Vision through Healthy Aging Eyes
Abstract
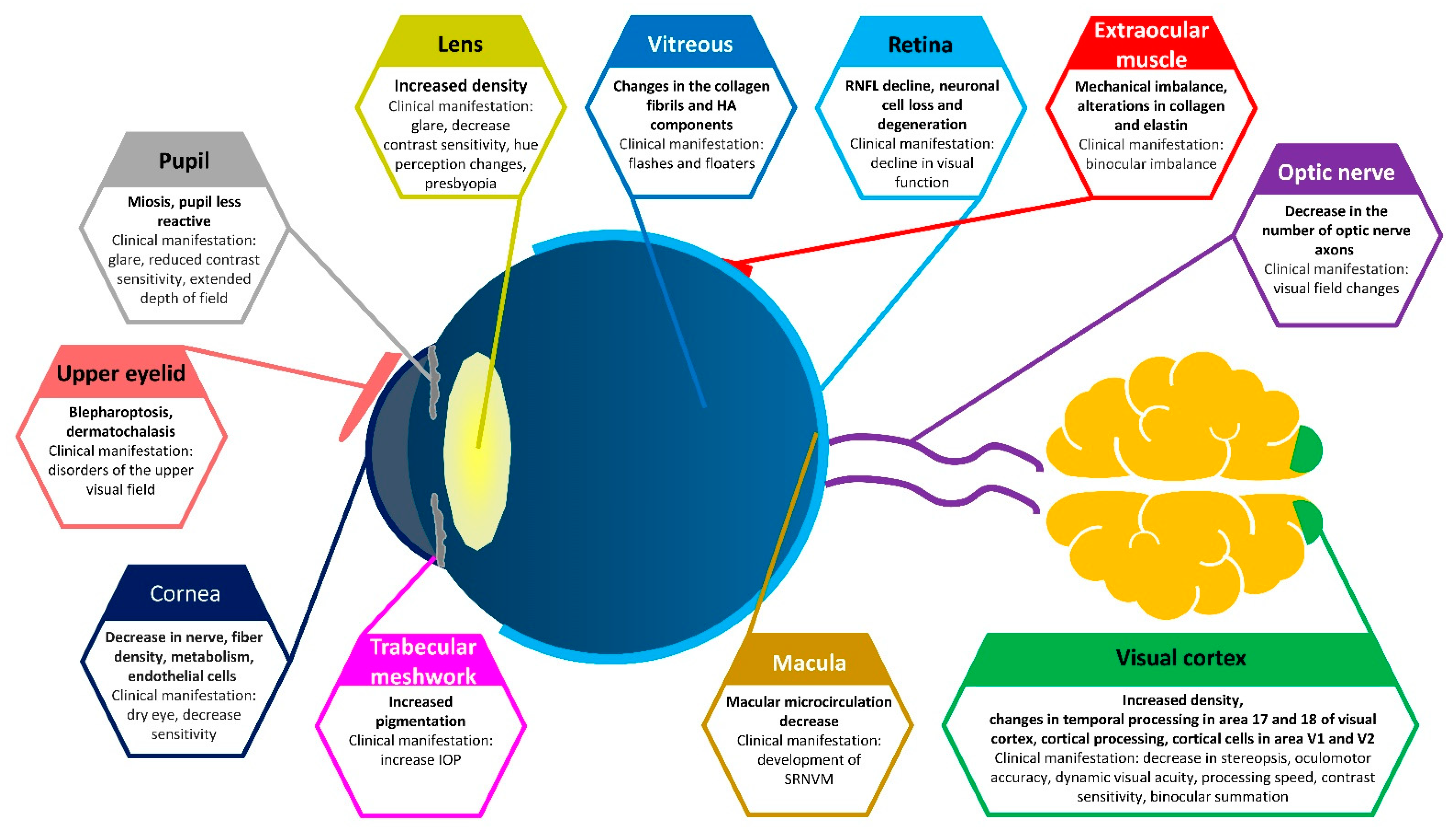
1. Introduction
2. Visual Acuity
3. Dry Eye
4. Binocular Vision
5. Ocular Motility
6. Visual Field
7. Presbyopia
8. Contrast Sensitivity
9. Dark Adaptation
10. Glare Recovery
11. Color Vision
12. Speed of Visual Processing
13. Developing Modes of Intervention and Prevention
14. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moarefi, M.A.; Bafna, S.; Wiley, W. A review of presbyopia treatment with corneal inlays. Ophthalmol. Ther. 2017, 6, 55–65. [Google Scholar] [CrossRef]
- Saftari, L.N.; Kwon, O.-S. Ageing vision and falls: A review. J. Physiol. Anthropol. 2018, 37, 11. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Davies, L.N. Presbyopia: Effectiveness of correction strategies. Prog. Retin. eye Res. 2019, 68, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.D. Neural bases of visual deficits during aging. Vis. Res. 1993, 33, 2589–2609. [Google Scholar] [CrossRef]
- Devaney, K.O.; Johnson, H.A. Neuron loss in the aging visual cortex of man. J. Gerontol. 1980, 35, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.; Millican, C.L.; Allen, K.; Kalina, R. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3278–3296. [Google Scholar]
- Curcio, C.A. Photoreceptor topography in ageing and age-related maculopathy. Eye 2001, 15, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. Retinal photoreceptor density decreases with age. Ophthalmology 1995, 102, 1853–1859. [Google Scholar] [CrossRef]
- Andersen, G.J.; Ni, R.; Bower, J.D.; Watanabe, T. Perceptual learning, aging, and improved visual performance in early stages of visual processing. J. Vis. 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Sekuler, R.; Hutman, L.P. Spatial vision and aging. I: Contrast sensitivity. J. Gerontol. 1980, 35, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Haegerstrom-Portnoy, G. Short-wavelength-sensitive-cone sensitivity loss with aging: A protective role for macular pigment? JOSA A 1988, 5, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Haegerstrom-Portnoy, G.; Schneck, M.E.; Brabyn, J.A. Seeing into old age: Vision function beyond acuity. Optom. Vis. Sci. 1999, 76, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Dall’Orto, G.C.; Facchin, A.; Bellatorre, A.; Maffioletti, S.; Serio, M. Measurement of visual acuity with a digital eye chart: Optotypes, presentation modalities and repeatability. J. Optom. 2021, 14, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, J.; Laakkonen, E.; Laatikainen, L. Random measurement error in visual acuity measurement in clinical settings. Acta Ophthalmol. Scand. 2005, 83, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Moutray, T.N.; Jackson, A.J. Uniformity of visual acuity measures in published studies. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4321–4327. [Google Scholar] [CrossRef]
- Barbur, J.L.; Konstantakopoulou, E. Changes in color vision with decreasing light level: Separating the effects of normal aging from disease. JOSA A 2012, 29, A27–A35. [Google Scholar] [CrossRef]
