The Action Cycle Theory of Perception and Mental Imagery
Abstract
1. Historical Context
“Yet, the most striking fact is that perceptual experience is far richer than available retinal images; and though neural signaling is slow, it is not usually delayed in time. From these shadowy ghosts in our eyes we see hard solid objects with properties beyond optics. This depends on knowledge of objects, and how they interact, allowing behavior to be appropriate to what is known or assumed, rather than limited to what is being sensed. This is where knowledge comes in, as the past enriches the present, and allows some prediction into the future”.[4]
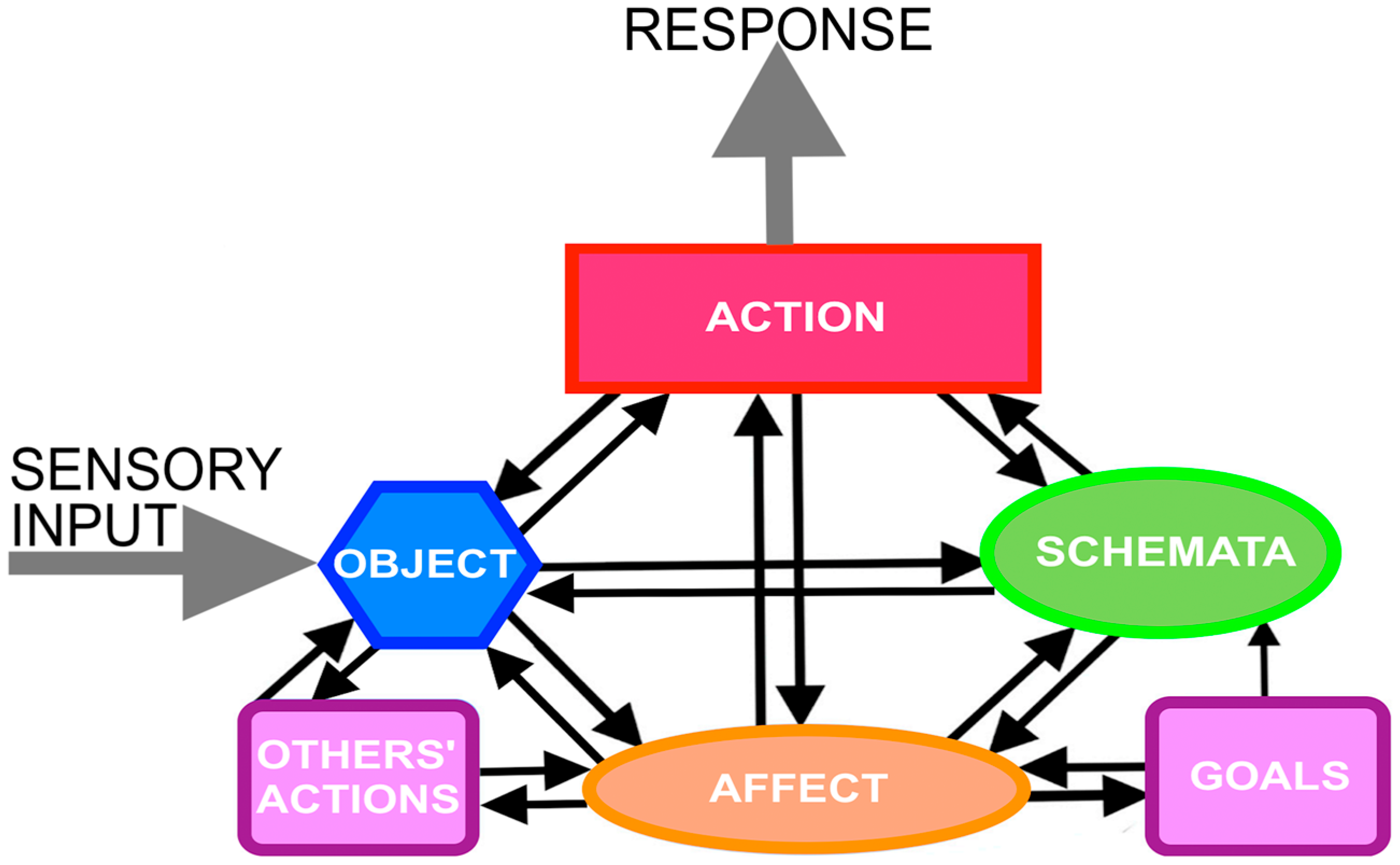
2. Action Cycle Theory
3. Schemata
4. Objects
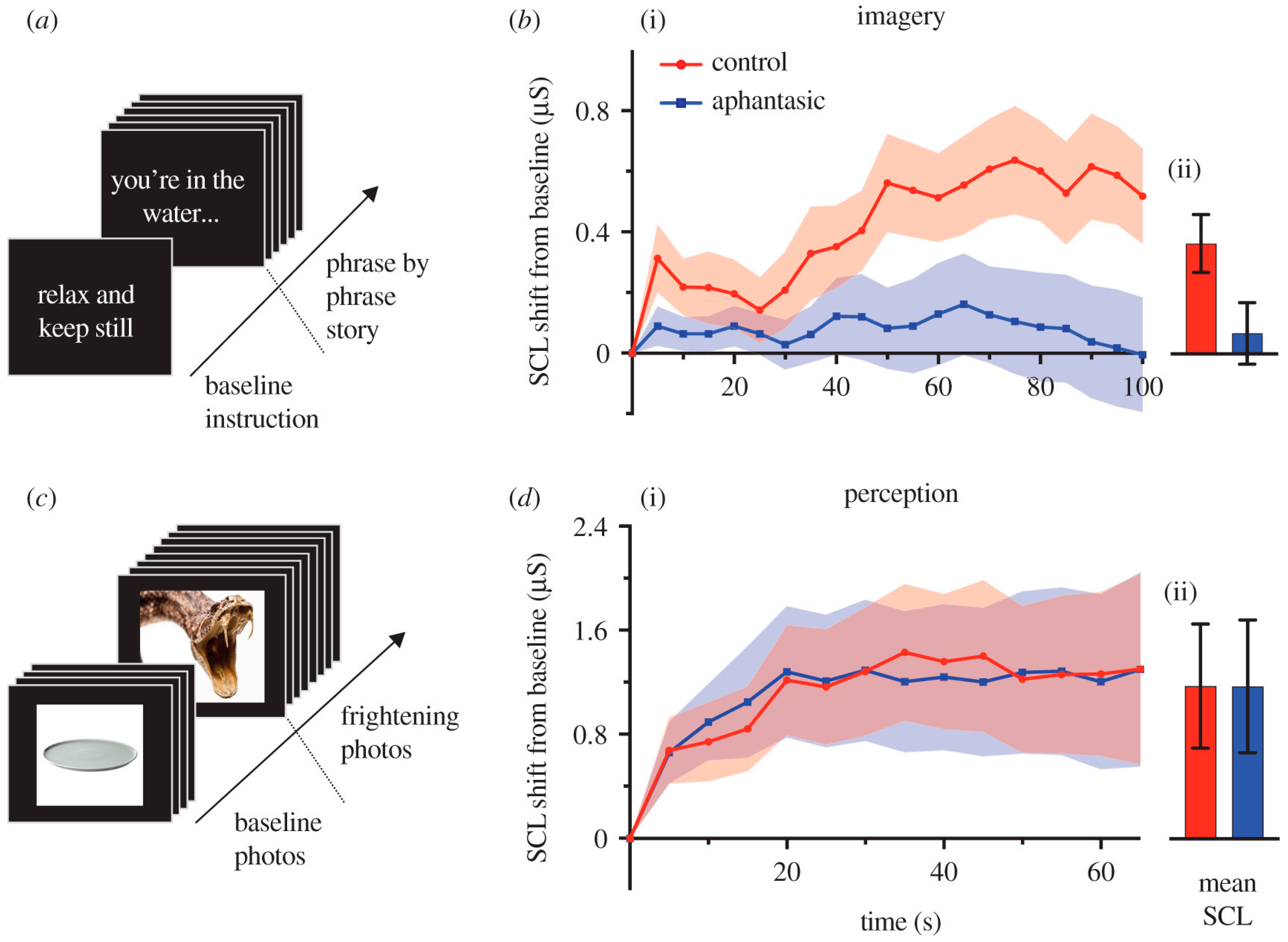
5. Action
6. Affect
7. Goals
8. Others’ Behavior
9. Limitations
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Descartes, R. Principles of Philosophy; 1644; Simon and Shuster: New York, NY, USA, 2012. [Google Scholar]
- O’Regan, J.K. Solving the real mysteries of visual perception: The world as an outside memory. Can. J. Psychol. Can. de Psychol. 1992, 46, 461–488. [Google Scholar] [CrossRef]
- von Helmholtz, H. Treatise on Physiological Optics; Translated from the Third German Edition Edited by James P. C. Southall; Courier Corporation: Chelmsford, MA, USA, 2013; Volume III, p. 209. [Google Scholar]
- Gregory, R.L. The Medawar Lecture 2001: Knowledge for Vision: Vision for Knowledge. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Bruner, J.S.; Postman, L. Emotional selectivity in perception and reaction. J. Personal. 1947, 16, 69–77. [Google Scholar] [CrossRef]
- Hubel, D.H.; Torsten, N.W. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 1962, 160, 106. [Google Scholar] [CrossRef]
- Neisser, U. Cognitive Psychology: Classic Edition; Psychology Press: London, UK, 2014. [Google Scholar]
- Marks, D.F. On the relationship between imagery, body, and mind. In Imagery: Current Developments; Routledge: Abingdon-on-Thames, UK, 1990; pp. 7–38. [Google Scholar]
- Marks, D.F. Consciousness, mental imagery and action. Br. J. Psychol. 1999, 90, 567–585. [Google Scholar] [CrossRef]
- Thomas Nigel, J.T. Are theories of imagery theories of imagination? An active perception approach to conscious mental content. Cogn. Sci. 1999, 23, 207–245. [Google Scholar] [CrossRef]
- Jeannerod, M.; Decety, J. Mental motor imagery: A window into the representational stages of action. Curr. Opin. Neurobiol. 1995, 5, 727–732. [Google Scholar] [CrossRef]
- O’Regan, J.K.; Noë, A. A sensorimotor account of vision and visual consciousness. Behav. Brain Sci. 2001, 24, 939–973. [Google Scholar] [CrossRef]
