New Evidence for Retrospectively Cued Perception
Abstract
1. Introduction
2. Experiment 1
2.1. Materials and Methods
2.1.1. Participants
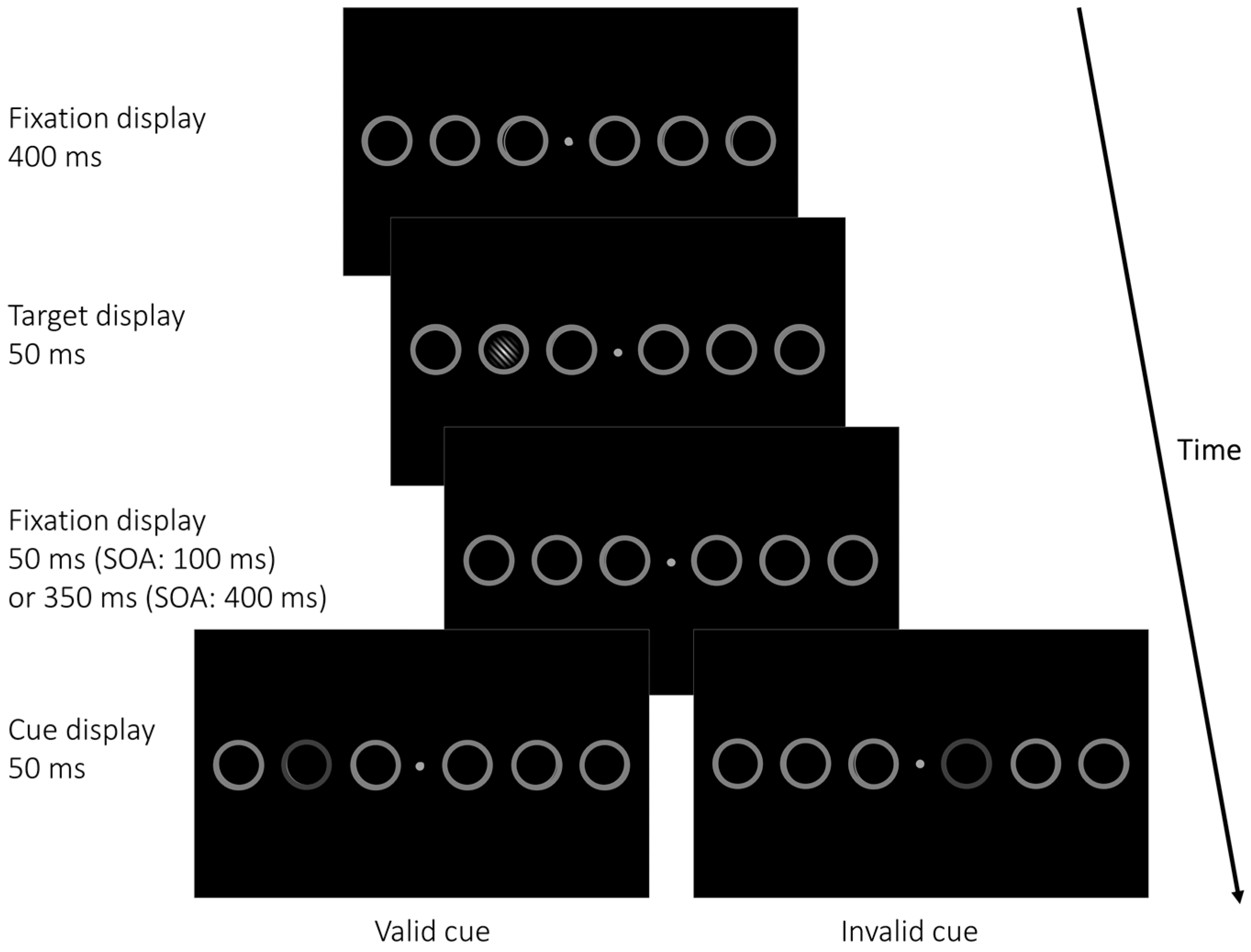
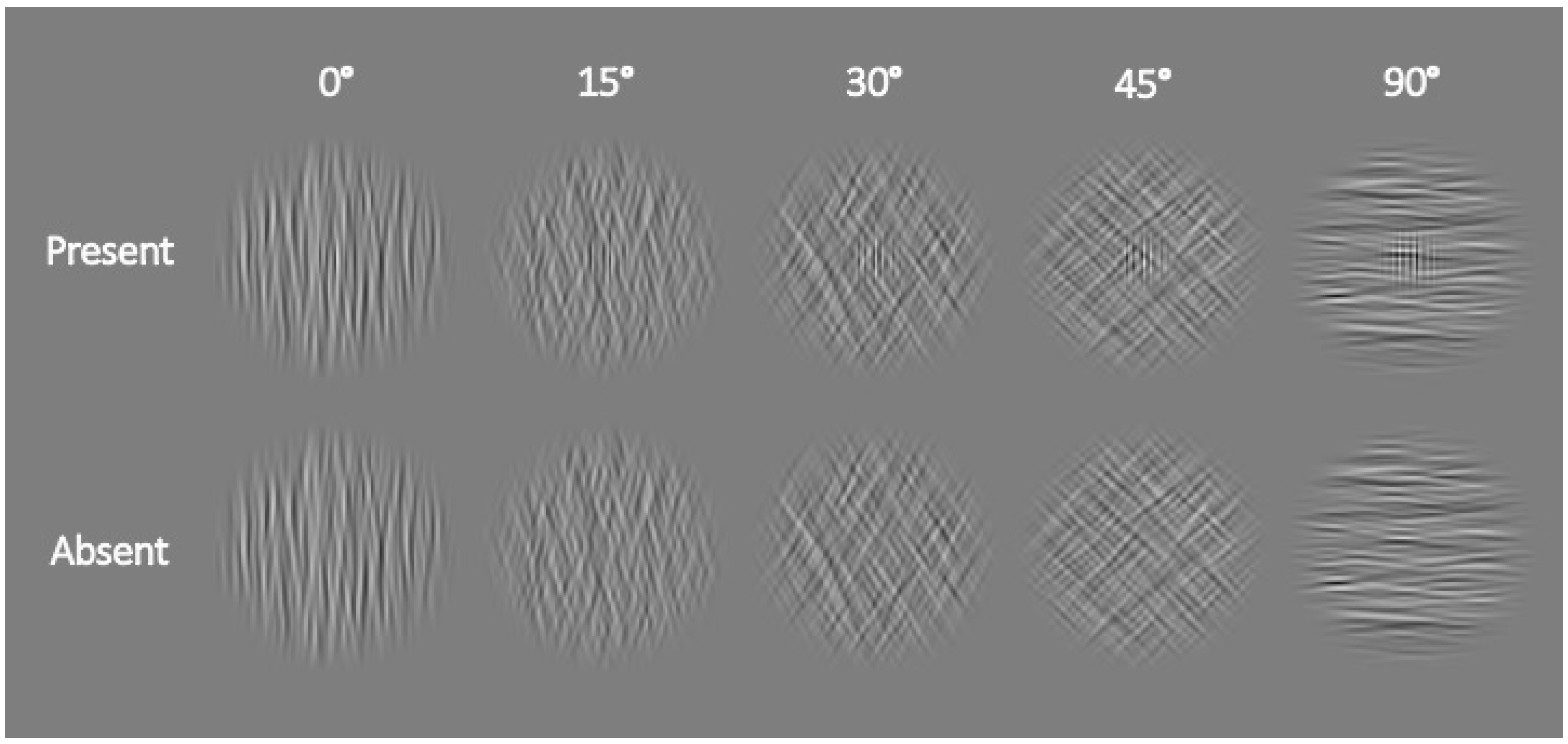
2.1.2. Apparatus, Stimuli, and Procedure
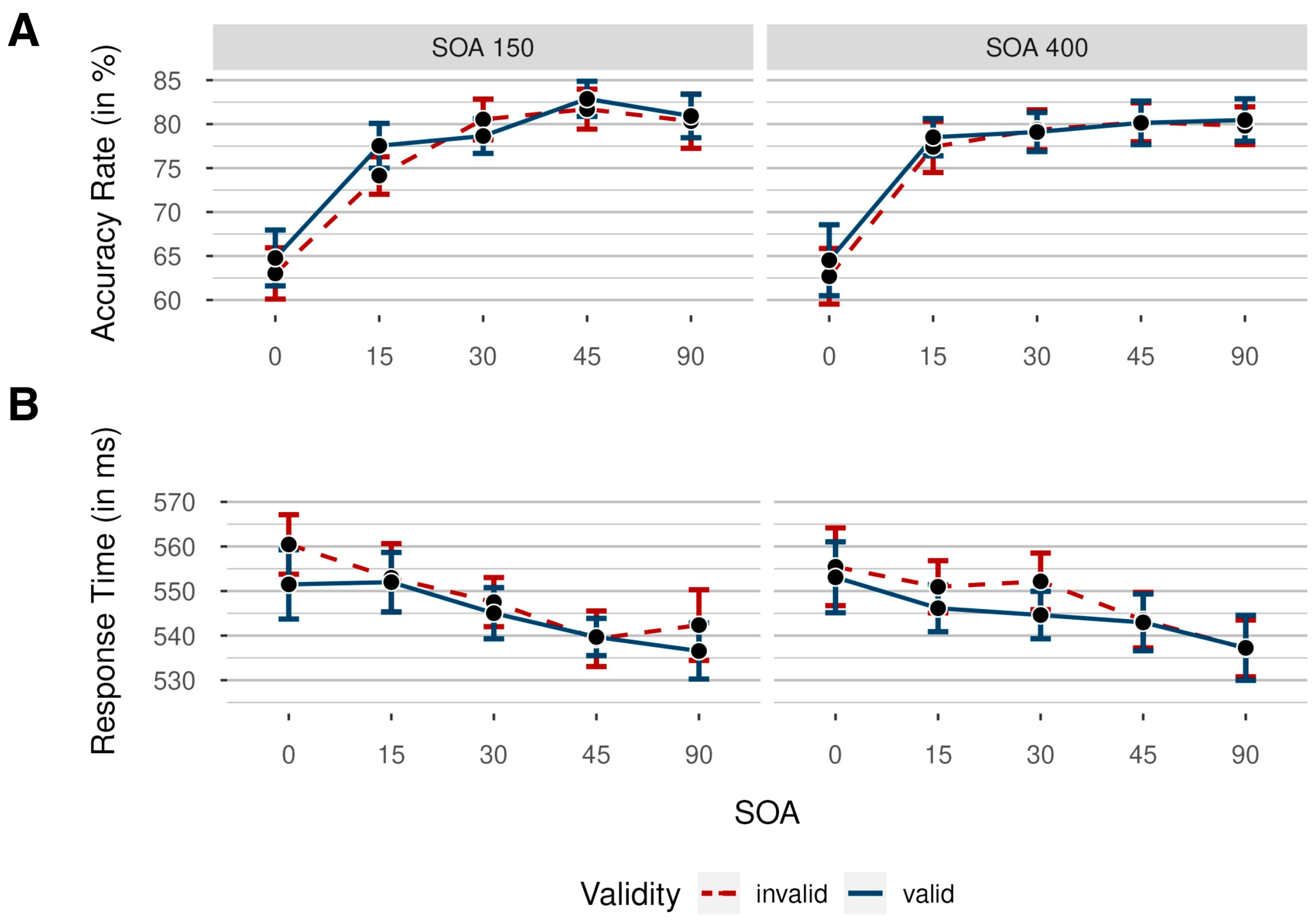
2.2. Results
2.2.1. Accuracy Rates
2.2.2. Response Times
2.3. Discussion
3. Experiment 2
3.1. Materials and Methods
3.1.1. Participants
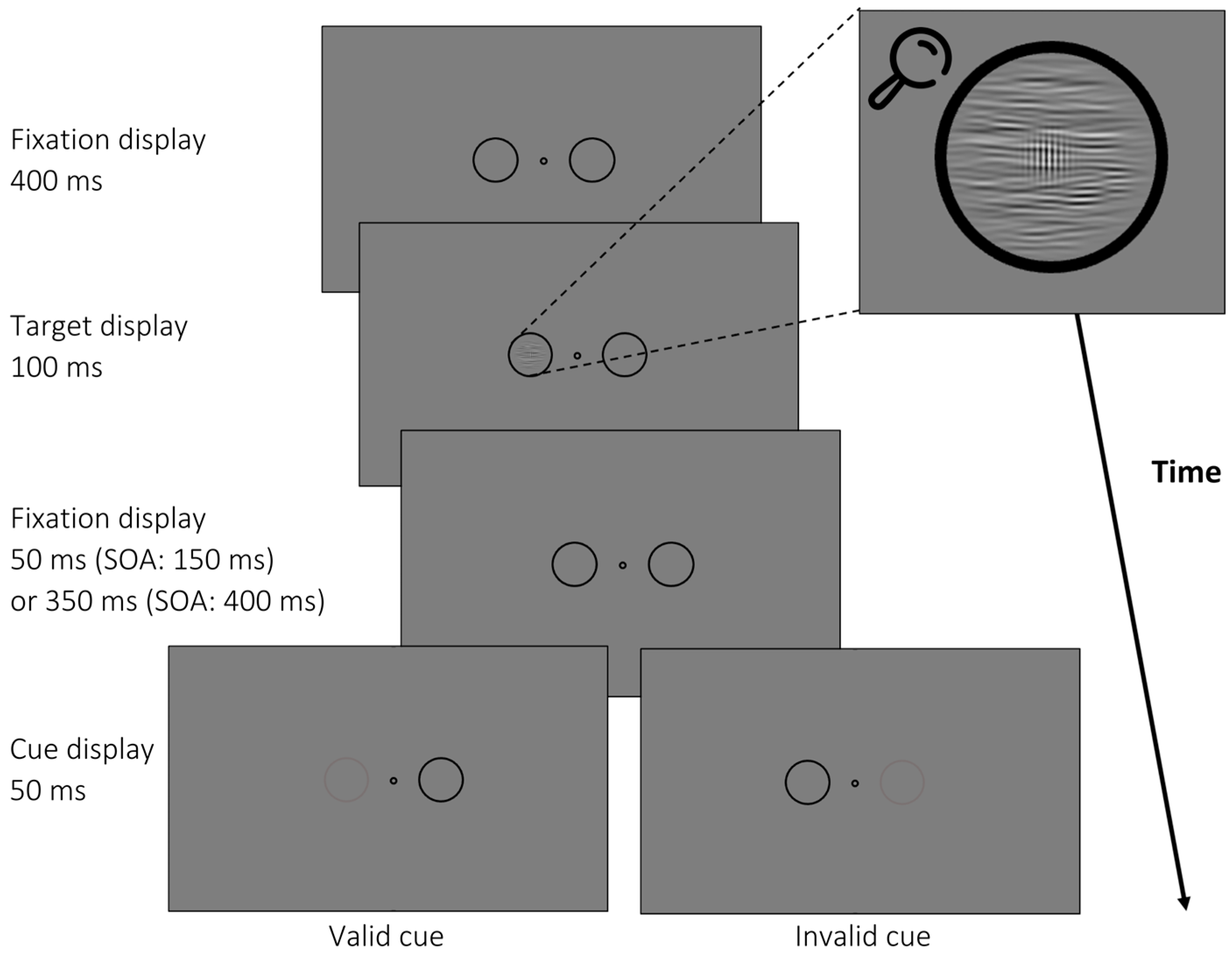
3.1.2. Apparatus, Stimuli, and Procedure
3.1.3. Data Analysis
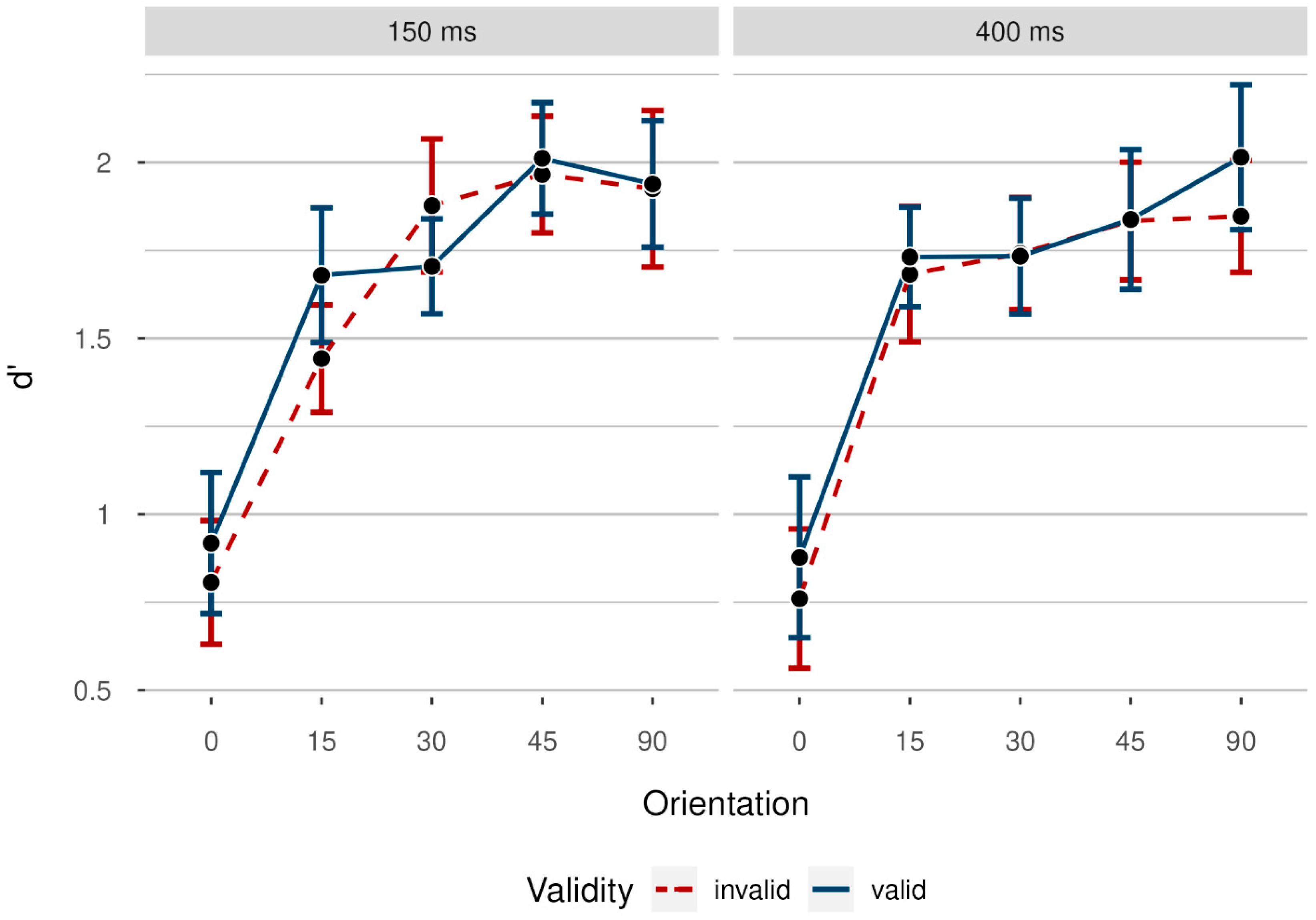
3.2. Results
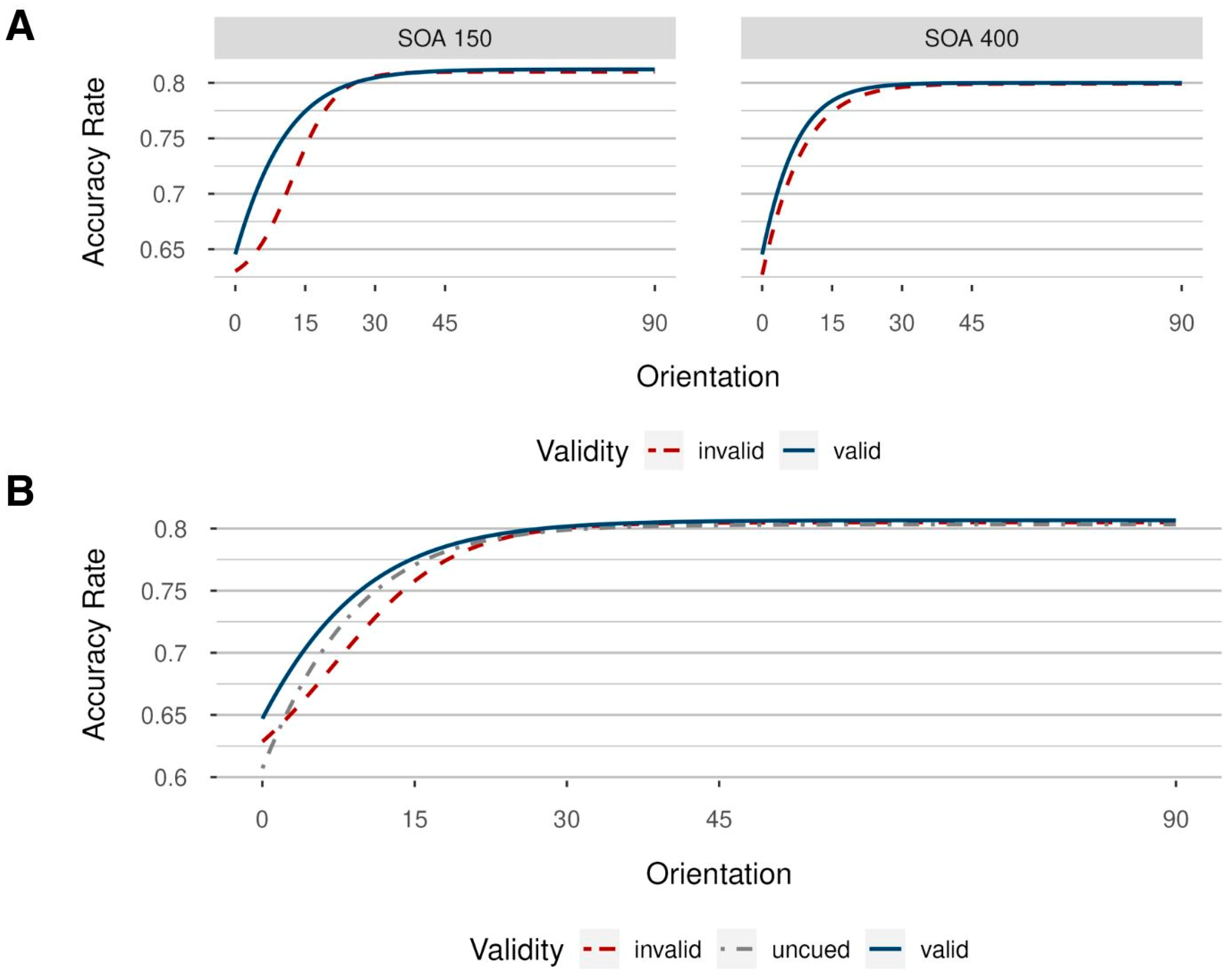
3.2.1. Accuracy Rates
3.2.2. Response Times
3.3. Discussion
4. General Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Effect | F (df) | p | BFIncl | |
|---|---|---|---|---|
| (Intercept) | 269.32 (1, 23) | <0.001 * | 0.92 | |
| Validity | 2.24 (1, 23) | 0.148 | 0.09 | 0.323 |
| SOA | 0.45 (1, 23) | 0.508 | 0.02 | 0.176 |
| Orientation | 47.68 (4, 92) | <0.001 * | 0.67 | >100 |
| Validity × SOA | 0.09 (1, 23) | 0.762 | <0.01 | 0.18 |
| SOA × Orientation | 2.48 (4, 92) | 0.050 | 0.10 | 0.404 |
| Validity × Orientation | 1.60 (4, 92) | 0.180 | 0.07 | 0.175 |
| Validity × SOA × Orientation | 0.89 (4, 92) | 0.472 | 0.04 | 0.216 |
| SOA | Validity | AICLog | BICLog | AICGauss | BICGauss |
|---|---|---|---|---|---|
| 150 ms | valid | 135.0752 | 139.7874 | 135.0790 | 139.7910 |
| invalid | 137.3606 | 142.0728 | 137.3626 | 142.0748 | |
| 400 ms | valid | 136.4380 | 141.1502 | 136.4400 | 141.1522 |
| invalid | 137.8132 | 142.5254 | 137.8144 | 142.5266 |
| SOA | Validity | α | β | γ | λ | LL |
|---|---|---|---|---|---|---|
| 150 ms | valid | −12.2038 | 0.1109 | <0.001 | 0.1878 | −63.5376 |
