Application of Eye Tracking Technology in Medicine: A Bibliometric Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Bibliometric Analysis
3. Results
3.1. Trends Regarding Most Investigated Disorders
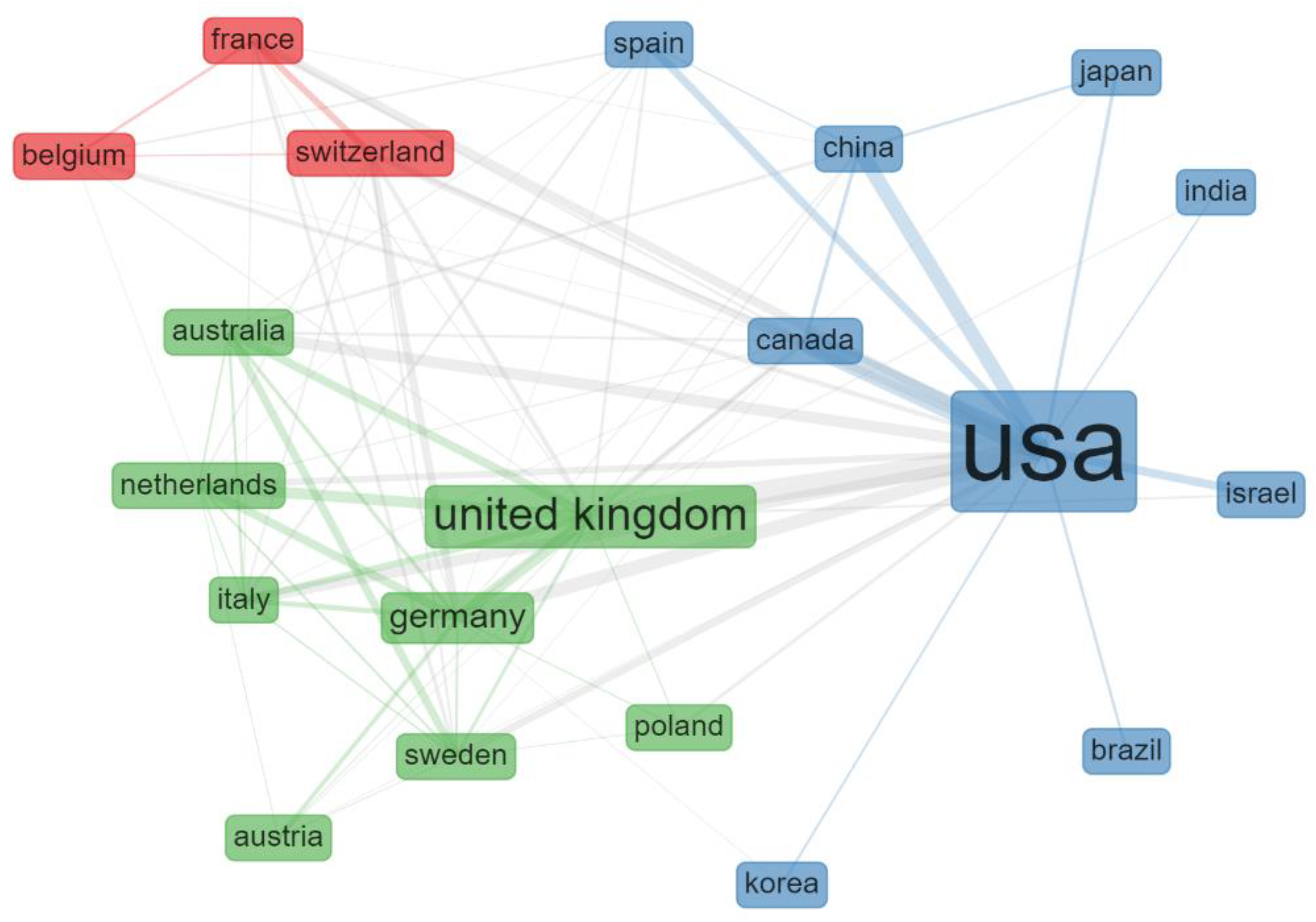
3.2. Countries and Institutions
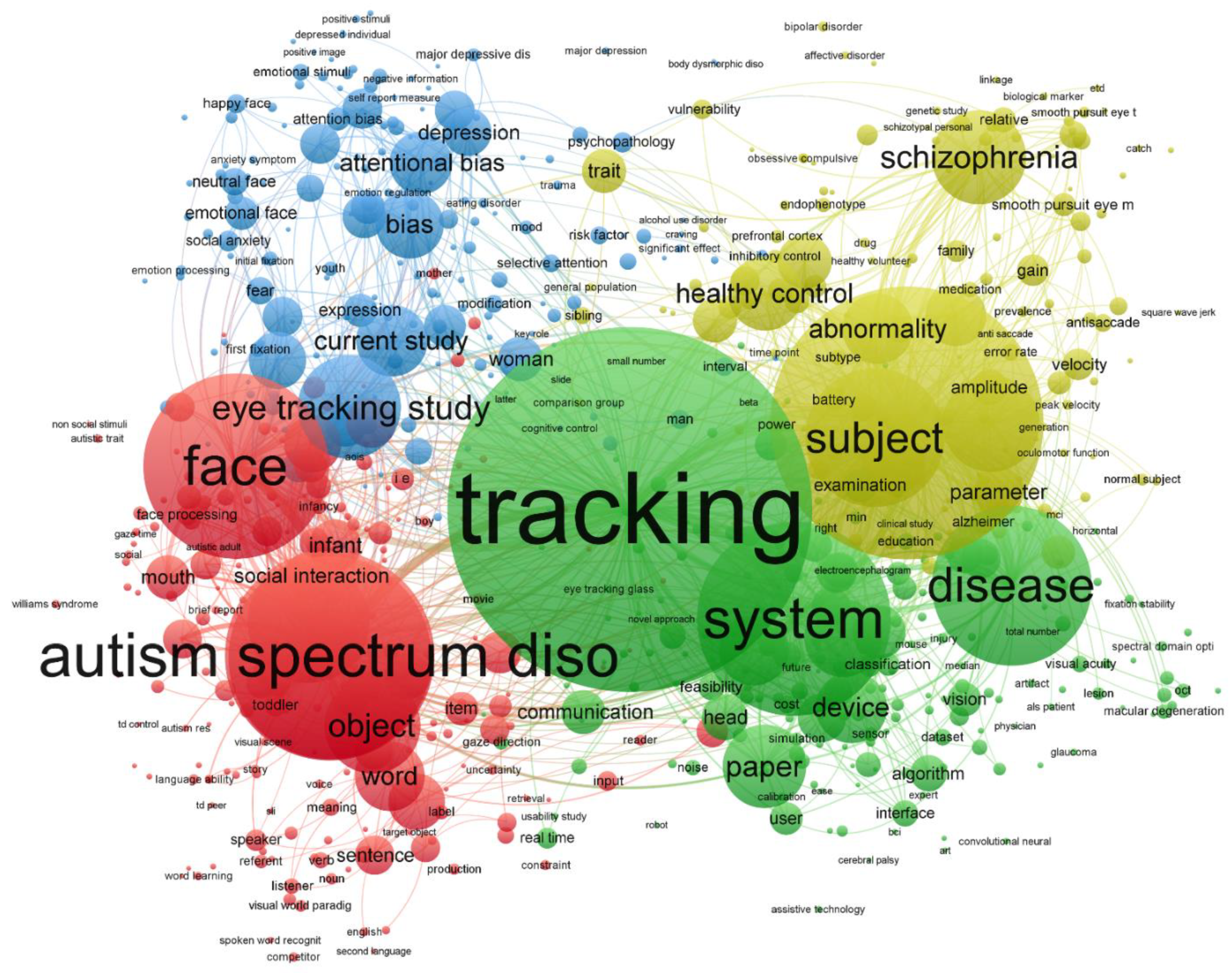
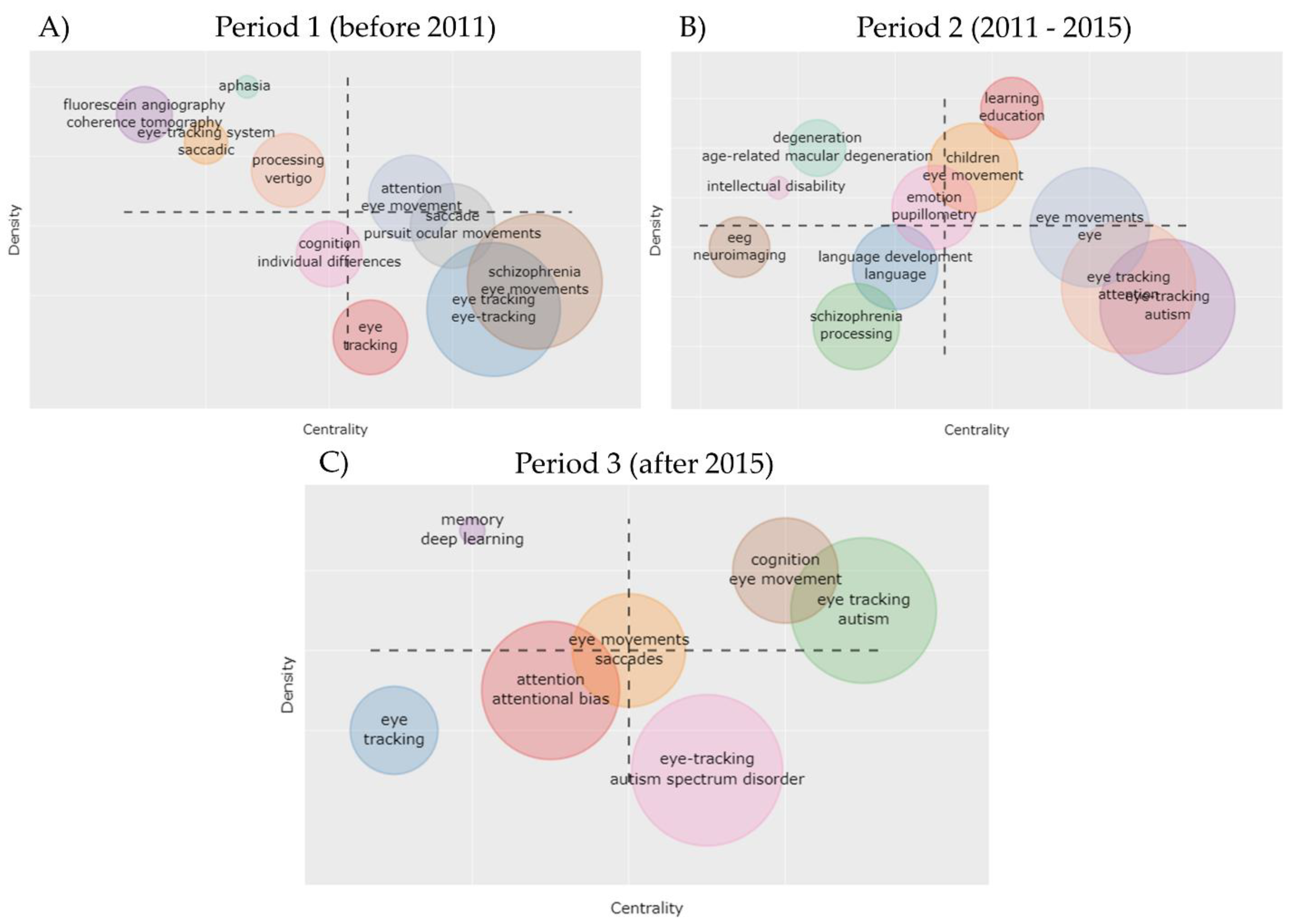
3.3. Trends in Topics
3.4. Case Study: Autism Spectrum Disorders
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bix, L.; Sundar, R.P.; Bello, N.M.; Peltier, C.; Weatherspoon, L.J.; Becker, M.W. To See or Not to See: Do Front of Pack Nutrition Labels Affect Attention to Overall Nutrition Information? PLoS ONE 2015, 10, e0139732. [Google Scholar] [CrossRef]
- McNeill, A.; Gravely, S.; Hitchman, S.C.; Bauld, L.; Hammond, D.; Hartmann-Boyce, J. Tobacco packaging design for reducing tobacco use. Cochrane Database Syst. Rev. 2017, 4, CD011244. [Google Scholar] [CrossRef] [Green Version]
- Zammarchi, G.; Frigau, L.; Mola, F. Markov chain to analyze web usability of a university website using eye tracking data. Stat. Anal. Data Min. 2021, 14, 331–341. [Google Scholar] [CrossRef]
