Low-Concentration Atropine Monotherapy vs. Combined with MiSight 1 Day Contact Lenses for Myopia Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Principles
2.2. Inclusion and Exclusion Criteria
2.3. Treatment Components
2.4. Follow-Up Visits
2.5. Cessation of Atropine Therapy
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Method Strengths and Weaknesses and Rebound
4.2. Peripheral Defocus Design
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brennan, N.A.; Toubouti, Y.M.; Cheng, X.; Bullimore, M.A. Efficacy in myopia control. Prog. Retin. Eye Res. 2021, 83, 100923. [Google Scholar] [CrossRef]
- Chamberlain, P.; Bradley, A.; Arumugam, B.; Hammond, D.; McNally, J.; Logan, N.S.; Jones, D.; Ngo, C.; Peixoto-de-Matos, S.C.; Hunt, C. Long-term effect of dual-focus contact lenses on myopia progression in children: A 6-year multicenter clinical trial. Optom. Vis. Sci. 2022, 99, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rodriguez, D.V.; Moreno-Montoya, J.; Álvarez-Peregrina, C. Prevalence of Myopia in America: A Systematic Review and Meta-Analysis. Cienc. Tecnol. Salud Vis. Ocul. 2021, 19, 72–86. [Google Scholar] [CrossRef]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Group, C. Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET). Investig. Ophthalmol. Vis. Sci. 2013, 54, 7871. [Google Scholar]
- Iribarren, R.; Galán, M.M.; Szeps, A.; Irigaray, L.F.; Kotlik, C.; Rodríguez, G.; Aguirre, R. Consensus on progressive myopia management. Oftalmol. Clín. Exp. 2022, 15, e137–e156. [Google Scholar]
- Hou, W.; Norton, T.T.; Hyman, L.; Gwiazda, J.; Group, C. Axial elongation in myopic children and its association with myopia progression in the Correction of Myopia Evaluation Trial (COMET). Eye Contact Lens Sci. Clin. Pract. 2018, 44, 248. [Google Scholar] [CrossRef]
- Morgan, I.G.; Jan, C.L. China Turns to School Reform to Control the Myopia Epidemic: A Narrative Review. Asia-Pac. J. Ophthalmol. 2022, 11, 27–35. [Google Scholar] [CrossRef]
- Hou, X.-W.; Wang, Y.; Ke, C.; Pan, C.-W. Metabolomics facilitates the discovery of metabolic profiles and pathways for myopia: A systematic review. Eye 2022, 1, 1–8. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.; Chen, Y.; He, M. Myopia prediction: A systematic review. Eye 2022, 36, 921–929. [Google Scholar] [CrossRef]