| invalid | 12.2555 | 0.2162 | 0.6175 | 0.19 | −64.0803 | |
| 400 ms | valid | −8.7449 | 0.1633 | <0.001 | 0.2 | −64.2190 |
| invalid | −9.1801 | 0.1407 | <0.001 | 0.2 | −64.9066 |
| Validity | α | β | γ | λ | LL |
|---|---|---|---|---|---|
| Valid | −11.3916 | 0.1229 | <0.001 | 0.1933 | −63.8869 |
| Invalid | −8.3230 | 0.1357 | <0.001 | 0.1968 | −64.8702 |
| uncued | 6.9628 | 0.1689 | 05742 | 0.1950 | −64.8172 |
References
- Drissi-Daoudi, L.; Doerig, A.; Herzog, M.H. Feature integration within discrete time windows. Nat. Commun. 2019, 10, 4901. [Google Scholar] [CrossRef]
- Exner, S. Experimentelle Untersuchung der einfachsten psychischen Processe [Experimental investigation of the simplest psychic processes]. Arch. Gesamte Physiol. Menschen Tiere 1875, 11, 403–432. [Google Scholar] [CrossRef]
- Herzog, M.H.; Fahle, M. Effects of grouping in contextual modulation. Nature 2002, 415, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Koster, N.; Mattler, U.; Albrecht, T. Visual experience forms a multidimensional pattern that is not reducible to a single measure: Evidence from metacontrast masking. J. Vis. 2020, 20, 2. [Google Scholar] [CrossRef]
- Sergent, C.; Wyart, V.; Babo-Rebelo, M.; Cohen, L.; Naccache, L.; Tallon-Baudry, C. Cueing attention after the stimulus is gone can retrospectively trigger conscious perception. Curr. Biol. 2013, 23, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Thibault, L.; van den Berg, R.; Cavanagh, P.; Sergent, C. Retrospective attention gates discrete conscious access to past sensory stimuli. PLoS ONE 2016, 11, e0148504. [Google Scholar] [CrossRef] [PubMed]
- Werner, H. Studies on contour: 1. Qualitative analyses. Am. J. Psychol. 1935, 47, 40–64. [Google Scholar] [CrossRef]