- Liversedge, S.P.; Findlay, J.M. Saccadic eye movements and cognition. Trends Cogn. Sci. 2000, 4, 6–14. [Google Scholar] [CrossRef]
- Persico, A.M.; Ricciardello, A.; Cucinotta, F. The psychopharmacology of autism spectrum disorder and Rett syndrome. Handb. Clin. Neurol. 2019, 165, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Howard, P.L.; Zhang, L.; Benson, V. What Can Eye Movements Tell Us about Subtle Cognitive Processing Differences in Autism? Vision 2019, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, K.; Miura, K.; Kasai, K.; Hashimoto, R. Eye movement characteristics in schizophrenia: A recent update with clinical implications. Neuropsychopharmacol. Rep. 2020, 40, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Giel, K.E.; Friederich, H.C.; Teufel, M.; Hautzinger, M.; Enck, P.; Zipfel, S. Attentional processing of food pictures in individuals with anorexia nervosa--an eye-tracking study. Biol. Psychiatry 2011, 69, 661–667. [Google Scholar] [CrossRef]
- Walle, K.M.; Nordvik, J.E.; Becker, F.; Espeseth, T.; Sneve, M.H.; Laeng, B. Unilateral neglect post stroke: Eye movement freqauencies indicate directional hypokinesia while fixation distributions suggest compensational mechanism. Brain Behav. 2019, 9, e01170. [Google Scholar] [CrossRef] [PubMed]