- Marks, D.F. Individual differences in the vividness of visual imagery and their effect on function. Funct. Nat. Imag. 1972, 83–108. [Google Scholar]
- Marks, D.F. Visual imagery differences in the recall of pictures. Br. J. Psychol. 1973, 64, 17–24. [Google Scholar] [CrossRef]
- Andrade, J.; May, J.; Deeprose, C.; Baugh, S.J.; Ganis, G. Assessing vividness of mental imagery: The Plymouth Sensory Imagery Questionnaire. Br. J. Psychol. 2014, 105, 547–563. [Google Scholar] [CrossRef]
- Isaac, A.; Marks, D.F.; Russell, D.G. An instrument for assessing imagery of movement: The Vividness of Movement Imagery Questionnaire (VMIQ). J. Ment. Imag. 1986, 10, 23–30. [Google Scholar]
- Croijmans, I.; Wang, Q.J. Do you want a description with that wine? The role of wine mental imagery in consumer’s desire to drink using the revised Vividness of Wine Imagery Questionnaire (VWIQ-II). J. Sens. Stud. 2022, 37, e12712. [Google Scholar] [CrossRef]
- Marks, D.F. New directions for mental imagery research. J. Ment. Imag. 1995, 19, 153–167. [Google Scholar]
- Campos, A.; Pérez-Fabello, M.J. Psychometric quality of a revised version Vividness of Visual Imagery Questionnaire. Percept. Mot. Ski. 2009, 108, 798–802. [Google Scholar] [CrossRef]
- Allbutt, J.; Ling, J.; Rowley, M.; Shafiullah, M. Vividness of visual imagery and social desirable responding: Correlations of the vividness of visual imagery questionnaire with the balanced inventory of desirable responding and the Marlowe–Crowne scale. Behav. Res. Methods 2011, 43, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Runge, M.; Bakhilau, V.; Omer, F.; D’Angiulli, A. Trial-by-Trial Vividness Self-Reports Versus VVIQ: A Meta-Analytic Comparison of Behavioral, Cognitive and Neurological Correlations. Imagin. Cogn. Personal. 2015, 35, 137–165. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.J.; Yacoub, E.; Ugurbil, K. The WU-Minn human connectome project: An overview. Neuroimage 2013, 80, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Craddock, C.; Jbabdi, S.; Yan, C.-G.; Vogelstein, J.; Castellanos, F.; Di Martino, A.; Kelly, C.; Heberlein, K.; Colcombe, S.; Milham, M.P. Imaging human connectomes at the macroscale. Nat. Methods 2013, 10, 524–539. [Google Scholar] [CrossRef]
- Ganis, G.; Thompson, W.L.; Kosslyn, S.M. Brain areas underlying visual mental imagery and visual perception: An fMRI study. Cogn. Brain Res. 2004, 20, 226–241. [Google Scholar] [CrossRef]
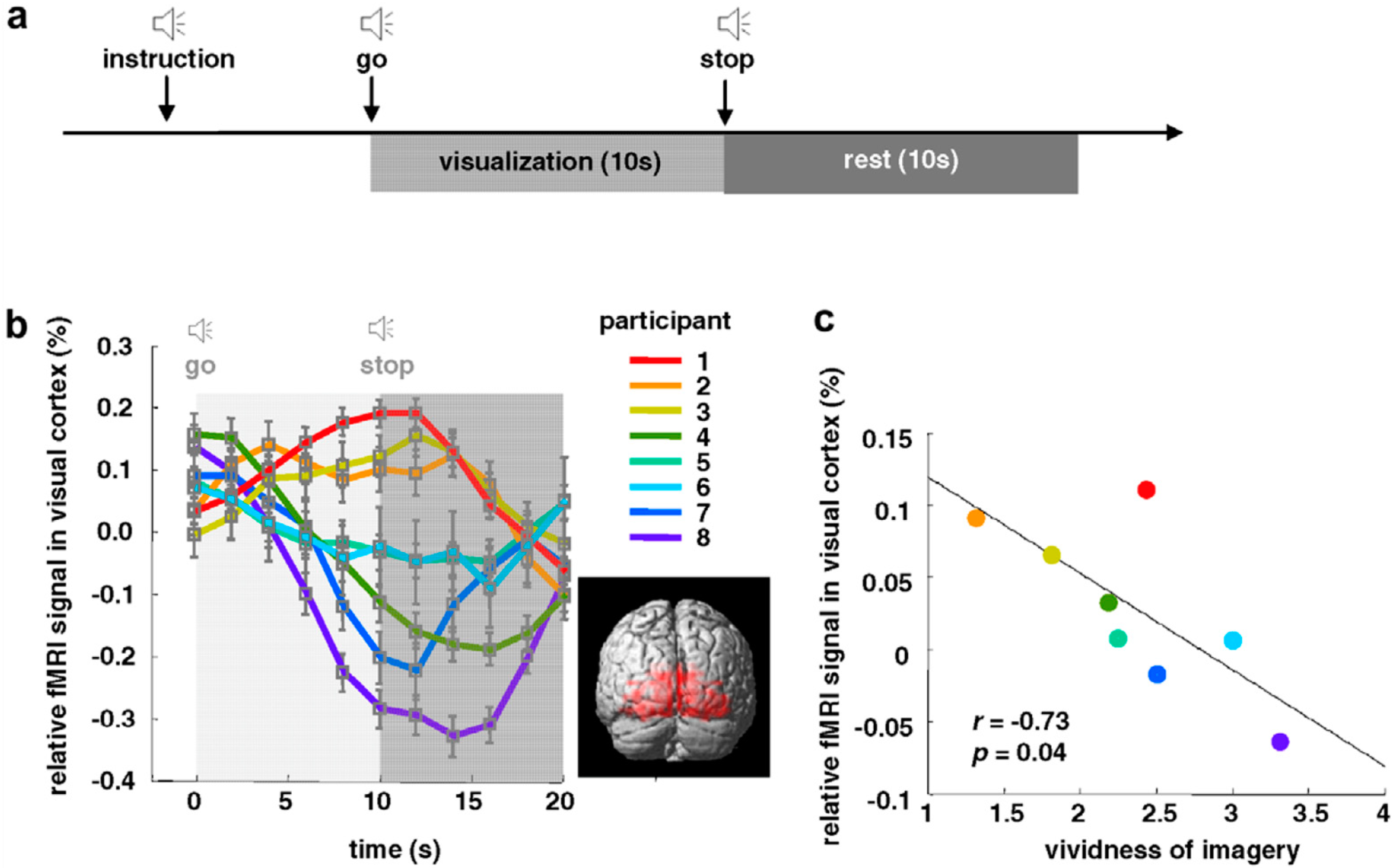
- Cui, X.; Jeter, C.B.; Yang, D.; Montague, P.R.; Eagleman, D.M. Vividness of mental imagery: Individual variability can be measured objectively. Vis. Res. 2007, 47, 474–478. [Google Scholar] [CrossRef]
