens during accommodation: A finite element study. Clin. Exp. Optom. 2015, 98, 126–137. [Google Scholar] [CrossRef]
- Abolmaali, A.; Schachar, R.A.; Le, T. Sensitivity study of human crystalline lens accommodation. Comput. Methods Programs Biomed. 2007, 85, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Burd, H.J.; Judge, S.J.; Cross, J.A. Numerical modelling of the accommodating lens. Vis. Res. 2002, 42, 2235–2251. [Google Scholar] [CrossRef] [PubMed]
- Van De Sompel, D.; Kunkel, G.J.; Hersh, P.S.; Smits, A.J. Model of accommodation: Contributions of lens geometry and mechanical properties to the development of presbyopia. J. Cataract. Refract. Surg. 2010, 36, 1960–1971. [Google Scholar] [CrossRef]
- Schachar, R.A. Insights into the age-related decline in the amplitude of accommodation of the human lens using a non-linear finite-element model. Br. J. Ophthalmol. 2006, 90, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Burd, H.J.; Wilde, G.S. Finite element modelling of radial lentotomy cuts to improve the accommodation performance of the human lens. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 727–737. [Google Scholar] [CrossRef] [PubMed]
- De La Hoz, A.; Martinez-Enriquez, E.; Marcos, S. Estimation of Crystalline Lens Material Properties From Patient Accommodation Data and Finite Element Models. Investig. Ophthalmol. Vis. Sci. 2023, 64, 31. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.A.; Dubbelman, M.; van der Heijde, G.L.; Heethaar, R.M. Estimating the external force acting on the human eye lens during accommodation by finite element modelling. Vis. Res. 2006, 46, 3642–3650. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Venetsanos, D.T.; Hoshino, M.; Uesugi, K.; Yagi, N.; Pierscionek, B.K. A Modeling Approach for Investigating Opto-Mechanical Relationships in the Human Eye Lens. IEEE Trans. Biomed. Eng. 2020, 67, 999–1006. [Google Scholar] [CrossRef]
- Brown, N. The change in shape and internal form of the lens of the eye on accommodation. Exp. Eye Res. 1973, 15, 441–459. [Google Scholar] [CrossRef]
- Dubbelman, M.; Van der Heijde, G.L.; Weeber, H.A. Change in shape of the aging human crystalline lens with accommodation. Vis. Res. 2005, 45, 117–132. [Google Scholar] [CrossRef]
- Chien, C.H.M.; Huang, T.; Schachar, R.A. A mathematical expression for the human crystalline lens. Compr. Ther. 2003, 29, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Urs, R.; Manns, F.; Ho, A.; Borja, D.; Amelinckx, A.; Smith, J.; Jain, R.; Augusteyn, R.; Parel, J.M. Shape of the isolated ex-vivo human crystalline lens. Vis. Res. 2009, 49, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.F. Elastic constants of the human lens capsule. J. Physiol. 1969, 201, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Krag, S.; Olsen, T.; Andreassewf, T.T. Biomechanical Characteristics of the Human Anterior Lens Capsule in Relation to Age. Investig. Ophthalmol. Vis. Sci. 1997, 38, 357–363. [Google Scholar]
- Wang, K.; Hoshino, M.; Uesugi, K.; Yagi, N.; Pierscionek, B.K. Contributions to Accommodative Loss of Ageing Human Lens by Shape and Stiffness: An Assessment using Finite Element Models. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2019, 36, B116–B122. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, P.N.; Burke, P. Three-dimensional architecture of the suspensory apparatus of the lens of the Rhesus monkey. Exp. Eye Res. 1977, 25, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Enriquez, E.; Pérez-Merino, P.; Velasco-Ocana, M.; Marcos, S. OCT-based full crystalline lens shape change during accommodation in vivo. Biomed. Opt. Express 2017, 8, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Fromm, M.; Halabi, F.A.; Besdo, S.; Lubatschowski, H.; Ripken, T.; Krüger, A. Measurement of Ex Vivo Porcine Lens Shape During Simulated Accommodation, Before and After fs-Laser Treatment. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5332. [Google Scholar] [CrossRef]
