Retinal Nerve Fiber Layer Changes after Intraocular Silicone Oil Tamponade in Rhegmatogenous Retinal Detachment
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nemet, A.; Moshiri, A.; Yiu, G.; Loewenstein, A.; Moisseiev, E. A Review of Innovations in Rhegmatogenous Retinal Detachment Surgical Techniques. J. Ophthalmol. 2017, 2017, 4310643. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, F.; Aylward, B. Rhegmatogenous retinal detachment: A reappraisal of its pathophysiology and treatment. Ophthalmic Res. 2013, 51, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Elzarrug, H.; Miller, K.M.; Fei, Y.; Daifalla, A.E.M. Risk Factors for Postoperative Retinal Detachment Following Cataract Surgery. Open J. Ophthalmol. 2019, 9, 141–150. [Google Scholar] [CrossRef]
- Van Leeuwen, R.; Haarman, A.E.G.; Van De Put, M.A.J.; Klaver, C.C.W.; Los, L.I. Association of Rhegmatogenous Retinal Detachment Incidence with Myopia Prevalence in the Netherlands. JAMA Ophthalmol. 2021, 139, 85–92. [Google Scholar] [CrossRef]
- Nagaraj, K. Pneumoretinopexy in Unusual Situations: Our Experience. Delhi J. Ophthalmol. 2021, 31, 43–46. [Google Scholar] [CrossRef]
- Chan, C.K.; Lin, S.G.; Nuthi, A.S.D.; Salib, D.M. Pneumatic Retinopexy for the Repair of Retinal Detachments: A Comprehensive Review (1986–2007). Surv. Ophthalmol. 2008, 53, 443–478. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.C.; Balasopoulou, A.; Κokkinos, P.; Pagoulatos, D.; Plotas, P.; Makri, O.E.; Georgakopoulos, C.D.; Vantarakis, A.; Li, Y.; Liu, J.J.; et al. A new normal with cataract surgery during COVID-19 pandemic. BMC Ophthalmol. 2017, 17, 1. [Google Scholar] [CrossRef]
- Wang, A.; Snead, M.P. Scleral buckling—A brief historical overview and current indications. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 467–478. [Google Scholar] [CrossRef]
- Fallico, M.; Alosi, P.; Reibaldi, M.; Longo, A.; Bonfiglio, V.; Avitabile, T.; Russo, A. Scleral Buckling: A Review of Clinical Aspects and Current Concepts. J. Clin. Med. 2022, 11, 314. [Google Scholar] [CrossRef]
- Watanabe, A.; Ishida, M.; Takeyama, A.; Ichikawa, Y.; Mizushima, A.; Imamura, Y. Surgical Success Rate of Scleral Buckling Surgery and Postoperative Incidence of Cystoid Macular Edema: 10 Years of Experience at a Single Academic Hospital. J. Clin. Med. 2022, 11, 5321. [Google Scholar] [CrossRef]
- Sultan, Z.N.; Agorogiannis, E.I.; Iannetta, D.; Steel, D.; Sandinha, T. Rhegmatogenous retinal detachment: A review of current practice in diagnosis and management. BMJ Open Ophthalmol. 2020, 5, e000474. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.; Kaiser, R. Pars Plana Vitrectomy for Rhegmatogenous Retinal Detachment. Expert Tech. Ophthalmic Surg. 2015, 2, 239. [Google Scholar] [CrossRef]
- Vaziri, K.; Schwartz, S.G.; Kishor, K.S.; Flynn, H.W. Tamponade in the surgical management of retinal detachment. Clin. Ophthalmol. 2016, 10, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Casini, G.; Loiudice, P.; De Cillà, S.; Radice, P.; Nardi, M. Sulfur hexafluoride (SF6) versus perfluoropropane (C3F8) tamponade and short term face-down position for macular hole repair: A randomized prospective study. Int. J. Retin. Vitr. 2016, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Giridhar, A.; Gopalakrishnan, M. Sulfurhexafluoride (SF6) versus perfluoropropane (C3F8) gas as tamponade in macular hole surgery. Retina 2017, 37, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J. Vitreous in Health and Disease; Springer Science+Business Media: New York, NY, USA, 2014; ISBN 978-1-4939-1085-4. [Google Scholar]
- Ryan, S.J.; Schachat, A.P.; Wilkinson Charles, P.; Hinton David, R.; Sadda SriniVas, R. ; Wiedemann Peter. Ryan’s Retina, 6th ed.; Elsevier: New York, NY, USA, 2018; Available online: https://www.clinicalkey.com.au/dura/browse/bookChapter/3-s2.0-C20141013440 (accessed on 2 June 2022).