- Dijkstra, N.; Zeidman, P.; Ondobaka, S.; van Gerven, M.A.J.; Friston, K. Distinct top-down and bottom-up brain connectivity during visual perception and imagery. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
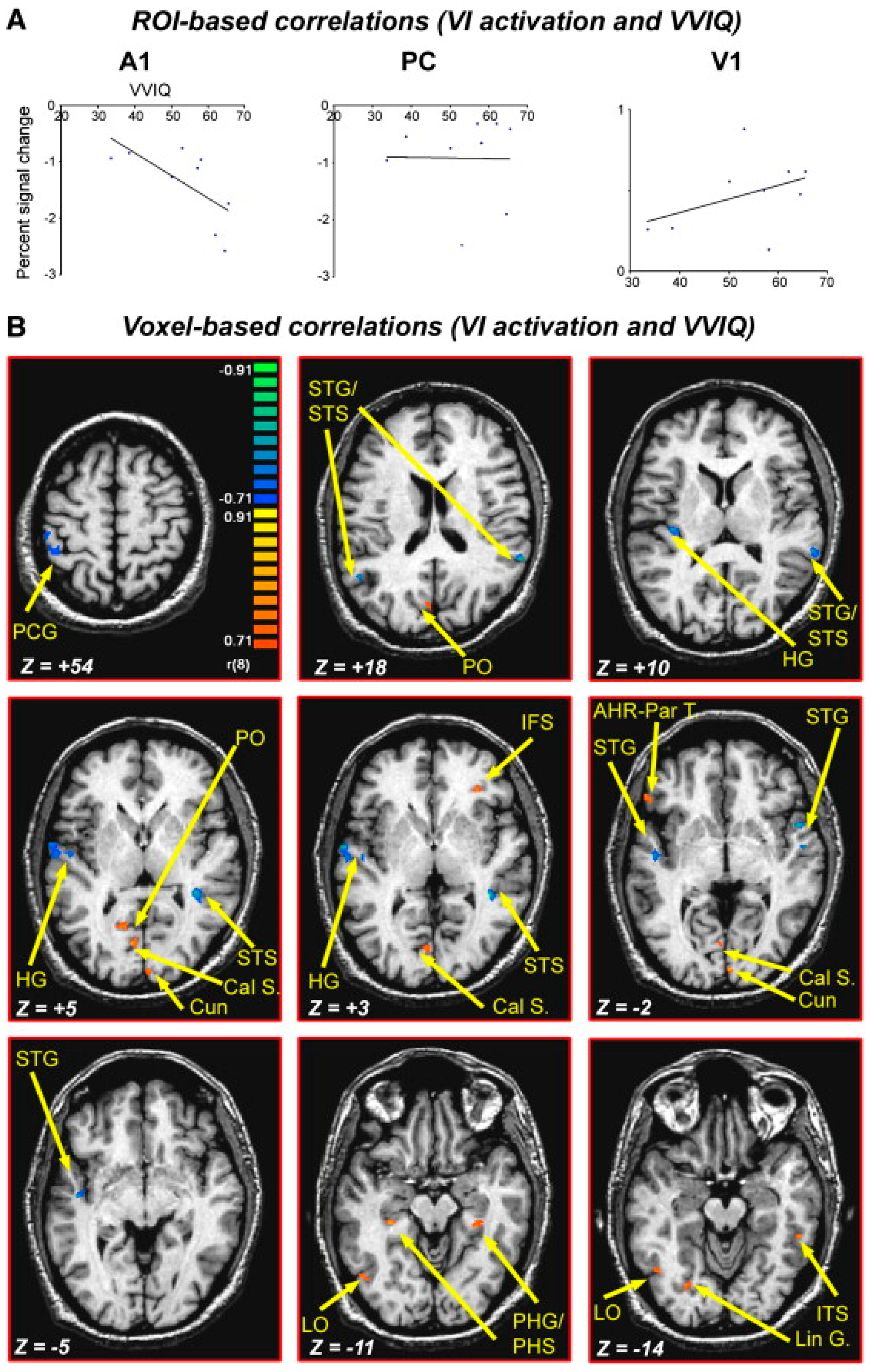
- Fulford, J.; Milton, F.; Salas, D.; Smith, A.; Simler, A.; Winlove, C.; Zeman, A. The neural correlates of visual imagery vividness—An fMRI study and literature review. Cortex 2018, 105, 26–40. [Google Scholar] [CrossRef]
- Mitchell, D.J.; Cusack, R. Semantic and emotional content of imagined representations in human occipitotemporal cortex. Sci. Rep. 2016, 6, 20232. [Google Scholar] [CrossRef]
- McKelvie, S.J. The VVIQ as a psychometric test of individual differences in visual imagery vividness: A critical quantitative review and plea for direction. J. Ment. Imag. 1995, 19, 1–106. [Google Scholar]
- McKelvie, S.J. The VVIQ and beyond: Vividness and its measurement. J. Ment. Imag. 1995, 19, 197–252. [Google Scholar]
- Marks, D.F. A General Theory of Behaviour; Sage: London, UK, 2018. [Google Scholar]
- Marks, D.F. I am conscious, therefore, I am: Imagery, affect, action, and a general theory of behavior. Brain Sci. 2019, 9, 107. [Google Scholar] [CrossRef]
- Chara, P.J., Jr.; Donald, A.H. An inquiry into the construct validity of the Vividness of Visual Imagery Questionnaire. Percept. Mot. Ski. 1989, 69, 127–136. [Google Scholar] [CrossRef]
- Friedlander, K.J.; Lenton, F.H.; Fine, P.A. A multifactorial model of visual imagery and its relationship to creativity and the vividness of Visual Imagery Questionnaire. Psychol. Aesthet. Creat. Arts 2022. [Google Scholar] [CrossRef]
- Rumelhart, D.E. Schemata: The building blocks of cognition. In Theoretical Issues in Reading Comprehension; Routledge: Abingdon-on-Thames, UK, 2017; pp. 33–58. [Google Scholar]
- Emmott, C.; Alexander, M. Schemata; de Gruyter: Berlin, Germany, 2014; Volume 1. [Google Scholar]
- Brewer, W.F.; Treyens, J.C. Treyens. Role of schemata in memory for places. Cogn. Psychol. 1981, 13, 207–230. [Google Scholar] [CrossRef]
- Head, H. Studies in Neurology; Oxford University Press: London, UK, 1920; Volume 2. [Google Scholar]
- Piaget, J. Language and Thought of the Child: Selected Works; Routledge: Abingdon-on-Thames, UK, 2005; Volume 5. [Google Scholar]
- Bartlett, F.C. Remembering: A Study in Experimental and Social Psychology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Norman, D.A.; Shallice, T. Attention to action. In Consciousness and Self-Regulation; Springer: Boston, MA, USA, 1986; pp. 1–18. [Google Scholar]
- Schmidt, R.A. A schema theory of discrete motor skill learning. Psychol. Rev. 1975, 82, 225. [Google Scholar] [CrossRef]