- Hunfalvay, M.; Murray, N.P.; Roberts, C.M.; Tyagi, A.; Barclay, K.W.; Carrick, F.R. Oculomotor Behavior as a Biomarker for Differentiating Pediatric Patients with Mild Traumatic Brain Injury and Age Matched Controls. Front. Behav. Neurosci. 2020, 14, 581819. [Google Scholar] [CrossRef]
- Archibald, N.K.; Hutton, S.B.; Clarke, M.P.; Mosimann, U.P.; Burn, D.J. Visual exploration in Parkinson’s disease and Parkinson’s disease dementia. Brain 2013, 136, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Boucart, M.; Bubbico, G.; Szaffarczyk, S.; Pasquier, F. Animal spotting in Alzheimer’s disease: An eye tracking study of object categorization. J. Alzheimer’s Dis. 2014, 39, 181–189. [Google Scholar] [CrossRef]
- Waltman, L.; van Eck, N.J.; Noyons, E.C.M. A unified approach to mapping and clustering of bibliometric networks. J. Informetr. 2010, 4, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Van Eck, N.J.; Waltman, L. How to normalize cooccurrence data? An analysis of some well-known similarity measures. J. Am. Soc. Inf. Sci. Technol. 2009, 60, 1635–1651. [Google Scholar]
- Borg, I.; Groenen, P.J.F. Modern Multidimensional Scaling: Theory and Applications, 2nd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Van Eck, N.J.; Waltman, L.; Dekker, R.; Van den Berg, J. A comparison of two techniques for bibliometric mapping: Multidimensional scaling and VOS. J. Am. Soc. Inf. Sci. Technol. 2010, 61, 2405–2416. [Google Scholar] [CrossRef] [Green Version]
- Waltman, L.; Van Eck, N.J. A smart local moving algorithm for large-scale modularity-based community detection. Eur. Phys. J. B. 2013, 86, 1–14. [Google Scholar] [CrossRef]
- Newman, M.E.; Girvan, M. Finding and evaluating community structure in networks. Phys. Rev. E 2004, 69, 026113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, M.E.J. Detecting community structure in networks. Eur. Phys. 2004, 38, 321–330. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wordcloud2 R Package. Available online: https://github.com/lchiffon/wordcloud2 (accessed on 1 November 2021).
- OSF. Available online: https://osf.io/chtjs/?view_only=3ef84f55d5264fd1900cfdfb2c29da8d (accessed on 1 November 2021).