- Wertheimer, M. Experimentelle Studien über das Sehen von Bewegung [Experimental studies on the seeing of motion]. Z. Psychol. 1912, 61, 161–265. [Google Scholar]
- Harrison, S.A.; Tong, F. Decoding reveals the contents of visual working memory in early visual areas. Nature 2009, 458, 632–635. [Google Scholar] [CrossRef]
- Luck, S.J.; Vogel, E.K. Visual working memory capacity: From psychophysics and neurobiology to individual differences. Trends Cogn. Sci. 2013, 17, 391–400. [Google Scholar] [CrossRef]
- Panichello, M.F.; Buschman, T.J. Shared mechanisms underlie the control of working memory and attention. Nature 2021, 592, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Serences, J.T. Neural mechanisms of information storage in visual short-term memory. Vis. Res. 2016, 128, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Serences, J.T.; Ester, E.F.; Vogel, E.K.; Awh, E. Stimulus-specific delay activity in human primary visual cortex. Psychol. Sci. 2009, 20, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Sligte, I.G.; Scholte, H.S.; Lamme, V.A. Are there multiple visual short-term memory stores? PLoS ONE 2008, 3, e1699. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Kravitz, D.J. Visual working memory directly alters perception. Nat. Hum. Behav. 2019, 3, 827–836. [Google Scholar] [CrossRef]
- Pasternak, T.; Greenlee, M.W. Working memory in primate sensory systems. Nat. Rev. Neurosci. 2005, 6, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.H.; Kammer, T.; Scharnowski, F. Time slices: What is the duration of a percept? PLoS Biol. 2016, 14, e1002433. [Google Scholar] [CrossRef]
- Herzog, M.H.; Drissi-Daoudi, L.; Doerig, A. All in Good Time: Long-lasting postdictive effects reveal discrete perception. Trends Cogn. Sci. 2020, 24, 826–837. [Google Scholar] [CrossRef]