- Ito, M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008, 9, 304–313. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Nadel, L. Précis of O’Keefe & Nadel’s The hippocampus as a cognitive map. Behav. Brain Sci. 1979, 2, 487–494. [Google Scholar]
- Ghaem, O.; Mellet, E.; Crivello, F.; Tzourio, N.; Mazoyer, B.; Berthoz, A.; Denis, M. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport 1997, 8, 739–744. [Google Scholar] [CrossRef]
- O’Keefe, J.; Burgess, N.; Donnett, J.G.; Jeffery, K.J.; Maguire, E.A. Place cells, navigational accuracy, and the human hippocampus. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1998, 353, 1333–1340. [Google Scholar] [CrossRef]
- Maguire, E.A.; Burgess, N.; Donnett, J.G.; Frackowiak, R.S.J.; Frith, C.D.; O’Keefe, J. Knowing where and getting there: A human navigation network. Science 1998, 280, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: How the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 2017, 20, 1434–1447. [Google Scholar] [CrossRef]
- Rolls, E.T.; Wirth, S. Spatial representations in the primate hippocampus, and their functions in memory and navigation. Prog. Neurobiol. 2018, 171, 90–113. [Google Scholar] [CrossRef]
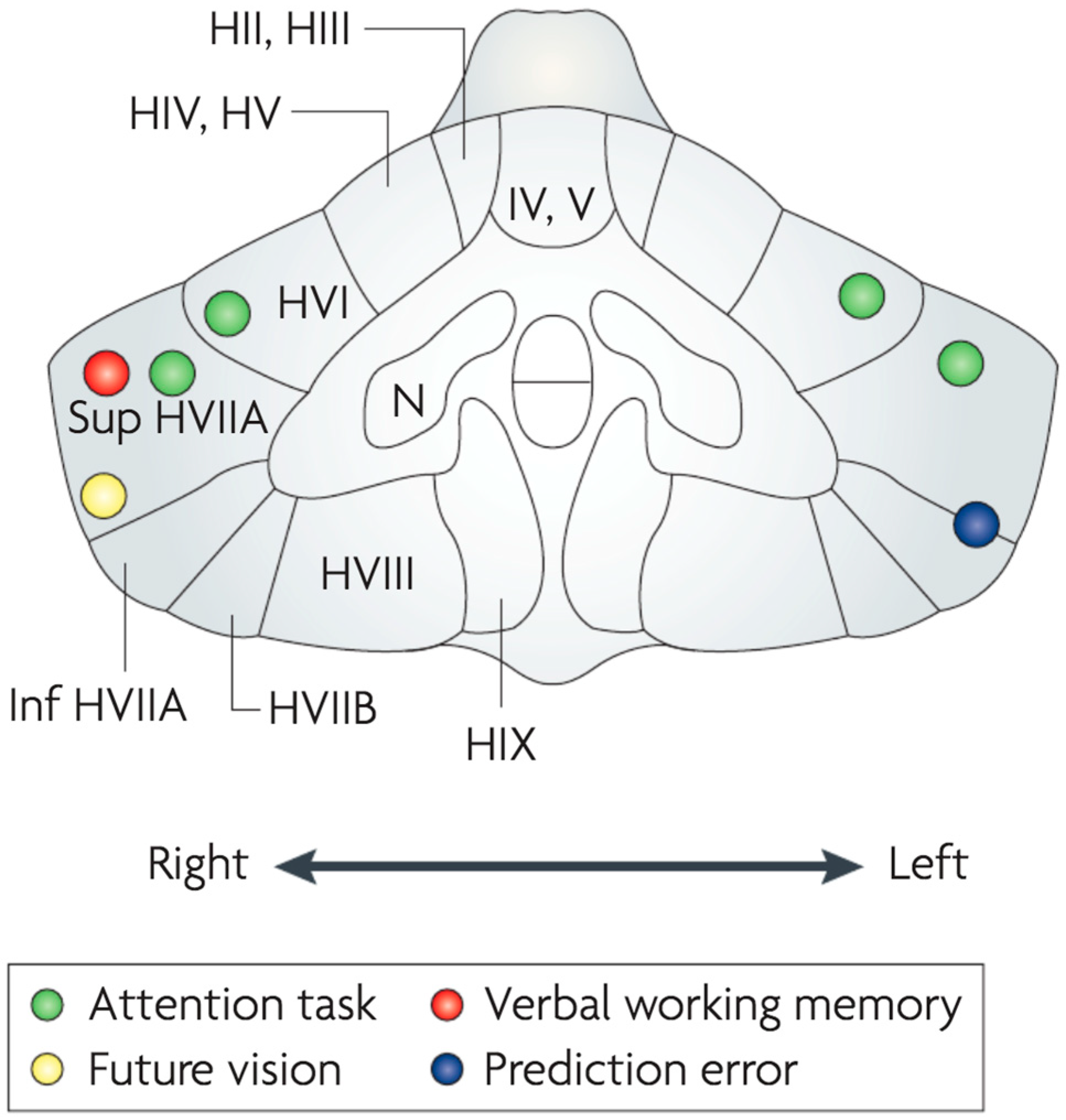
- Parkins, E. Cerebellum and Cerebrum in Homeostatic Control and Cognition: A Systems Approach to an Integrated Psychology; Routledge: Abingdon-on-Thames, UK, 2021. [Google Scholar]
- Tabi, Y.A.; Maio, M.R.; Attaallah, B.; Dickson, S.; Drew, D.; Idris, M.I.; Kienast, A.; Klar, V.; Nobis, L.; Plant, O.; et al. Vividness of visual imagery questionnaire scores and their relationship to visual short-term memory performance. Cortex 2022, 146, 186–199. [Google Scholar] [CrossRef]
- Tullo, M.G.; Almgren, H.; Van de Steen, F.; Sulpizio, V.; Marinazzo, D.; Galati, G. Individual differences in mental imagery modulate effective connectivity of scene-selective regions during resting state. Brain Struct. Funct. 2022, 227, 1831–1842. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Maguire, E.A.; Spiers, H.; Good, C.D.; Hartley, T.; Frackowiak, R.; Burgess, N. Navigation expertise and the human hippocampus: A structural brain imaging analysis. Hippocampus 2003, 13, 250–259. [Google Scholar] [CrossRef]
- Bridge, H.; Harrold, S.; Holmes, E.A.; Stokes, M.; Kennard, C. Vivid visual mental imagery in the absence of the primary visual cortex. J. Neurol. 2012, 259, 1062–1070. [Google Scholar] [CrossRef]
- Spagna, A.; Hajhajate, D.; Liu, J.; Bartolomeo, P. Visual mental imagery engages the left fusiform gyrus, but not the early visual cortex: A meta-analysis of neuroimaging evidence. Neurosci. Biobehav. Rev. 2021, 122, 201–217. [Google Scholar] [CrossRef]