- Callon, M.; Courtial, J.P.; Laville, F. Co-word analysis as a tool for describing the network of interactions between basic and technological research: The case of polymer chemsitry. Scientometrics 1991, 22, 155–205. [Google Scholar] [CrossRef]
- Batagelj, V.; Cerinsek, M. On bibliographic networks. Scientometrics 2013, 96, 845–864. [Google Scholar] [CrossRef] [Green Version]
- Dalton, K.M.; Nacewicz, B.M.; Alexander, A.L.; Davidson, R.J. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol. Psychiatry 2007, 61, 512–520. [Google Scholar] [CrossRef]
- Dalton, K.M.; Nacewicz, B.M.; Johnstone, T.; Schaefer, H.S.; Gernsbacher, M.A.; Goldsmith, H.H.; Alexander, A.L.; Davidson, R.J. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005, 8, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.; Carr, K.; Klin, A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch. Gen. Psychiatry 2008, 65, 946–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riby, D.M.; Hancock, P.J. Do faces capture the attention of individuals with Williams syndrome or autism? Evidence from tracking eye movements. J. Autism Dev. Disord. 2009, 39, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Speer, L.L.; Cook, A.E.; McMahon, W.M.; Clark, E. Face processing in children with autism: Effects of stimulus contents and type. Autism 2007, 11, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Young, G.S.; Merin, N.; Rogers, S.J.; Ozonoff, S. Gaze behavior and affect at 6 months: Predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Dev. Sci. 2009, 12, 798–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nacewicz, B.M.; Dalton, K.M.; Johnstone, T.; Long, M.T.; McAuliff, E.M.; Oakes, T.R.; Alexander, A.L.; Davidson, R.J. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch. Gen. Psychiatry 2006, 63, 1417–1428. [Google Scholar] [CrossRef] [Green Version]
- van der Geest, J.N.; Kemner, C.; Verbaten, M.N.; van Engeland, H. Gaze behavior of children with pervasive developmental disorder toward human faces: A fixation time study. J. Child Psychol. Psychiatry 2002, 43, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.; Spezio, M.L.; Piven, J.; Adolphs, R. Looking you in the mouth: Abnormal gaze in autism resulting from impaired top-down modulation of visual attention. Soc. Cogn. Affect. Neurosci. 2006, 1, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Tanaka, K.; Endo, Y.; Yamane, Y.; Yamamoto, T.; Nakano, Y.; Ohta, H.; Kato, N.; Kitazawa, S. Atypical gaze patterns in children and adults with autism spectrum disorders dissociated from developmental changes in gaze behaviour. Proc. Biol. Sci. 2010, 277, 2935–2943. [Google Scholar] [CrossRef] [Green Version]
- Merin, N.; Young, G.S.; Ozonoff, S.; Rogers, S.J. Visual Fixation Patterns during Reciprocal Social Interaction Distinguish a Subgroup of 6-Month-Old Infants At-Risk for Autism from Comparison Infants. J. Autism Dev. Disord. 2007, 37, 108–121. [Google Scholar] [CrossRef]
- Chawarska, K.; Shic, F. Looking but not seeing: Atypical visual scanning and recognition of faces in 2 and 4-year-old children with autism spectrum disorder. J. Autism Dev. Disord. 2009, 39, 1663–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Jiang, M.; Duchesne, X.M.; Laugeson, E.A.; Kennedy, D.P.; Adolphs, R.; Zhao, Q. Atypical Visual Saliency in Autism Spectrum Disorder Quantified through Model-Based Eye Tracking. Neuron 2015, 88, 604–616. [Google Scholar] [CrossRef] [Green Version]
- Chawarska, K.; Macari, S.; Shic, F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol. Psychiatry 2013, 74, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Riby, D.M.; Hancock, P.J. Viewing it differently: Social scene perception in Williams syndrome and autism. Neuropsychologia 2008, 46, 2855–2860. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Watson, S.; Leekam, S.R.; Benson, V.; Frank, M.C.; Findlay, J.M. Eye-movements reveal attention to social information in autism spectrum disorder. Neuropsychologia 2009, 47, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Sasson, N.J.; Turner-Brown, L.M.; Holtzclaw, T.N.; Lam, K.S.; Bodfish, J.W. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Res. 2008, 1, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Chawarska, K.; Macari, S.; Shic, F. Context modulates attention to social scenes in toddlers with autism. J. Child Psychol. Psychiatry 2012, 53, 903–913. [Google Scholar] [CrossRef] [Green Version]