- Fekete, T.; Van de Cruys, S.; Ekroll, V.; van Leeuwen, C. In the interest of saving time: A critique of discrete perception. Neurosci. Conscious. 2018, 2018, niy003. [Google Scholar] [CrossRef]
- Griffin, I.C.; Nobre, A.C. Orienting attention to locations in internal representations. J. Cogn. Neurosci. 2003, 15, 1176–1194. [Google Scholar] [CrossRef] [PubMed]
- Landman, R.; Spekreijse, H.; Lamme, V.A.F. The role of figure–ground segregation in change blindness. Psychon. Bull. Rev. 2004, 11, 254–261. [Google Scholar] [CrossRef]
- Ruff, C.C.; Kristjánsson, R.; Driver, J. Readout from iconic memory and selective spatial attention involve similar neural processes. Psychol. Sci. 2007, 18, 901–909. [Google Scholar] [CrossRef]
- Sperling, G. The information available in brief visual presentations. Psychol. Monogr. Gen. Appl. 1960, 74, 1–29. [Google Scholar] [CrossRef]
- Souza, A.S.; Oberauer, K. In search of the focus of attention in working memory: 13 years of the retro-cue effect. Atten. Percept. Psychophys. 2016, 78, 1839–1860. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.M.; Nobre, A.C.; Clark, I.A.; Cravo, A.M.; Stokes, M.G. Attention restores discrete items to visual short-term memory. Psychol. Sci. 2013, 24, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.E.; Walther, L.; Wallis, G.; Stokes, M.G.; Nobre, A.C. Temporal dynamics of attention during encoding versus maintenance of working memory: Complementary views from event-related potentials and alpha-band oscillations. J. Cogn. Neurosci. 2015, 27, 492–508. [Google Scholar] [CrossRef] [PubMed]