- Rhee, M.K. Update on Myopia Control: The US Perspective. Eye Contact Lens Sci. Clin. Pract. 2022, 48, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Erdinest, N.; London, N.; Levinger, N.; Lavy, I.; Pras, E.; Morad, Y. Treatment of Rapid Progression of Myopia: Case Series and Literature Review. Case Rep. Ophthalmol. 2021, 12, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Liu, Y.-L.; Ma, I.-H.; Su, C.-C.; Lin, C.-W.; Lin, L.L.-K.; Hsiao, C.K.; Wang, I.-J. Evolution of the prevalence of myopia among Taiwanese schoolchildren: A review of survey data from 1983 through 2017. Ophthalmology 2021, 128, 290–301. [Google Scholar] [CrossRef]
- Zhao, C.; Cai, C.; Ding, Q.; Dai, H. Efficacy and safety of atropine to control myopia progression: A systematic review and meta-analysis. BMC Ophthalmol. 2020, 20, 478. [Google Scholar] [CrossRef]
- Hiraoka, T. Myopia Control with Orthokeratology: A Review. Eye Contact Lens Sci. Clin. Pract. 2022, 48, 100–104. [Google Scholar] [CrossRef]
- Xu, S.; Li, Z.; Zhao, W.; Zheng, B.; Jiang, J.; Ye, G.; Feng, Z.; Long, W.; He, L.; He, M.; et al. Effect of atropine, orthokeratology and combined treatments for myopia control: A 2-year stratified randomised clinical trial. Br. J. Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Khorrami-Nejad, M.; Naghdi, T.; Gheibi, A. Latest Updates on Pharmacological Management of Myopia Control; A review article. J. Mod. Rehabil. 2022, 16, 214–221. [Google Scholar]
- Hieda, O.; Hiraoka, T.; Fujikado, T.; Ishiko, S.; Hasebe, S.; Torii, H.; Takahashi, H.; Nakamura, Y.; Sotozono, C.; Oshika, T.; et al. Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn. J. Ophthalmol. 2021, 65, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Chia, A.; Lu, Q.-S.; Tan, D. Five-year clinical trial on atropine for the treatment of myopia 2: Myopia control with atropine 0.01% eyedrops. Ophthalmology 2016, 123, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Pancham, K.; Kalika, B. Role of Short Term Open Eye Orthok Lens Wear in Inducing Myopia Control Changes in Eyes with Moderate Myopia. Indian J. Forensic Med. Toxicol. 2021, 15, 851–857. [Google Scholar]
- Tan, Q.; Ng, A.L.; Cheng, G.P.; Woo, V.C.; Cho, P. Combined atropine with orthokeratology for myopia control: Study design and preliminary results. Curr. Eye Res. 2019, 44, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, J.-M.; De-Hita-Cantalejo, C.; Baustita-Llamas, M.-J.; Sánchez-González, M.C.; Capote-Puente, R. The Combined Effect of Low-dose Atropine with Orthokeratology in Pediatric Myopia Control: Review of the Current Treatment Status for Myopia. J. Clin. Med. 2020, 9, 2371. [Google Scholar] [CrossRef] [PubMed]
- Azuara-Blanco, A.; Logan, N.; Strang, N.; Saunders, K.; Allen, P.M.; Weir, R.; Doherty, P.; Adams, C.; Gardner, E.; Hogg, R.; et al. Low-dose (0.01%) atropine eye-drops to reduce progression of myopia in children: A multicentre placebo-controlled randomised trial in the UK (CHAMP-UK)—Study protocol. Br. J. Ophthalmol. 2020, 104, 950–955. [Google Scholar] [CrossRef]