- Aziz, T.; Fan, H.; Khan, F.U.; Haroon, M.; Cheng, L. Modified silicone oil types, mechanical properties and applications. Polym. Bull. 2019, 76, 2129–2145. [Google Scholar] [CrossRef]
- Barca, F.; Caporossi, T.; Rizzo, S. Silicone oil: Different physical proprieties and clinical applications. BioMed Res. Int. 2014, 2014, 502143. [Google Scholar] [CrossRef]
- Knorr, H.L.; Seltsam, A.; Holbach, L.; Naumann, G.O. Intraocular silicone oil tamponade. A clinico-pathologic study of 36 enucleated eyes. Ophthalmologe 1996, 93, 130–138. [Google Scholar]
- Biswas, J.; Verma, A.; Davda, M.D.; Ahuja, S.; Pushparaj, V. Intraocular tissue migration of silicone oil after silicone oil tamponade: A histopathological study of enucleated silicone oil-filled eyes. Indian J. Ophthalmol. 2008, 56, 425–428. [Google Scholar] [CrossRef]
- Wickham, L.; Asaria, R.H.; Alexander, R.; Luthert, P.; Charteris, D.G. Immunopathology of intraocular silicone oil: Enucleated eyes. Br. J. Ophthalmol. 2007, 91, 253–257. [Google Scholar] [CrossRef]
- Papp, A.; Kiss, E.B.; Tímár, Ö.; Szabó, E.; Berecki, Á.; Tóth, J.; Páli, J. Long-term exposure of the rabbit eye to silicone oil causes optic nerve atrophy. Brain Res. Bull. 2007, 74, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, J.E.; Shin, Y.I.; Lee, K.M.; Jo, Y.J.; Kim, J.Y. Longitudinal changes in retinal nerve fiber layer thickness after vitrectomy for rhegmatogenous retinal detachment. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5471–5474. [Google Scholar] [CrossRef] [PubMed]
- Ichsan, A.M.; Tajuddin, A.S.; Muhiddin, H.S.; Budu; Umar, B.T.; Waspodo, N. Visual acuity and contrast sensitivity outcomes based on photoreceptor layer after retinal reattachment surgery. Edorium J. Ophthalmol. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Soheilian, M.; Mazareei, M.; Mohammadpour, M.; Rahmani, B. Comparison of silicon oil removal with various viscosities after complex retinal detachment surgery. BMC Ophthalmol. 