- Cooper, B.A.; Ward, M.; Gowland, C.A.; McIntosh, J.M. The use of the Lanthony New Color Test in determining the effects of aging on color vision. J. Gerontol. 1991, 46, P320–P324. [Google Scholar] [CrossRef]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. Retinal pigment epithelial cell count, distribution, and correlations in normal human eyes. Am. J. Ophthalmol. 1996, 121, 181–189. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Ghadban, R.; Martinez, J.M.; Diehl, N.N.; Mohney, B.G. The incidence and clinical characteristics of adult-onset convergence insufficiency. Ophthalmology 2015, 122, 1056–1059. [Google Scholar] [CrossRef]
- Cioplean, D.; Raluca, L.N. Age related strabismus. Rom. J. Ophthalmol. 2016, 60, 54–58. [Google Scholar]
- Brazel, C.Y.; Rao, M.S. Aging and neuronal replacement. Ageing Res. Rev. 2004, 3, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Leuba, G.; Kraftsik, R. Changes in volume, surface estimate, three-dimensional shape and total number of neurons of the human primary visual cortex from midgestation until old age. Anat. Embryol. 1994, 190, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Jorge, L.; Canário, N.; Quental, H.; Bernardes, R.; Castelo-Branco, M. Is the retina a mirror of the aging brain? Aging of neural retina layers and primary visual cortex across the lifespan. Front. Aging Neurosci. 2020, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Leuba, G.; Garey, L. Evolution of neuronal numerical density in the developing and aging human visual cortex. Hum. Neurobiol. 1987, 6, 11–18. [Google Scholar] [PubMed]
- Long, G.M.; Crambert, R.F. The nature and basis of age-related changes in dynamic visual acuity. Psychol. Aging 1990, 5, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Borland, R.; Nicholson, A. Visual motor co-ordination and dynamic visual acuity. Br. J. Clin. Pharmacol. 1984, 18, 69S–72S. [Google Scholar] [CrossRef]
- Ishigaki, H.; Miyao, M. Implications for dynamic visual acuity with changes in age and sex. Percept. Mot. Ski. 1994, 78, 363–369. [Google Scholar] [CrossRef]
- Vale, A.; Buckley, J.G.; Elliott, D.B. Gait alterations negotiating a raised surface induced by monocular blur. Optom. Vis. Sci. 2008, 85, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M. Aging, driving and vision. Clin. Exp. Optom. 2002, 85, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, V.; Enoch, J.M. Vernier acuity and aging. Int. Ophthalmol. 1995, 19, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Good, W.V.; Norcia, A.M. Validation study of VEP vernier acuity in normal-vision and amblyopic adults. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4070–4078. [Google Scholar] [CrossRef] [PubMed]
- Barzegaran, E.; Norcia, A.M. Neural sources of letter and Vernier acuity. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Odell, N.V.; Leske, D.A.; Hatt, S.R.; Adams, W.E.; Holmes, J.M. The effect of Bangerter filters on optotype acuity, Vernier acuity, and contrast sensitivity. J. Am. Assoc. Pediatric Ophthalmol. Strabismus 2008, 12, 555–559. [Google Scholar] [CrossRef]
- Pistilli, M.; Maguire, M.G.; Greiner, J.V.; Asbell, P.A. The Association between Signs and Symptoms in Patients with Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, 924. [Google Scholar]