- Upadhyay, A.; Beuerman, R.W. Biological Mechanisms of Atropine Control of Myopia. Eye Contact Lens Sci. Clin. Pract. 2020, 46, 129. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, S.; Momeni-Moghaddam, H.; Abdolahian, M.; Piñero, D.P. Agreement of wavefront-based refraction, dry and cycloplegic autorefraction with subjective refraction. J. Optom. 2020, 15, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Shi, L.; Xu, K.; Hong, D. Clinical evaluation of autorefraction and subjective refraction with and without cycloplegia in Chinese school-aged children: A cross-sectional study. Transl. Pediatr. 2022, 11, 933. [Google Scholar] [CrossRef]
- Mukash, S.N.; Kayembe, D.L.; Mwanza, J.-C. Agreement between retinoscopy, autorefractometry and subjective refraction for determining refractive errors in Congolese children. Clin. Optom. 2021, 13, 129–136. [Google Scholar] [CrossRef]
- Yam, J.C.; Jiang, Y.; Tang, S.M.; Law, A.K.; Chan, J.J.; Wong, E.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J. Low-concentration atropine for myopia progression (LAMP) study: A randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 2019, 126, 113–124. [Google Scholar] [CrossRef]
- Li, F.F.; Zhang, Y.; Zhang, X.; Yip, B.H.K.; Tang, S.M.; Kam, K.W.; Young, A.L.; Chen, L.J.; Tham, C.C.; Pang, C.P. Age Effect on Treatment Responses to 0.05%, 0.025%, and 0.01% Atropine: Low-Concentration Atropine for Myopia Progression Study. Ophthalmology 2021, 128, 1180–1187. [Google Scholar] [CrossRef]
- Yam, J.C.; Zhang, X.J.; Zhang, Y.; Wang, Y.M.; Tang, S.M.; Li, F.F.; Kam, K.W.; Ko, S.T.; Yip, B.H.; Young, A.L. Three-Year clinical trial of low-concentration atropine for myopia progression (lamp) study: Continued versus washout: Phase 3 report. Ophthalmology 2022, 129, 308–321. [Google Scholar] [CrossRef]
- Huang, J.; Mutti, D.O.; Jones-Jordan, L.A.; Walline, J.J. Bifocal & Atropine in Myopia (BAM) Study: Baseline Data and Methods. Optom. Vis. Sci. 2019, 96, 335. [Google Scholar] [PubMed]
- Erdinest, N.; London, N.; Levinger, N.; Morad, Y. Myopia control with combination low-dose atropine and peripheral Defocus soft contact lenses: A case series. Case Rep. Ophthalmol. 2021, 12, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Anstice, N.S.; Phillips, J.R. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology 2011, 118, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, P.; Peixoto-de-Matos, S.C.; Logan, N.S.; Ngo, C.; Jones, D.; Young, G. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom. Vis. Sci. 2019, 96, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Walline, J.J.; Giannoni, A.G.; Sinnott, L.T.; Chandler, M.A.; Huang, J.; Mutti, D.O.; Jones-Jordan, L.A.; Berntsen, D.A.; BLINK Study Group. A randomized trial of soft multifocal contact lenses for myopia control: Baseline data and methods. Optom. Vis. Sci. 2017, 94, 856. [Google Scholar] [CrossRef] [PubMed]