2006, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Kartasasmita, A.; Kusdiono, W.; Virgana, R.; Boesorie, S. Emulsification Analysis of 1000 cs and 5000 cs Silicone Oil after Rhegmatogenous Retinal Detachment Vitrectomy Surgery. Open J. Ophthalmol. 2017, 7, 231–239. [Google Scholar] [CrossRef]
- Scott, I.U.; Flynn, H.W.; Murray, T.G.; Smiddy, W.E.; Davis, J.L.; Feuer, W.J. Outcomes of complex retinal detachment repair using 1000- vs 5000-centistoke silicone oil. Arch. Ophthalmol. 2005, 123, 473–478. [Google Scholar] [CrossRef]
- Bolukbasi, S. The Effect of Silicone Oil Endotamponade on Subfoveal Choroidal Thickness after Pars Plana Vitrectomy for Rhegmatogenous Retinal Detachment. Beyoglu Eye J. 2019, 4, 97–101. [Google Scholar] [CrossRef]
- Nassar, G.A.; Youssef, M.M.; Hassan, L.M.; Makled, H.S. Retinal Sensitivity before and after Silicone Oil Removal Using Microperimetry. J. Ophthalmol. 2019, 2019, 2723491. [Google Scholar] [CrossRef]
- Abu Al-Naga, A.B.; Khalil, H.F.; Mostafa, M.M.; Gomaa, B.I.K. Macular Microstructure Assessment by Optical Coherence Tomography and Fundus Fluorescein Angiography before and after Silicone Oil Removal. Egypt. J. Hosp. Med. 2019, 75, 3083–3092. [Google Scholar] [CrossRef]
- Brănişteanu, D.C.; Moraru, A.; Bîlha, A. Anatomical results and complications after silicone oil removal. Rom. J. Ophthalmol. 2017, 61, 261–266. [Google Scholar] [CrossRef]
- Saleh, O.A.; Fleissig, E.; Barr, C.C. Outcomes after the Use of Silicone Oil in Complex Retinal Detachment Repair. J. Vitreoretin. Dis. 2020, 4, 96–102. [Google Scholar] [CrossRef]
- Issa, R.; Xia, T.; Zarbin, M.A.; Bhagat, N. Silicone oil removal: Post-operative complications. Eye 2020, 34, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Jawad, M.; Khan, B.; Shah, M.A.; Qayum, I.; Aftab, M. Changes of Intraocular Pressure in Vitrectomised Eyes after Removal of Silicone Oil. J. Ayub Med. Coll. Abbottabad 2016, 28, 327–330. [Google Scholar] [PubMed]