- Marks, D.F. Visual imagery differences and eye movements in the recall of pictures. Percept. Psychophys. 1973, 14, 407–412. [Google Scholar] [CrossRef]
- Johansson, R.; Holsanova, J.; Homqvist, K. The dispersion of eye movements during visual imagery is related to individual differences in spatial imagery ability. In Proceedings of the Annual Meeting of the Cognitive Science Society, Boston, MA, USA, 20–23 July 2011; Volume 33. [Google Scholar]
- Amedi, A.; Malach, R.; Pascual-Leone, A. Negative BOLD differentiates visual imagery and perception. Neuron 2005, 48, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Revina, Y.; Maus, G.W. Stronger perceptual filling-in of spatiotemporal information in the blind spot compared with artificial gaps. J. Vis. 2020, 20, 20. [Google Scholar] [CrossRef]
- Michotte, A.; Thinès, G.; Crabbé, G. Les Complements Amodaux des Structures Perceptives; Publications Universitaires: Paris, France, 1964. [Google Scholar]
- Nanay, B. The importance of amodal completion in everyday perception. i-Perception 2018, 9, 2041669518788887. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Huber, D.E.; Rieth, C.A.; Tian, J.; Lee, K. Detecting faces in pure noise images: A functional MRI study on top-down perception. Neuroreport 2008, 19, 229–233. [Google Scholar] [CrossRef]
- Salge, J.H.; Pollmann, S.; Reeder, R.R. Anomalous visual experience is linked to perceptual uncertainty and visual imagery vividness. Psychol. Res. 2021, 85, 1848–1865. [Google Scholar] [CrossRef]
- James, K.H.; Gauthier, I. Letter processing automatically recruits a sensory–motor brain network. Neuropsychologia 2006, 44, 2937–2949. [Google Scholar] [CrossRef]
- Gibbs, R.W., Jr.; Berg, E.A. Mental imagery and embodied activity. J. Ment. Imag. 2002, 26, 1–30. [Google Scholar]
- Newton, N. Foundations of Understanding; New York Institute of Technology: New York, NY, USA, 1996; pp. 1–221. [Google Scholar]
- Gilboa, A.; Marlatte, H. Neurobiology of schemas and schema-mediated memory. Trends Cogn. Sci. 2017, 21, 618–631. [Google Scholar] [CrossRef]
- Kaiser, D.; Turini, J.; Cichy, R.M. A neural mechanism for contextualizing fragmented inputs during naturalistic vision. elife 2019, 8, e48182. [Google Scholar] [CrossRef]
- Shaw, W.A. The Relation of Muscular Action Potentials to Imaginal Weight Lifting; Archives of Psychology (Columbia University): New York, NY, USA, 1940. [Google Scholar]
- Hishitani, S.; Miyazaki, T.; Motoyama, H. Some mechanisms responsible for the vividness of mental imagery: Suppressor, closer, and other functions. J. Ment. Imag. 2011, 35, 5–32. [Google Scholar]