- Ong, E.S.; Felix, E.R.; Levitt, R.C.; Feuer, W.J.; Sarantopoulos, C.D.; Galor, A. Epidemiology of discordance between symptoms and signs of dry eye. Br. J. Ophthalmol. 2018, 102, 674–679. [Google Scholar] [CrossRef]
- Martin, E.; Oliver, K.M.; Pearce, E.I.; Tomlinson, A.; Simmons, P.; Hagan, S. Effect of tear supplements on signs, symptoms and inflammatory markers in dry eye. Cytokine 2018, 105, 37–44. [Google Scholar] [CrossRef]
- Kawashima, M.; Sano, K.; Takechi, S.; Tsubota, K. Impact of lifestyle intervention on dry eye disease in office workers: A randomized controlled trial. J. Occup. Health 2018, 60, 281–288. [Google Scholar] [CrossRef]
- Gonzales, J.A.; Chou, A.; Rose-Nussbaumer, J.R.; Bunya, V.Y.; Criswell, L.A.; Shiboski, C.H.; Lietman, T.M. How Are Ocular Signs and Symptoms of Dry Eye Associated With Depression in Women With and Without Sjögren Syndrome? Am. J. Ophthalmol. 2018, 191, 42–48. [Google Scholar] [CrossRef]
- Mann, A.; Campbell, D.; Mirza, Z.; Hunt, O.; Wolffsohn, J.S.; Tighe, B.J. Clinical and biochemical analysis of the ageing tear film. Br. J. Ophthalmol. 2020, 104, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Lambiase, A. Neurotrophic factors and corneal nerve regeneration. Neural Regen. Res. 2017, 12, 1220–1224. [Google Scholar]
- Tummanapalli, S.S.; Willcox, M.D.; Issar, T.; Kwai, N.; Poynten, A.M.; Krishnan, A.V.; Pisarcikova, J.; Markoulli, M. The effect of age, gender and body mass index on tear film neuromediators and corneal nerves. Curr. Eye Res. 2020, 45, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Alassane, S.; Binquet, C.; Bretillon, L.; Acar, N.; Arnould, L.; Muselier-Mathieu, A.; Delcourt, C.; Bron, A.M.; Creuzot-Garcher, C. Dry eye disease in the elderly in a French population-based study (the Montrachet study: Maculopathy, Optic Nerve, nuTRition, neurovAsCular and HEarT diseases): Prevalence and associated factors. Ocul. Surf. 2018, 16, 112–119. [Google Scholar] [CrossRef]
- Essa, L.; Laughton, D.; Wolffsohn, J.S. Can the optimum artificial tear treatment for dry eye disease be predicted from presenting signs and symptoms? Contact Lens Anterior Eye 2018, 41, 60–68. [Google Scholar] [CrossRef]
- Colorado, L.; Edwards, K.; Dinh, L.; Ha, S.; Liu, D.; Luu, A.; Trang, S.; Yu-Ting, T.H.; Schmid, K.L. Lifestyle factors and menstrual cycle phases: Impact on dry eye signs and symptoms. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4903. [Google Scholar]
- Baudouin, C.; Irkeç, M.; Messmer, E.M.; Benítez-del-Castillo, J.M.; Bonini, S.; Figueiredo, F.C.; Geerling, G.; Labetoulle, M.; Lemp, M.; Rolando, M.; et al. Clinical impact of inflammation in dry eye disease: Proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018, 96, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Potvin, R.; Makari, S.; Rapuano, C.J. Tear film osmolarity and dry eye disease: A review of the literature. Clin. Ophthalmol. 2015, 9, 2039–2047. [Google Scholar] [CrossRef]
- Rolando, M.; Barabino, S. The Subtle Role of Para-inflammation in Modulating the Progression of Dry Eye Disease. Ocul. Immunol. Inflamm. 2021, 16, 1–6. [Google Scholar]