- Wolf, S.; Schön, V.; Meier, P.; Wiedemann, P. Silicone oil-RMN3 mixture (“heavy silicone oil”) as internal tamponade for complicated retinal detachment. Retina 2003, 23, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Er, D.; Öner, H.; Kaya, M.; Dönmez, O. Evaluation of the effects of silicone oil on the macula with optical coherence tomography in patients with rhegmatogenous retinal detachment. Turkish J. Ophthalmol. 2021, 51, 218–224. [Google Scholar] [CrossRef]
- Faisal, S.M.; Tahir, M.A.; Achakzai, N.U.; Abbasi, S. Aziz-Ur-rehman Effect of Silicon Oil Tamponade and Its Removal on Central Macular Thickness in Cases of Rhegmatogenous Retinal Detachment Using Swept Source OCT. Pakistan J. Ophthalmol. 2022, 38, 234–238. [Google Scholar] [CrossRef]
- Bae, K.; Lee, S.M.; Kang, S.W.; Kim, E.S.; Yu, S.Y.; Kim, K.T. Atypical epiretinal tissue in full-thickness macular holes: Pathogenic and prognostic significance. Br. J. Ophthalmol. 2019, 103, 251–256. [Google Scholar] [CrossRef]
- Rabina, G.; Azem, N.; Barequet, D.; Barak, A.; Loewenstein, A.; Schwartz, S. Silicone Oil Tamponade Effect on Macular Layer Thickness and Visual Acuity. Retina 2020, 40, 998–1004. [Google Scholar] [CrossRef]
- Rabina, G. Full-thickness macular hole in age-related macular degeneration patients with two distinct entities. J. Retin. Vitr. Dis. 2021, 41, 2066–2072. [Google Scholar] [CrossRef]
- Kheir, W.J.; Mehanna, C.-J.; Koaik, M.; Bashshur, Z. Macular Changes on Optical Coherence Tomography Before, during, and after Silicone Oil Tamponade for Macula-On Retinal Detachment: A Case Series. J. Vitreoretin. Dis. 2018, 2, 297–301. [Google Scholar] [CrossRef]