- Isaac, A.R.; Marks, D.F. Individual differences in mental imagery experience: Developmental changes and specialization. Br. J. Psychol. 1994, 85, 479–500. [Google Scholar] [CrossRef] [PubMed]
- Wöllner, C. Anticipated sonic actions and sounds in performance. In The Oxford Handbook of Sound and Imagination, Volume 2; Oxford University Press: Oxford, UK, 2019; p. 37. [Google Scholar]
- Sevdalis, N.; Moran, A.; Arora, S. Mental imagery and mental practice applications in surgery: State of the art and future directions. In Multisensory Imagery; Springer: New York, NY, USA, 2013. [Google Scholar]
- Di Pellegrino, G.; Fadiga, L.; Fogassi, L.; Gallese, V.; Rizzolatti, G. Understanding motor events: A neurophysiological study. Exp. Brain Res. 1992, 91, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M.; Woods, R.P.; Brass, M.; Bekkering, H.; Mazziotta, J.C.; Rizzolatti, G. Cortical mechanisms of human imitation. Science 1999, 286, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Mukamel, R.; Ekstrom, A.D.; Kaplan, J.; Iacoboni, M.; Fried, I. Single-neuron responses in humans during execution and observation of actions. Curr. Biol. 2010, 20, 750–756. [Google Scholar] [CrossRef]
- Cook, R.; Bird, G.; Catmur, C.; Press, C.; Heyes, C. Mirror neurons: From origin to function. Behav. Brain Sci. 2014, 37, 177–192. [Google Scholar] [CrossRef]
- Belkacem, A.N.; Jamil, N.; Palmer, J.A.; Ouhbi, S.; Chen, C. Brain computer interfaces for improving the quality of life of older adults and elderly patients. Front. Neurosci. 2020, 14, 692. [Google Scholar] [CrossRef] [PubMed]
- Leeuwis, N.; Paas, A.; Alimardani, M. Vividness of visual imagery and personality impact motor-imagery brain computer interfaces. Front. Hum. Neurosci. 2021, 15, 634748. [Google Scholar] [CrossRef]
- Thompson, M.C. Critiquing the concept of BCI illiteracy. Sci. Eng. Ethics 2019, 25, 1217–1233. [Google Scholar] [CrossRef] [PubMed]
- Mane, R.; Chouhan, T.; Guan, C. BCI for stroke rehabilitation: Motor and beyond. J. Neural Eng. 2020, 17, 041001. [Google Scholar] [CrossRef] [PubMed]
- Blumen, H.M.; Holtzer, R.; Brown, L.L.; Gazes, Y.; Verghese, J. Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Hum. Brain Mapp. 2014, 8, 4090–4104. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, E.; McKinnon, M.C.; Levine, B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 2006, 44, 2189–2208. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J. A bio-informational theory of emotional imagery. Psychophysiology 1979, 16, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Imbriano, G.; Sussman, T.J.; Jin, J.; Mohanty, A. The role of imagery in threat-related perceptual decision making. Emotion 2020, 20, 1495. [Google Scholar] [CrossRef]