- Gunseli, E.; van Moorselaar, D.; Meeter, M.; Olivers, C.N.L. The reliability of retro-cues determines the fate of noncued visual working memory representations. Psychon. Bull. Rev. 2015, 22, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- van Moorselaar, D.; Gunseli, E.; Theeuwes, J.; Olivers, C.N.L. The time course of protecting a visual memory representation from perceptual interference. Front. Hum. Neurosci. 2015, 8, 1053. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, G.; Patel, S.S.; Bedell, H.E.; Ogmen, H. Moving ahead through differential visual latency. Nature 1998, 396, 424. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Lit, A. Luminance-dependent visual latency for the Hess effect, the Pulfrich effect, and simple reaction time. Vis. Res. 1983, 23, 171–179. [Google Scholar] [CrossRef]
- Jonides, J. Voluntary versus automatic control over the mind’s eye’s movement. In Attention and Performance IX; Long, J.B., Baddeley, A., Eds.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1981; pp. 187–203. [Google Scholar]
- Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Morimoto, Y.; Noguchi, Y. Retrospective triggering of conscious perception by an interstimulus interaction. J. Vis. 2016, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Trujillo, J.C.; Treue, S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr. Biol. 2004, 14, 744–751. [Google Scholar] [CrossRef]
- Treue, S.; Trujillo, J.C.M. Feature-based attention influences motion processing gain in macaque visual cortex. Nature 1999, 399, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Trujillo, J.C.; Treue, S. Attentional modulation strength in cortical area MT depends on stimulus contrast. Neuron 2002, 35, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.H.; Heeger, D.J. The normalization model of attention. Neuron 2009, 61, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Montaser-Kouhsari, L.; Carrasco, M.; Heeger, D.J. When size matters: Attention affects performance by contrast or response gain. Nat. Neurosci. 2010, 13, 1554–1559. [Google Scholar] [CrossRef]