- Pierce, K.; Conant, D.; Hazin, R.; Stoner, R.; Desmond, J. Preference for geometric patterns early in life as a risk factor for autism. Arch. Gen. Psychiatry 2011, 68, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elison, J.T.; Paterson, S.J.; Wolff, J.J.; Reznick, J.S.; Sasson, N.J.; Gu, H.; Botteron, K.N.; Dager, S.R.; Estes, A.M.; Evans, A.C.; et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am. J. Psychiatry 2013, 170, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Z.; Di Martino, J.M.; Aiello, R.; Baker, J.; Carpenter, K.; Compton, S.; Davis, N.; Eichner, B.; Espinosa, S.; Flowers, J.; et al. Computational Methods to Measure Patterns of Gaze in Toddlers with Autism Spectrum Disorder. JAMA Pediatr. 2021, 175, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.S.; Franco, F.O.; Revers, M.C.; Silva, A.F.; Portolese, J.; Brentani, H.; Machado-Lima, A.; Nunes, F.L.S. Computer-aided autism diagnosis based on visual attention models using eye tracking. Sci. Rep. 2021, 11, 10131. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, Q.; Wu, Y.; Yi, L.; Wei, K. Automatic classification of children with autism spectrum disorder by using a computerized visual-orienting task. Psych J. 2021, 10, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Carette, R.; Cilia, F.; Dequen, G.; Bosche, J.; Guerin, J.L.; Vandromme, L.; Carette, R.; Cilia, F.; Dequen, G.; Bosche, J.; et al. Automatic Autism Spectrum Disorder Detection Thanks to Eye-Tracking and Neural Network-Based Approach. Internet Things (Iot) Technol. Healthc. 2017, 225, 75–81. [Google Scholar]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Applying Machine Learning to Kinematic and Eye Movement Features of a Movement Imitation Task to Predict Autism Diagnosis. Sci. Rep. 2020, 10, 8346. [Google Scholar] [CrossRef]
- Hayes, J.; Ford, T.; Rafeeque, H.; Russell, G. Clinical practice guidelines for diagnosis of autism spectrum disorder in adults and children in the UK: A narrative review. BMC Psychiatry 2018, 18, 222. [Google Scholar] [CrossRef] [Green Version]
- Frazier, T.W.; Coury, D.L.; Sohl, K.; Wagner, K.E.; Uhlig, R.; Hicks, S.D.; Middleton, F.A. Evidence-based use of scalable biomarkers to increase diagnostic efficiency and decrease the lifetime costs of autism. Autism Res. 2021, 14, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Colombo, J. Larger tonic pupil size in young children with autism spectrum disorder. Dev. Psychobiol. 2009, 51, 207–211. [Google Scholar] [CrossRef]
- Anderson, C.J.; Colombo, J.; Unruh, K.E. Pupil and salivary indicators of autonomic dysfunction in autism spectrum disorder. Dev. Psychobiol. 2013, 55, 465–482. [Google Scholar] [CrossRef] [Green Version]
- Camero, R.; Martinez, V.; Gallego, C. Gaze Following and Pupil Dilation as Early Diagnostic Markers of Autism in Toddlers. Children (Basel) 2021, 8, 113. [Google Scholar] [CrossRef]
- Buffle, P.; Cavadini, T.; Posada, A.; Gentaz, E. A study on visual preference for social stimuli in typical Ecuadorian preschoolers as a contribution to the identification of autism risk factors. Sci. Rep. 2021, 11, 8461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Francis, S.M.; Srishyla, D.; Conelea, C.; Zhao, Q.; Jacob, S.; Jiang, M.; Francis, S.M.; Srishyla, D.; Conelea, C.; et al. Classifying Individuals with ASD through Facial Emotion Recognition and Eye-Tracking. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6063–6068. [Google Scholar]