- Köchel, A.; Plichta, M.M.; Schäfer, A.; Leutgeb, V.; Scharmüller, W.; Fallgatter, A.J.; Schienle, A. Affective perception and imagery: A NIRS study. Int. J. Psychophysiol. 2011, 80, 192–197. [Google Scholar] [CrossRef]
- Wicken, M.; Keogh, R.; Pearson, J. The critical role of mental imagery in human emotion: Insights from fear-based imagery and aphantasia. Proc. R. Soc. B 2021, 288, 20210267. [Google Scholar] [CrossRef] [PubMed]
- Manyande, A.; Berg, S.; Gettins, D.; Stanford, S.; Mazhero, S.; Marks, D.F.; Salmon, P. Preoperative rehearsal of active coping imagery influences subjective and hormonal responses to abdominal surgery. Psychosom. Med. 1995, 57, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Aggarwal, R.; Moran, A.; Sirimanna, P.; Crochet, P.; Darzi, A.; Kneebone, R.; Sevdalis, N. Mental practice: Effective stress management training for novice surgeons. J. Am. Coll. Surg. 2011, 212, 225–233. [Google Scholar] [CrossRef]
- Louridas, M.; Bonrath, E.M.; Sinclair, D.A.; Dedy, N.J.; Grantcharov, T.P. Randomized clinical trial to evaluate mental practice in enhancing advanced laparoscopic surgical performance. J. Br. Surg. 2015, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Conway, M.; Meares, K.; Standart, S. Images and goals. Memory 2004, 12, 525–531. [Google Scholar] [CrossRef]
- D’Argembeau, A.; Van der Linden, M. Individual differences in the phenomenology of mental time travel: The effect of vivid visual imagery and emotion regulation strategies. Conscious. Cogn. 2006, 15, 342–350. [Google Scholar] [CrossRef]
- Libby, L.K.; Shaeffer, E.M.; Eibach, R.P.; Slemmer, J.A. Picture yourself at the polls: Visual perspective in mental imagery affects self-perception and behavior. Psychol. Sci. 2007, 18, 199–203. [Google Scholar] [CrossRef]
- Boomsma, C.; Pahl, S.; Andrade, J. Imagining change: An integrative approach toward explaining the motivational role of mental imagery in pro-environmental behavior. Front. Psychol. 2016, 7, 1780. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-S.; Sung, Y.-H.; Wu, C.-C.; Ho, L.-C.; Chiou, W.-B. Using episodic future thinking to pre-experience climate change increases pro-environmental behavior. Environ. Behav. 2020, 52, 60–81. [Google Scholar] [CrossRef]
- Marks, D.F. Mental imagery: Pure and applied. Jpn. J. Ment. Imag. 2010, 8, 1–9. [Google Scholar]
- Bonini, L.; Rotunno, C.; Arcuri, E.; Gallese, V. Mirror neurons 30 years later: Implications and applications. Trends Cogn. Sci. 2022, 26, 767–781. [Google Scholar] [CrossRef]