- Strasburger, H.; Rentschler, I.; Jüttner, M. Peripheral vision and pattern recognition: A review. J. Vis. 2011, 11, 13. [Google Scholar] [CrossRef]
- Baldassi, S.; Verghese, P. Attention to locations and features: Different top-down modulation of detector weights. J. Vis. 2005, 5, 7. [Google Scholar] [CrossRef]
- Ling, S.; Blake, R. Suppression during binocular rivalry broadens orientation tuning. Psychol. Sci. 2009, 20, 1348–1355. [Google Scholar] [CrossRef]
- Stolte, M.; Bahrami, B.; Lavie, N. High perceptual load leads to both reduced gain and broader orientation tuning. J. Vis. 2014, 14, 9. [Google Scholar] [CrossRef][Green Version]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behav. Res. Methods 2012, 44, 314–324. [Google Scholar] [CrossRef]
- Collewijn, H.; Erkelens, C.J.; Steinman, R.M. Binocular co-ordination of human horizontal saccadic eye movements. J. Physiol. 1988, 404, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Pélisson, D.; Prablanc, C. Kinematics of centrifugal and centripetal saccadic eye movements in man. Vis. Res. 1988, 28, 87–94. [Google Scholar] [CrossRef]
- MATLAB. MATLAB and Statistics Toolbox Release; The MathWorks, Inc.: Natick, MA, USA, 2022. [Google Scholar]
- Prins, N.; Kingdom, F.A.A. Applying the Model-Comparison Approach to Test Specific Research Hypotheses in Psychophysical Research Using the Palamedes Toolbox. Front. Psychol. 2018, 9, 1250. [Google Scholar] [CrossRef]