- Mutti, D.O.; Sinnott, L.T.; Reuter, K.S.; Walker, M.K.; Berntsen, D.A.; Jones-Jordan, L.A.; Walline, J.J.; Bifocal Lenses In Nearsighted Kids (BLINK) Study Group. Peripheral Refraction and Eye Lengths in Myopic Children in the Bifocal Lenses In Nearsighted Kids (BLINK) Study. Transl. Vis. Sci. Technol. 2019, 8, 17. [Google Scholar] [CrossRef]
- Sankaridurg, P. Contact lenses to slow progression of myopia. Clin. Exp. Optom. 2017, 100, 432–437. [Google Scholar] [CrossRef]
- Wu, P.-C.; Chuang, M.-N.; Choi, J.; Chen, H.; Wu, G.; Ohno-Matsui, K.; Jonas, J.B.; Cheung, C.M.G. Update in myopia and treatment strategy of atropine use in myopia control. Eye 2019, 33, 3–13. [Google Scholar] [CrossRef]
- Cheung, S.W.; Cho, P.; Fan, D. Asymmetrical increase in axial length in the two eyes of a monocular orthokeratology patient. Optom. Vis. Sci. 2004, 81, 653–656. [Google Scholar] [CrossRef]
- Lyu, Y.; Ji, N.; Fu, A.-C.; Wang, W.-Q.; Wei, L.; Qin, J.; Zhao, B.-X. Comparison of administration of 0.02% atropine and orthokeratology for myopia control. Eye Contact Lens Sci. Clin. Pract. 2021, 47, 81–85. [Google Scholar] [CrossRef]
- Lin, H.-J.; Wan, L.; Tsai, F.-J.; Tsai, Y.-Y.; Chen, L.-A.; Tsai, A.L.; Huang, Y.-C. Overnight orthokeratology is comparable with atropine in controlling myopia. BMC Ophthalmol. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xiong, R.; Jin, L.; Chen, Q.; Wang, D.; Chen, S.; Chen, X.; Ha, J.; Li, Y.; Qu, Y.; et al. Longitudinal changes in lens thickness and lens power among persistent non-myopic and myopic children. Investig. Ophthalmol. Vis. Sci. 2022, 63, 10. [Google Scholar] [CrossRef]
- Rozema, J.; Dankert, S.; Iribarren, R.; Lanca, C.; Saw, S.-M. Axial growth and lens power loss at myopia onset in Singaporean children. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3091–3099. [Google Scholar] [CrossRef]
- Huang, J.; Wen, D.; Wang, Q.; McAlinden, C.; Flitcroft, I.; Chen, H.; Saw, S.M.; Chen, H.; Bao, F.; Zhao, Y. Efficacy comparison of 16 interventions for myopia control in children: A network meta-analysis. Ophthalmology 2016, 123, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Zeri, F.; Di Vizio, A.; Ruffinato, T.; Naroo, S. Contact lens use in sport: Practice and perception in Italy. Contact Lens Anterior Eye 2018, 41, S32–S33. [Google Scholar] [CrossRef]