- Suzuki, M.; Massingale, M.L.; Ye, F.; Godbold, J.; Elfassy, T.; Vallabhajosyula, M.; Asbell, P.A. Tear osmolarity as a biomarker for dry eye disease severity. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4557–4561. [Google Scholar] [CrossRef] [PubMed]
- Stahl, U.; Willcox, M.; Stapleton, F. Osmolality and tear film dynamics. Clin. Exp. Optom. 2012, 95, 3–11. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Eftimov, P.; Yokoi, N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp. Eye Res. 2017, 163, 17–28. [Google Scholar] [CrossRef]
- Rantamäki, A.H.; Javanainen, M.; Vattulainen, I.; Holopainen, J.M. Do lipids retard the evaporation of the tear fluid? Investig. Ophthalmol. Vis. Sci. 2012, 53, 6442–6447. [Google Scholar] [CrossRef]
- Mathers, W.D.; Lane, J.A.; Zimmerman, M.B. Tear film changes associated with normal aging. Cornea 1996, 15, 229–234. [Google Scholar] [CrossRef]
- Den, S.; Shimizu, K.; Ikeda, T.; Tsubota, K.; Shimmura, S.; Shimazaki, J. Association between meibomian gland changes and aging, sex, or tear function. Cornea 2006, 25, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Nien, C.J.; Paugh, J.R.; Massei, S.; Wahlert, A.J.; Kao, W.W.; Jester, J.V. Age-related changes in the meibomian gland. Exp. eye Res. 2009, 89, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Inoue, K.; Amano, S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008, 115, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Fasanella, V.; Agnifili, L.; Mastropasqua, R.; Brescia, L.; di Staso, F.; Ciancaglini, M.; Mastropasqua, L. In vivo laser scanning confocal microscopy of human meibomian glands in aging and ocular surface diseases. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Mehra, D.; Galor, A. Digital screen use and dry eye: A review. Asia-Pac. J. Ophthalmol. 2020, 9, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Berdahl, J.P.; Chan, C.C.; Rocha, K.M.; Yeu, E.; Ayres, B.; Farid, M.; Lee, W.B.; Beckman, K.A.; Kim, T.; et al. The corneal endothelium: Clinical review of endothelial cell health and function. J. Cataract. Refract. Surg. 2021, 47, 1218–1226. [Google Scholar] [CrossRef]
- Galgauskas, S.; Krasauskaite, D.; Pajaujis, M.; Juodkaite, G.; Asoklis, R.-S. Central corneal thickness and corneal endothelial characteristics in healthy, cataract, and glaucoma patients. Clin. Ophthalmol. 2012, 6, 1195–1199. [Google Scholar] [CrossRef][Green Version]
- Roszkowska, A.M.; Colosi, P.; D’Angelo, P.; Ferreri, G. Age-related modifications of the corneal endothelium in adults. Int. Ophthalmol. 2004, 25, 163–166. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Saboo, U.S.; Abud, T.B.; Dohlman, T.H.; Arnoldner, M.A.; Hamrah, P.; Dana, R. Reduced corneal endothelial cell density in patients with dry eye disease. Am. J. Ophthalmol. 2015, 159, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Borroni, D.; Gadhvi, K.; Wojcik, G.; Pennisi, F.; Vallabh, N.A.; Galeone, A.; Ruzza, A.; Arbabi, E.; Menassa, N.; Kaye, S.; et al. The influence of speed during stripping in Descemet membrane endothelial keratoplasty tissue preparation. Cornea 2020, 39, 1086–1090. [Google Scholar] [CrossRef]