- Molenberghs, P.; Cunnington, R.; Mattingley, J.B. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 2012, 36, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Filimon, F.; Nelson, J.D.; Hagler, D.J.; Sereno, M.I. Human cortical representations for reaching: Mirror neurons for execution, observation, and imagery. Neuroimage 2007, 37, 1315–1328. [Google Scholar] [CrossRef]
- Darwin, C. The Expression of the Emotions in Man and Animals; University of Chicago Press: Chicago, IL, USA, 1965. [Google Scholar]
- Wicker, B.; Keysers, C.; Plailly, J.; Royet, J.-P.; Gallese, V.; Rizzolatti, G. Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 2003, 40, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Bensafi, M.; Sobel, N.; Khan, R.M. Hedonic-specific activity in piriform cortex during odor imagery mimics that during odor perception. J. Neurophysiol. 2007, 98, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Aflalo, T.; Chivukula, S.; Zhang, C.; Rosario, E.R.; Pouratian, N.; Andersen, R.A. Cognition through internal models: Mirror neurons as one manifestation of a broader mechanism. bioRxiv 2022. [Google Scholar] [CrossRef]
- Lahuerta-Martín, S.; Llamas-Ramos, R.; Llamas-Ramos, I. Effectiveness of Therapies Based on Mirror Neuron System to Treat Gait in Patients with Parkinson’s Disease—A Systematic Review. J. Clin. Med. 2022, 11, 4236. [Google Scholar] [CrossRef]
- Patel, M. Action observation in the modification of postural sway and gait: Theory and use in rehabilitation. Gait Posture 2017, 58, 115–120. [Google Scholar] [CrossRef]
- Molokopoy, V.; D’Angiulli, A. Multidisciplinary Intersections on Artificial-Human Vividness: Phenomenology, Representation, and the Brain. Brain Sci. 2022, 12, 1495. [Google Scholar] [CrossRef]
- Runge, M.S.; Cheung, M.W.; D’Angiulli, A. Meta-analytic comparison of trial-versus questionnaire-based vividness reportability across behavioral, cognitive and neural measurements of imagery. Neurosci. Conscious. 2017, 2017, nix006. [Google Scholar] [CrossRef]
- Leboutillier, N.; Marks, D.F. Mental imagery and creativity: A meta-analytic review study. Br. J. Psychol. 2003, 94, 29–44. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marks, D.F. The Action Cycle Theory of Perception and Mental Imagery. Vision 2023, 7, 12. https://doi.org/10.3390/vision7010012
Marks DF. The Action Cycle Theory of Perception and Mental Imagery. Vision. 2023; 7(1):12. https://doi.org/10.3390/vision7010012
Chicago/Turabian StyleMarks, David F. 2023. "The Action Cycle Theory of Perception and Mental Imagery" Vision 7, no. 1: 12. https://doi.org/10.3390/vision7010012
APA StyleMarks, D. F. (2023). The Action Cycle Theory of Perception and Mental Imagery. Vision, 7(1), 12. https://doi.org/10.3390/vision7010012