- Lanca, C.; Yam, J.C.; Jiang, W.J.; Tham, Y.C.; Hassan Emamian, M.; Tan, C.S.; Guo, Y.; Liu, H.; Zhong, H.; Zhu, D.; et al. Near work, screen time, outdoor time and myopia in schoolchildren in the Sunflower Myopia AEEC Consortium. Acta Ophthalmol. 2022, 100, 302–311. [Google Scholar] [CrossRef] [PubMed]

| Groups | SV | A0.01% | A0.01% + DFCL | |
|---|---|---|---|---|
| n | 30 | 29 | 26 | |
| Gender | 53% male | 51% female | 62% female | |
| Age | Avg and SD | 9.08 ± 2.31 | 10.93 ± 1.94 | 11.12 ± 1.99 |
| Range | 9–15.5 | 8–15 | 9–15.5 | |
| SV vs. A0.01% | p < 0.01 | |||
| SV vs. A0.01% + DFCL | p < 0.01 | |||
| A0.01% vs. A0.01% + DFCL | p > 0.05 | |||
| VA and SD | (LogMar) | 0.055 ± 0.065 | 0.097 ± 0.07 | 0.171 ± 0.259 |
| SE (D) | AVG | 5.06 | 4.81 | 4.14 |
| SD | 1.77 | 2.12 | 1.35 | |
| Min | 2.375 | 1.25 | 1.625 | |
| Max | 8.875 | 10.875 | 6.00 | |
| SV vs. A0.01% | p > 0.05 | |||
| SV vs. A0.01% + DFCL | p < 0.05 | |||
| A0.01% vs. A0.01% + DFCL | p > 0.05 | |||
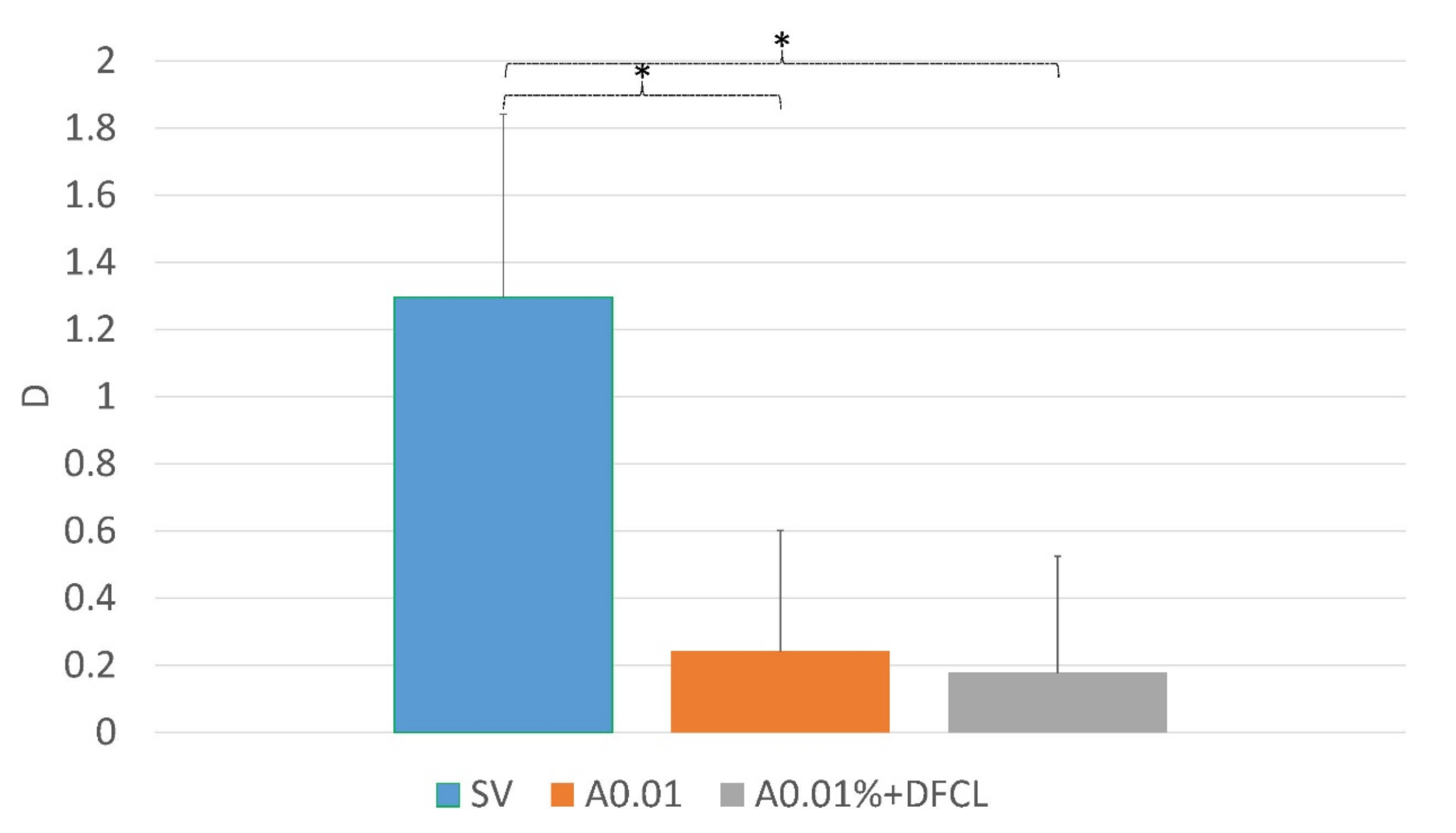
| Myopia Progression SE (D) | |||
|---|---|---|---|
| Groups | Year 1 | Year 2 | Year 3 |
| SV | 1.19 ± 0.43 | 1.25 ± 0.52 | 1.13 ± 0.36 |
| A0.01% | 0.44 ± 0.21 | 0.51 ± 0.39 | 0.24 ± 0.35 |
| A0.01% + DFCL | 0.35 ± 0.26 | 0.44 ± 0.40 | 0.18 ± 0.34 |
| SV vs. A0.01% | p < 0.001 | p < 0.001 | p < 0.001 |
| SV vs. A0.01% + DFCL | p < 0.001 | p < 0.001 | p < 0.001 |
| A0.01% vs. A0.01% + DFCL | p > 0.05 | p > 0.05 | p > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdinest, N.; London, N.; Lavy, I.; Landau, D.; Ben Ephraim Noyman, D.; Levinger, N.; Morad, Y. Low-Concentration Atropine Monotherapy vs. Combined with MiSight 1 Day Contact Lenses for Myopia Management. Vision 2022, 6, 73. https://doi.org/10.3390/vision6040073
Erdinest N, London N, Lavy I, Landau D, Ben Ephraim Noyman D, Levinger N, Morad Y. Low-Concentration Atropine Monotherapy vs. Combined with MiSight 1 Day Contact Lenses for Myopia Management. Vision. 2022; 6(4):73. https://doi.org/10.3390/vision6040073
Chicago/Turabian StyleErdinest, Nir, Naomi London, Itay Lavy, David Landau, Dror Ben Ephraim Noyman, Nadav Levinger, and Yair Morad. 2022. "Low-Concentration Atropine Monotherapy vs. Combined with MiSight 1 Day Contact Lenses for Myopia Management" Vision 6, no. 4: 73. https://doi.org/10.3390/vision6040073
APA StyleErdinest, N., London, N., Lavy, I., Landau, D., Ben Ephraim Noyman, D., Levinger, N., & Morad, Y. (2022). Low-Concentration Atropine Monotherapy vs. Combined with MiSight 1 Day Contact Lenses for Myopia Management. Vision, 6(4), 73. https://doi.org/10.3390/vision6040073