- Borroni, D.; de Lossada, C.R.; Parekh, M.; Gadhvi, K.; Bonzano, C.; Romano, V.; Levis, H.J.; Tzamalis, A.; Steger, B.; Rechichi, M.; et al. Tips, Tricks, and Guides in Descemet Membrane Endothelial Keratoplasty Learning Curve. J. Ophthalmol. 2021, 2021, 1819454. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, Q.; Sun, H.; Zhang, Y.; Tighe, S.; Xu, L.; Zhu, Y. Advances in culture, expansion and mechanistic studies of corneal endothelial cells: A systematic review. J. Biomed. Sci. 2019, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef] [PubMed]
- Rocha-de-Lossada, C.; Rachwani-Anil, R.; Borroni, D.; Sánchez-González, J.-M.; Esteves-Marques, R.; Soler-Ferrández, F.-L.; Gegúndez-Fernández, J.-A.; Romano, V.; Livny, E.; Calvo-de-Mora, M.R. New Horizons in the Treatment of Corneal Endothelial Dysfunction. J. Ophthalmol. 2021, 2021, 6644114. [Google Scholar] [CrossRef]
- Ayaki, M.; Negishi, K.; Kawashima, M.; Uchino, M.; Kaido, M.; Tsubota, K. Age is a determining factor of dry eye-related signs and symptoms. Diagnostics 2020, 10, 193. [Google Scholar] [CrossRef]
- Prabha, J.L. Tear secretion—a short review. Journal of Pharmaceutical Sciences and Research 2014, 6, 6155, 155–157. [Google Scholar]
- Roka, N.; Shrestha, S. Assessment of tear secretion and tear film instability in cases with pterygium and normal subjects. Nepal. J. Ophthalmol. 2013, 5, 16–23. [Google Scholar] [CrossRef]
- Eter, N.; Göbbels, M. A new technique for tear film fluorophotometry. Br. J. Ophthalmol. 2002, 86, 616–619. [Google Scholar] [CrossRef]
- Bron, A.J.; Willshire, C. Tear Osmolarity in the Diagnosis of Systemic Dehydration and Dry Eye Disease. Diagnostics 2021, 11, 387. [Google Scholar] [CrossRef]
- Singh, S.; Shanbhag, S.S.; Basu, S. Tear secretion from the lacrimal gland: Variations in normal versus dry eyes. Br. J. Ophthalmol. 2021, 106, 1–5. [Google Scholar]
- Sagaser, S.; Butterfield, R.; Kosiorek, H.; Kusne, Y.; Maldonado, J.; Fautsch, M.P.; Patel, D.; Shen, J.F. Effects of Intense Pulsed Light on Tear Film TGF-β and Microbiome in Ocular Rosacea with Dry Eye. Clin. Ophthalmol. 2021, 15, 323–330. [Google Scholar] [CrossRef]
- Fan, Q.; Pazo, E.E.; You, Y.; Zhang, C.; Zhang, C.; Xu, L.; He, W. Subjective quality of vision in evaporative dry eye patients after intense pulsed light. Photobiomodulation Photomed. Laser Surg. 2020, 38, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Suwal, A.; Hao, J.-l.; Zhou, D.-d.; Liu, X.-f.; Suwal, R.; Lu, C.-w. Use of Intense Pulsed Light to Mitigate Meibomian Gland Dysfunction for Dry Eye Disease. Int. J. Med. Sci. 2020, 17, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, A.N.; Smith, S.D.; Siedlecki, A.R.; Hayek, S.M.; Sayegh, R.R. Ocular pain response to treatment in dry eye patients. Ocul. Surf. 2020, 18, 305–311. [Google Scholar]
- Osei, K.A.; Cox, S.M.; Nichols, K.K. Dry Eye Disease Practice in Ghana: Diagnostic Perspectives. Treat. Modalities Chall. Optom. Vis. Sci. 2020, 97, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, J.H.; Shin, Y.U.; Hwang, S.; Lim, H.W. Differences in eye movement range based on age and gaze direction. Eye 2019, 33, 1145–1151. [Google Scholar] [CrossRef]
- Marandi, R.Z.; Gazerani, P. Aging and eye tracking: In the quest for objective biomarkers. Future Neurol. 2019, 14. [Google Scholar] [CrossRef]