- Vargas-Cuentas, N.I.; Roman-Gonzalez, A.; Gilman, R.H.; Barrientos, F.; Ting, J.; Hidalgo, D.; Jensen, K.; Zimic, M. Developing an eye-tracking algorithm as a potential tool for early diagnosis of autism spectrum disorder in children. PLoS ONE 2017, 12, e0188826. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, M.; Manyakov, N.V.; Bangerter, A.; Kaliukhovich, D.A.; Jagannatha, S.; Ness, S.; Pandina, G. Learning Scan Paths of Eye Movement in Autism Spectrum Disorder. Stud. Health Technol. Inform. 2020, 270, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.W.; Lin, F.; Song, C.; Xu, X.W.; Hartley-McAndrew, M.; Doody, K.R.; Xu, W.Y.; Cho, K.W.; Lin, F.; Song, C.; et al. Gaze-Wasserstein: A Quantitative Screening Approach to Autism Spectrum Disorders. In Proceedings of the 2016 IEEE Wireless Health (WH), Bethesda, MD, USA, 25–27 October 2016; pp. 14–21. [Google Scholar]
- Falck-Ytter, T.; Nystrom, P.; Gredeback, G.; Gliga, T.; Bolte, S.; The EASE Team. Reduced orienting to audiovisual synchrony in infancy predicts autism diagnosis at 3 years of age. J. Child Psychol. Psychiatry 2018, 59, 872–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujioka, T.; Inohara, K.; Okamoto, Y.; Masuya, Y.; Ishitobi, M.; Saito, D.N.; Jung, M.; Arai, S.; Matsumura, Y.; Fujisawa, T.X.; et al. Gazefinder as a clinical supplementary tool for discriminating between autism spectrum disorder and typical development in male adolescents and adults. Mol. Autism 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, S.; Takahashi, T.; Tsukamoto, K.; Matsumura, M.; Takigawa, R.; Sakai, Y.; Maniwa, S.; Murphy, L.; Taketani, T. The feasibility of Gazefinder under 12 months of age infants. Sci. Rep. 2021, 11, 10009. [Google Scholar] [CrossRef]
- Tsuchiya, K.J.; Hakoshima, S.; Hara, T.; Ninomiya, M.; Saito, M.; Fujioka, T.; Kosaka, H.; Hirano, Y.; Matsuo, M.; Kikuchi, M.; et al. Diagnosing Autism Spectrum Disorder without Expertise: A Pilot Study of 5- to 17-Year-Old Individuals Using Gazefinder. Front. Neurol. 2020, 11, 603085. [Google Scholar] [CrossRef]
- Kang, J.; Han, X.; Song, J.; Niu, Z.; Li, X. The identification of children with autism spectrum disorder by SVM approach on EEG and eye-tracking data. Comput. Biol. Med. 2020, 120, 103722. [Google Scholar] [CrossRef]
- Thapaliya, S.; Jayarathna, S.; Jaime, M.; Thapaliya, S.; Jayarathna, S.; Jaime, M. Evaluating the EEG and Eye Movements for Autism Spectrum Disorder. In Proceedings of the IEEE International Conference on Big Data (BIG DATA), Seattle, WA, USA, 10–13 December 2018; pp. 2328–2336. [Google Scholar]
- Zhang, S.; Chen, D.; Tang, Y.; Zhang, L. Children ASD Evaluation through Joint Analysis of EEG and Eye-Tracking Recordings with Graph Convolution Network. Front. Hum. Neurosci. 2021, 15, 651349. [Google Scholar] [CrossRef]
- Li, J.; Zhong, Y.H.; Han, J.X.; Ouyang, G.X.; Li, X.L.; Liu, H.H.; Li, J.; Zhong, Y.; Han, J.; Ouyang, G.; et al. Classifying ASD children with LSTM based on raw videos. Neurocomputing 2020, 390, 226–238. [Google Scholar] [CrossRef]
- Bacon, E.C.; Moore, A.; Lee, Q.; Carter Barnes, C.; Courchesne, E.; Pierce, K. Identifying prognostic markers in autism spectrum disorder using eye tracking. Autism 2020, 24, 658–669. [Google Scholar] [CrossRef]
- Aresti-Bartolome, N.; Garcia-Zapirain, B. Cognitive rehabilitation system for children with autism spectrum disorder using serious games: A pilot study. Biomed. Mater. Eng. 2015, 26 (Suppl. 1), S811–S824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradshaw, J.; Shic, F.; Holden, A.N.; Horowitz, E.J.; Barrett, A.C.; German, T.C.; Vernon, T.W. The Use of Eye Tracking as a Biomarker of Treatment Outcome in a Pilot Randomized Clinical Trial for Young Children with Autism. Autism Res. 2019, 12, 779–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gepner, B.; Charrier, A.; Arciszewski, T.; Tardif, C. Slowness Therapy for Children with Autism Spectrum Disorder: A Blind Longitudinal Randomized Controlled Study. J. Autism Dev. Disord. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, L.; Kim, S.; Bastian, D.A.; Jung, J.; Iyengar, U.; Martinez, S.; Goin-Kochel, R.P.; Fonagy, P. Visual systemizing preference in children with autism: A randomized controlled trial of intranasal oxytocin. Dev. Psychopathol. 2018, 30, 511–521. [Google Scholar] [CrossRef] [Green Version]