- Zhong, J.; Tao, A.; Xu, Z.; Jiang, H.; Shao, Y.; Zhang, H.; Liu, C.; Wang, J. Whole eye axial biometry during accommodation using ultra-long scan depth optical coherence tomography. Am. J. Ophthalmol. 2014, 157, 1064–1069. [Google Scholar] [CrossRef]
- Neri, A.; Ruggeri, M.; Protti, A.; Leaci, R.; Gandolfi, S.A.; Macaluso, C. Dynamic imaging of accommodation by swept-source anterior segment optical coherence tomography. J. Cataract. Refract. Surg. 2015, 41, 501–510. [Google Scholar] [CrossRef]
- Fisher, R.F. The significance of the shape of the lens and capsular energy changes in accommodation. J. Physiol. 1969, 201, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Glasser, A.; Campbell, M.C.W. Presbyopia and the optical changes in the human crystalline lens with age. Vis. Res. 1998, 38, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Pierscionek, B.K. In Vitro Alteration of Human Lens Curvatures by Radial Stretching. Exp. Eye Res. 1993, 57, 629–635. [Google Scholar] [CrossRef]
- Dubbelman, M.; Van der Heijde, G.L.; Weeber, H.A.; Vrensen, G.F.J.M. Changes in the internal structure of the human crystalline lens with age and accommodation. Vis. Res. 2003, 43, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.L.; Davies, L.N. The Effect of Ageing on In Vivo Human Ciliary Muscle Morphology and Contractility. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1809. [Google Scholar] [CrossRef] [PubMed]
- Krag, S.; Andreassen, T.T. Mechanical Properties of the Human Posterior Lens Capsule. Investig. Ophthalmol. Vis. Sci. 2003, 44, 691. [Google Scholar] [CrossRef]
- Krag, S.; Thim, K.; Corydon, L.; Kyster, B. Biomechanical aspects of the anterior capsulotomy. J. Cataract. Refract. Surg. 1994, 20, 410–416. [Google Scholar] [CrossRef]

| Age | Young’s Modulus (pa) | Poisson’s Ratio | |
|---|---|---|---|
| Capsule | 11 | 0.73 × 106 | 0.47 |
| 29 | 1.27 × 106 | ||
| 45 | 1.45 × 106 | ||
| Cortex | 11 | 1875 | 0.49 |
| 29 | 3417 | ||
| 45 | 3980 | ||
| Nucleus | 11 | 569.5 | 0.49 |
| 29 | 547.4 | ||
| 45 | 996.6 |
| Study | Angles [Anterior, Equatorial, Posterior] | Anterior Radius Change (mm) | Posterior Radius Change (mm) | Optical Power Change (D) |
|---|---|---|---|---|
| Wang et al. [29] | [10, 0, 24] | 2.4 | 1.3 | 3 |
| [18, 0, 32] | 0.7 | 0.6 | – | |
| [26, 0, 40] | −0.7 | 0 | – | |
| This study | [10, 0, 14] | 3.2 | 0.48 | 7.12 |
| [0, 0, 0] | 3.4 | 0.49 | 7.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Pierscionek, B.; Weeber, H.; Canovas Vidal, C.; Rozema, J.J. The Effect of the Zonular Fiber Angle of Insertion on Accommodation. Vision 2024, 8, 45. https://doi.org/10.3390/vision8030045
Feng L, Pierscionek B, Weeber H, Canovas Vidal C, Rozema JJ. The Effect of the Zonular Fiber Angle of Insertion on Accommodation. Vision. 2024; 8(3):45. https://doi.org/10.3390/vision8030045
Chicago/Turabian StyleFeng, Liying, Barbara Pierscionek, Henk Weeber, Carmen Canovas Vidal, and Jos J. Rozema. 2024. "The Effect of the Zonular Fiber Angle of Insertion on Accommodation" Vision 8, no. 3: 45. https://doi.org/10.3390/vision8030045
APA StyleFeng, L., Pierscionek, B., Weeber, H., Canovas Vidal, C., & Rozema, J. J. (2024). The Effect of the Zonular Fiber Angle of Insertion on Accommodation. Vision, 8(3), 45. https://doi.org/10.3390/vision8030045