- Caramoy, A.; Foerster, J.; Allakhiarova, E.; Hoyng, C.B.; Dröge, K.; Kirchhof, B.; Fauser, S. Spectral-domain optical coherence tomography in subjects over 60 years of age, and its implications for designing clinical trials. Br. J. Ophthalmol. 2012, 96, 1325–1330. [Google Scholar] [CrossRef]
- Klettner, A.; Harms, A.; Waetzig, V.; Tode, J.; Purtskhvanidze, K.; Roider, J. Emulsified silicone oil is taken up by and induces pro-inflammatory response in primary retinal microglia. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1965–1974. [Google Scholar] [CrossRef] [PubMed]
- Takkar, B.; Azad, R.; Kamble, N.; Azad, S. Retinal nerve fiber layer changes following primary retinal detachment repair with silicone oil tamponade and subsequent oil removal. J. Ophthalmic Vis. Res. 2018, 13, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Mirza, E. Evaluation of The Effect of Intraocular Silicone Oil on Retinal Nerve Fiber Layer Thickness in Patients Treated with Pars Plana Vitrectomy and Endolaser for Rhegmatogenous Retinal Detachment. Retin. Vitr. 2018, 27, 231–238. [Google Scholar]
- Raczyńska, D.; Mitrosz, K.; Raczyńska, K.; Glasner, L. The Influence of Silicone Oil on the Ganglion Cell Complex after Pars Plana Vitrectomy for Rhegmatogenous Retinal Detachment. Curr. Pharm. Des. 2018, 24, 3476–3493. [Google Scholar] [CrossRef] [PubMed]
- Morescalchi, F.; Costagliola, C.; Duse, S.; Gambicorti, E.; Parolini, B.; Arcidiacono, B.; Romano, M.R.; Semeraro, F. Heavy silicone oil and intraocular inflammation. BioMed Res. Int. 2014, 2014, 574825. [Google Scholar] [CrossRef]
- Pichi, F.; Hay, S.; Abboud, E.B. Inner retinal toxicity due to silicone oil: A case series and review of the literature. Int. Ophthalmol. 2020, 40, 2413–2422. [Google Scholar] [CrossRef]
- Doslak, M.J. A Theoretical study of the effect of silicone oil on the electroretinogram. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1881–1884. [Google Scholar]