- Darnell, M.; Lamy, D. Spatial cueing effects do not always index attentional capture: Evidence for a priority accumulation framework. Psychol. Res. 2022, 86, 1547–1564. [Google Scholar] [CrossRef]
- Toledano, D.; Sasi, M.; Yuval-Greenberg, S.; Lamy, D. On the timing of overt attention deployment: Eye-movement evidence for the Priority Accumulation Framework. J. Exp. Psychol. Hum. Percept. Perform. 2023; in press. [Google Scholar]
- Yaron, I.; Lamy, D. Spatial cueing effects are not what we thought: On the timing of attentional deployment. J. Exp. Psychol. Hum. Percept. Perform. 2021, 47, 946–962. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.E.; Shapiro, K.L.; Arnell, K.M. Temporary suppression of visual processing in an RSVP task: An attentional blink? J. Exp. Psychol. Hum. Percept. Perform. 1992, 18, 849–860. [Google Scholar] [CrossRef]
- Tang, M.F.; Ford, L.; Arabzadeh, E.; Enns, J.T.; Visser, T.A.W.; Mattingley, J.B. Neural dynamics of the attentional blink revealed by encoding orientation selectivity during rapid visual presentation. Nat. Commun. 2020, 11, 434. [Google Scholar] [CrossRef]
- McLeod, P. Parallel processing and the psychological refractory period. Acta Psychol. 1977, 41, 381–396. [Google Scholar] [CrossRef]
- Chun, M.M.; Potter, M.C. A two-stage model for multiple target detection in rapid serial visual presentation. J. Exp. Psychol. Hum. Percept. Perform. 1995, 21, 109–127. [Google Scholar] [CrossRef]
- Olivers, C.N.L.; Meeter, M. A boost and bounce theory of temporal attention. Psychol. Rev. 2008, 115, 836–863. [Google Scholar] [CrossRef]
- Awh, E.; Belopolsky, A.V.; Theeuwes, J. Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends Cogn. Sci. 2012, 16, 437–443. [Google Scholar] [CrossRef]
- Chun, M.M.; Turk-Browne, N.B. Interactions between attention and memory. Curr. Opin. Neurobiol. 2007, 17, 177–184. [Google Scholar] [CrossRef]