| Country | Articles | Single Country Publications | Multiple Country Publications | % of International Collaborations |
|---|---|---|---|---|
| USA | 867 | 739 | 128 | 15% |
| UK | 234 | 153 | 81 | 35% |
| Germany | 178 | 122 | 56 | 31% |
| China | 132 | 93 | 39 | 30% |
| Canada | 108 | 84 | 24 | 22% |
| Australia | 89 | 59 | 30 | 34% |
| France | 85 | 69 | 16 | 19% |
| Italy | 81 | 57 | 24 | 30% |
| Netherlands | 73 | 53 | 20 | 27% |
| Japan | 62 | 59 | 3 | 5% |
| Sweden | 57 | 43 | 14 | 25% |
| Spain | 48 | 32 | 16 | 33% |
| Poland | 43 | 34 | 9 | 21% |
| Korea | 38 | 35 | 3 | 8% |
| Switzerland | 38 | 14 | 24 | 63% |
| Belgium | 29 | 15 | 14 | 48% |
| India | 29 | 23 | 6 | 21% |
| Brazil | 23 | 15 | 8 | 35% |
| Austria | 22 | 14 | 8 | 36% |
| Israel | 22 | 11 | 11 | 50% |
| Denmark | 15 | 10 | 5 | 33% |
| Finland | 13 | 4 | 9 | 69% |
| Ireland | 12 | 9 | 3 | 25% |
| Portugal | 11 | 6 | 5 | 45% |
| Country | Articles | Single Country Publications | Multiple Country Publications | % of International Collaborations |
|---|---|---|---|---|
| USA | 234 | 198 | 36 | 15% |
| UK | 88 | 58 | 30 | 34% |
| China | 52 | 36 | 16 | 31% |
| Australia | 31 | 19 | 12 | 39% |
| France | 31 | 24 | 7 | 23% |
| Sweden | 23 | 16 | 7 | 30% |
| Germany | 19 | 12 | 7 | 37% |
| Japan | 18 | 16 | 2 | 11% |
| Italy | 16 | 11 | 5 | 31% |
| Switzerland | 15 | 2 | 13 | 87% |
| Belgium | 13 | 7 | 6 | 46% |
| India | 13 | 10 | 3 | 23% |
| Canada | 12 | 7 | 5 | 42% |
| Netherlands | 11 | 9 | 2 | 18% |
| Article | Cit | Cit/Year | Main Topic | Ref. |
|---|---|---|---|---|
| Dalton et al., 2005 | 954 | 56.12 | Face processing | [27] |
| Jones et al., 2008 | 324 | 23.14 | Face processing | [28] |
| Chawarska et al., 2013 | 265 | 29.44 | Pictures with social scenes | [39] |
| Riby and Hacock, 2008 | 235 | 16.79 | Pictures with social scenes | [40] |
| Pierce et al., 2011 | 209 | 19.00 | Vision of images with geometric Patterns compared to social images | [44] |
| Speer et al., 2007 | 200 | 13.33 | Face processing | [30] |
| Fletcher-Watson et al., 2009 | 196 | 15.08 | Pictures with social scenes | [41] |
| Young et al., 2009 | 195 | 15.00 | Face processing | [31] |
| Nacewicz et al., 2006 | 184 | 11.50 | Discriminate facial expressions | [32] |
| Sasson et al., 2008 | 181 | 12.93 | Social or nonsocial images | [42] |
| Van Der Geest et al., 2002 | 166 | 8.30 | Face processing | [33] |
| Dalton et al., 2007 | 163 | 10.87 | Face processing | [26] |
| Riby et al., 2009 | 162 | 12.46 | Face processing | [29] |
| Neumann et al., 2006 | 156 | 9.75 | Face processing | [34] |
| Elison et al., 2013 | 147 | 16.33 | Oculomotor functioning and visual orienting | [45] |
| Nakano et al., 2010 | 147 | 12.25 | Gaze patterns on social and nonsocial stimuli | [35] |
| Chawarska et al., 2012 | 146 | 14.60 | Pictures with social scenes | [43] |
| Merlin et al., 2007 | 146 | 9.73 | Videos with social scenes | [36] |
| Chawarska and Shic, 2009 | 145 | 11.15 | Face processing | [37] |
| Wang et al., 2015 | 140 | 20.00 | Face processing | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zammarchi, G.; Conversano, C. Application of Eye Tracking Technology in Medicine: A Bibliometric Analysis. Vision 2021, 5, 56. https://doi.org/10.3390/vision5040056
Zammarchi G, Conversano C. Application of Eye Tracking Technology in Medicine: A Bibliometric Analysis. Vision. 2021; 5(4):56. https://doi.org/10.3390/vision5040056
Chicago/Turabian StyleZammarchi, Gianpaolo, and Claudio Conversano. 2021. "Application of Eye Tracking Technology in Medicine: A Bibliometric Analysis" Vision 5, no. 4: 56. https://doi.org/10.3390/vision5040056
APA StyleZammarchi, G., & Conversano, C. (2021). Application of Eye Tracking Technology in Medicine: A Bibliometric Analysis. Vision, 5(4), 56. https://doi.org/10.3390/vision5040056