- Christou, E.E.; Zafeiropoulos, P.; Bagli, E.; Katsanos, A.; Asproudis, I.; Stefaniotou, M. Electroretinogram changes before and after silicone oil removal in eyes with macula-off rhegmatogenous retinal detachment. Med. Hypothesis Discov. Innov. Optom. 2022, 3, 119–127. [Google Scholar] [CrossRef]
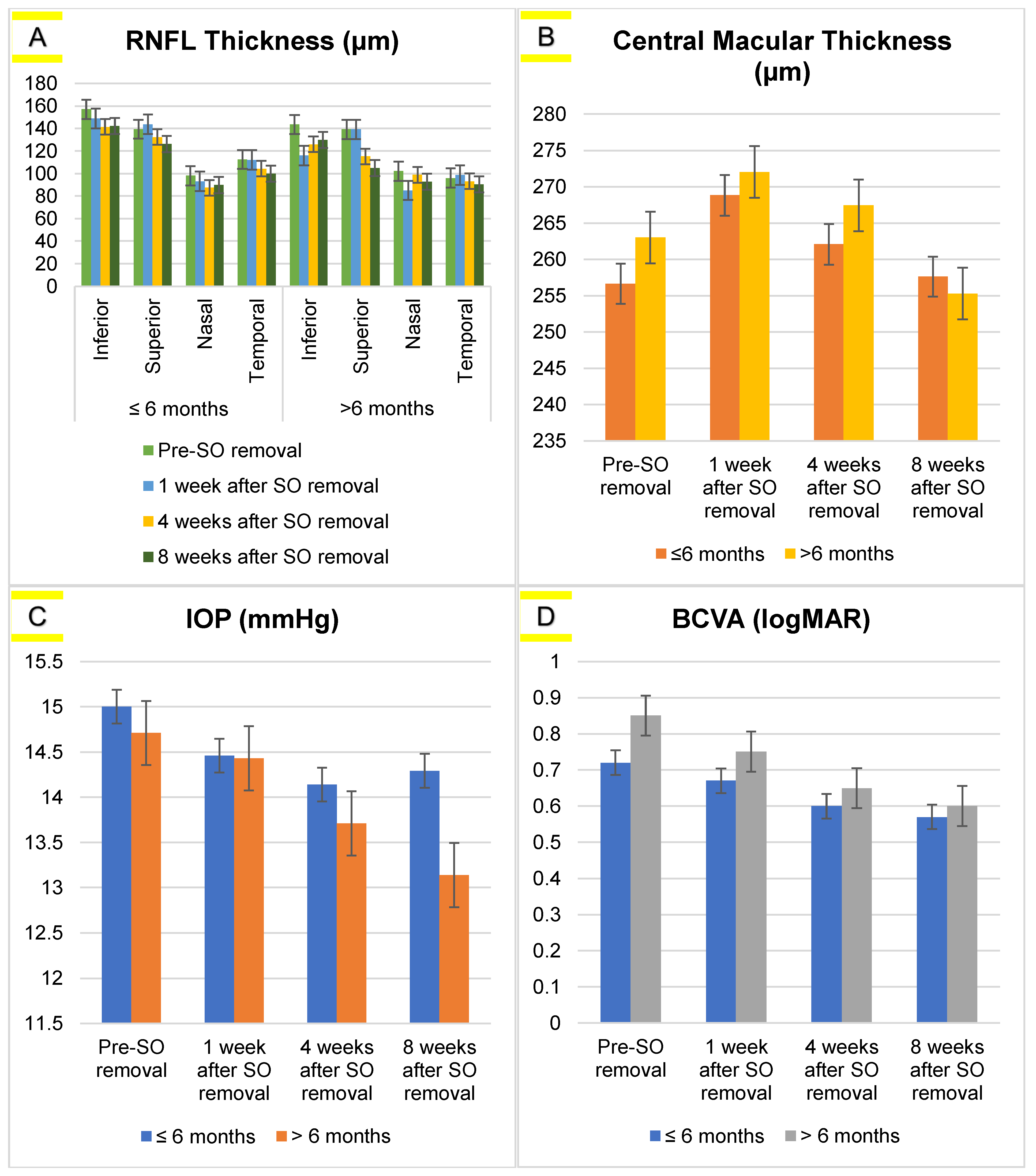
| Variables | Measurement Time | p-Value | |||
|---|---|---|---|---|---|
| Pre SO Removal | 1 wk Post SO Removal | 4 wk Post SO Removal | 8 wk Post SO Removal | ||
| RNFL (µm) | |||||
| Inferior | 154.31 ± 44.05 | 142.23 ± 38.46 | 138.34 ± 35.66 | 139.69 ± 36.38 | 0.17 |
| Superior | 139.31 ± 34.71 | 142.86 ± 42.86 | 128.91 ± 27.16 | 121.94 ± 25.47 | <0.001 * |
| Nasal | 98.97 ± 34.50 | 91.37 ± 28.54 | 89.77 ± 32.79 | 90.40 ± 31.43 | 0.34 |
| Temporal | 109.20 ± 44.92 | 109.43 ± 42.85 | 102.11 ± 31.79 | 97.86 ± 31.23 | 0.02 * |
| CMT (µm) | 265.91 ± 20.01 | 269.46 ± 18.52 | 263.14 ± 22.14 | 257.14 ± 22.17 | <0.001 * |
| IOP (mmHg) | 14.94 ± 2.74 | 14.46 ± 2.72 | 14.06 ± 2.51 | 14.06 ± 2.87 | 0.08 |
| BCVA (LogMAR) | 0.75 ± 0.33 | 0.69 ± 0.29 | 0.61 ± 0.29 | 0.58 ± 0.27 | <0.001 * |
| Variables | Group | Mean Difference | p-Value | 95% CI | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| BCVA ≤ 6 months group | 1 | 2 | 0.04 | 0.57 | −0.03 | 0.12 |
| 3 | 0.11 | <0.001 * | 0.04 | 0.19 | ||
| 4 | 0.14 | <0.001 * | 0.06 | 0.23 | ||
| 2 | 3 | 0.07 | 0.04 | 0.00 | 0.14 | |
| 4 | 0.10 | <0.001 * | 0.02 | 0.17 | ||
| 3 | 4 | 0.02 | 0.37 | −0.01 | 0.07 | |
| BCVA > 6 months group | 1 | 2 | 0.10 | 0.90 | −0.13 | 0.34 |
| 3 | 0.19 | 0.01 * | 0.04 | 0.35 | ||
| 4 | 0.25 | 0.07 | −0.02 | 0.52 | ||
| 2 | 3 | 0.09 | 0.39 | −0.06 | 0.25 | |
| 4 | 0.14 | 0.08 | −0.01 | 0.31 | ||
| 3 | 4 | 0.05 | 1.00 | −0.11 | 0.21 | |
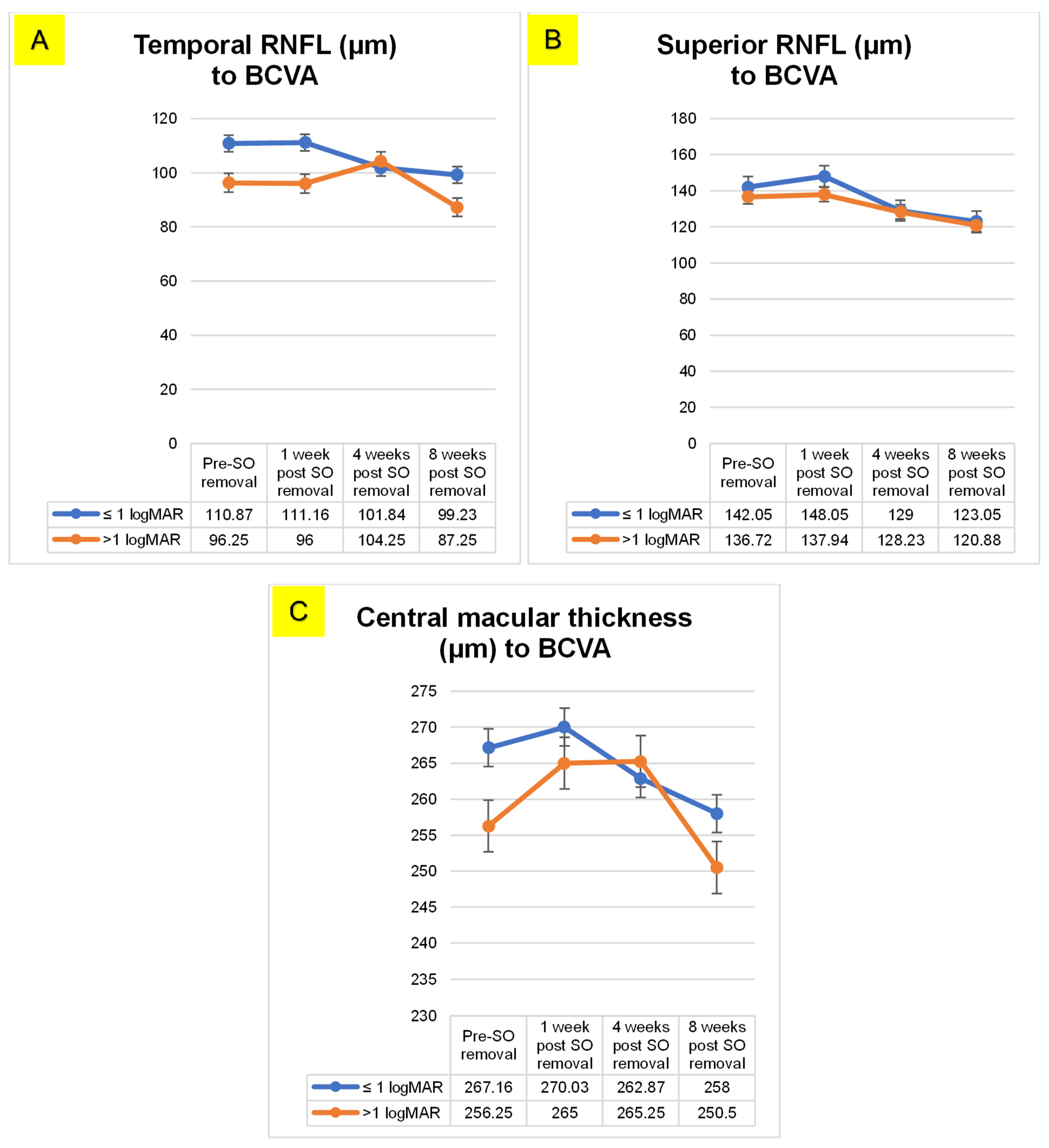
| CMT > 6 months group | 1 | 2 | −2.17 | 1.00 | −14.63 | 10.27 |