- Summerfield, C.; Egner, T. Expectation (and attention) in visual cognition. Trends Cogn. Sci. 2009, 13, 403–409. [Google Scholar] [CrossRef]
- Rausch, M.; Hellmann, S.; Zehetleitner, M. Modelling visibility judgments using models of decision confidence. Atten. Percept. Psychophys. 2021, 83, 3311–3336. [Google Scholar] [CrossRef] [PubMed]
- Zehetleitner, M.; Rausch, M. Being confident without seeing: What subjective measures of visual consciousness are about. Atten. Percept. Psychophys. 2013, 75, 1406–1426. [Google Scholar] [CrossRef] [PubMed]
- Scharlau, I. Leading, but not trailing, primes influence temporal order perception: Further evidence for an attentional account of perceptual latency priming. Percept. Psychophys. 2002, 64, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Scharlau, I.; Neumann, O. Perceptual latency priming by masked and unmasked stimuli: Evidence for an attentional interpretation. Psychol. Res. 2003, 67, 184–196. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaszkó, B.; Stolte, M.; Bachmann, L.; Ansorge, U. New Evidence for Retrospectively Cued Perception. Vision 2024, 8, 5. https://doi.org/10.3390/vision8010005
Szaszkó B, Stolte M, Bachmann L, Ansorge U. New Evidence for Retrospectively Cued Perception. Vision. 2024; 8(1):5. https://doi.org/10.3390/vision8010005
Chicago/Turabian StyleSzaszkó, Bence, Moritz Stolte, Lea Bachmann, and Ulrich Ansorge. 2024. "New Evidence for Retrospectively Cued Perception" Vision 8, no. 1: 5. https://doi.org/10.3390/vision8010005
APA StyleSzaszkó, B., Stolte, M., Bachmann, L., & Ansorge, U. (2024). New Evidence for Retrospectively Cued Perception. Vision, 8(1), 5. https://doi.org/10.3390/vision8010005