| 3 | 4.57 | 1.00 | −9.25 | 18.39 | ||
| 4 | 9.03 | 0.43 | −4.70 | 22.77 | ||
| 2 | 3 | 6.75 | 0.15 | −1.40 | 14.90 | |
| 4 | 11.21 | 0.04 * | 0.26 | 22.16 | ||
| 3 | 4 | 4.46 | 0.57 | −2.91 | 11.84 | |
| RNFL Superior < 6 months group | 1 | 2 | 8.25 | 0.06 | −0.53 | 17.03 |
| 3 | 15.57 | 0.04 * | 0.76 | 30.38 | ||
| 4 | 14.85 | 0.02 * | 2.00 | 27.70 | ||
| 2 | 3 | 7.32 | 0.16 | −3.13 | 17.78 | |
| 4 | 6.60 | 0.19 | −3.50 | 16.72 | ||
| 3 | 4 | −0.71 | 0.87 | −9.56 | 8.13 | |
| RNFL Temporal < 6 months group | 1 | 2 | −4.42 | 1.00 | −13.53 | 4.67 |
| 3 | 7.00 | 1.00 | −7.54 | 21.54 | ||
| 4 | 13.17 | 0.05 * | −0.30 | 26.65 | ||
| 2 | 3 | 11.42 | 0.19 | −2.96 | 25.82 | |
| 4 | 17.60 | 0.01 * | 3.06 | 31.15 | ||
| 3 | 4 | 6.17 | 0.03 * | 0.25 | 12.10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikmah, F.A.; Ichsan, A.M.; Islam, I.C.; Hendarto, J.; Muhiddin, H.S.; Budu. Retinal Nerve Fiber Layer Changes after Intraocular Silicone Oil Tamponade in Rhegmatogenous Retinal Detachment. Vision 2023, 7, 13. https://doi.org/10.3390/vision7010013
Chikmah FA, Ichsan AM, Islam IC, Hendarto J, Muhiddin HS, Budu. Retinal Nerve Fiber Layer Changes after Intraocular Silicone Oil Tamponade in Rhegmatogenous Retinal Detachment. Vision. 2023; 7(1):13. https://doi.org/10.3390/vision7010013
Chicago/Turabian StyleChikmah, Fitri Annur, Andi Muhammad Ichsan, Itzar Chaidir Islam, Joko Hendarto, Habibah Setyawati Muhiddin, and Budu. 2023. "Retinal Nerve Fiber Layer Changes after Intraocular Silicone Oil Tamponade in Rhegmatogenous Retinal Detachment" Vision 7, no. 1: 13. https://doi.org/10.3390/vision7010013
APA StyleChikmah, F. A., Ichsan, A. M., Islam, I. C., Hendarto, J., Muhiddin, H. S., & Budu. (2023). Retinal Nerve Fiber Layer Changes after Intraocular Silicone Oil Tamponade in Rhegmatogenous Retinal Detachment. Vision, 7(1), 13. https://doi.org/10.3390/vision7010013