- Birnbaum, M.H.; Soden, R.; Cohen, A.H. Efficacy of vision therapy for convergence insufficiency in an adult male population. J. Am. Optom. Assoc. 1999, 70, 225–232. [Google Scholar]
- Yekta, A.; Pickwell, L.; Jenkins, T. Binocular vision, age and symptoms. Ophthalmic Physiol. Opt. 1989, 9, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, M.C.; Sánchez-González, J.-M.; De-Hita-Cantalejo, C.; Vega-Holm, M.; Jiménez-Rejano, J.-J.; Gutiérrez-Sánchez, E. The effect of age on binocular vision normative values. J. Pediatric Ophthalmol. Strabismus 2020, 57, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.J.; Heinzel, S.; Pilotto, A.; Stetz, L.; Lachenmaier, S.; Gugolz, L.; Srulijes, K.; Eschweiler, G.W.; Sünkel, U.; Berg, D.; et al. The effect of age and gender on anti-saccade performance: Results from a large cohort of healthy aging individuals. Eur. J. Neurosci. 2020, 52, 4165–4184. [Google Scholar] [CrossRef] [PubMed]
- Pelak, V.S. Ocular motility of aging and dementia. Curr. Neurol. Neurosci. Rep. 2010, 10, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Wouters, R.J.; van den Bosch, W.A.; Mulder, P.G.; Lemij, H.G. Upper eyelid motility in blepharoptosis and in the aging eyelid. Investig. Ophthalmol. Vis. Sci. 2001, 42, 620–625. [Google Scholar]
- Paquette, C.; Fung, J. Old age affects gaze and postural coordination. Gait Posture 2011, 33, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Paterson, K.B.; McGowan, V.A.; Warrington, K.L.; Li, L.; Li, S.; Xie, F.; Chang, M.; Zhao, S.; Pagán, A.; White, S.J.; et al. Effects of normative aging on eye movements during reading. Vision 2020, 4, 7. [Google Scholar] [CrossRef]
- Seferlis, F.; Chimona, T.S.; Papadakis, C.E.; Bizakis, J.; Triaridis, S.; Skoulakis, C. Age related changes in ocular motor testing in healthy subjects. J. Vestib. Res. 2015, 25, 57–66. [Google Scholar] [CrossRef]
- Camacho, P.B.; Carbonari, R.; Shen, S.; Zadikoff, C.; Kramer, A.F.; López-Ortiz, C. Voluntary saccade training protocol in persons with Parkinson’s disease and healthy adults. Front. Aging Neurosci. 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. Aging and measures of processing speed. Biol. Psychol. 2000, 54, 35–54. [Google Scholar] [CrossRef]
- Swaminathan, S.S.; Greenberg, M.B.; Vanner, E.A.; Cavuoto, K.M.; Wellik, S.R.; Chang, T.C. The effect of patient characteristics and sleep quality on visual field performance reliability. J. Ophthalmol. 2018, 2018, 2731260. [Google Scholar] [CrossRef]
- Feng, J.; Craik, F.I.; Levine, B.; Moreno, S.; Naglie, G.; Choi, H. Differential age-related changes in localizing a target among distractors across an extended visual field. Eur. J. ageing 2017, 14, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Otaki, C.; Nakayama, T. Age-related changes in the normal visual field using colored targets. Nippon. Ganka Gakkai Zasshi 1992, 96, 1317–1324. [Google Scholar] [PubMed]
- Ramsey, D.J.; Arden, G. Hypoxia and dark adaptation in diabetic retinopathy: Interactions, consequences, and therapy. Curr. Diabetes Rep. 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Collin, H.B.; Han, C.; Khor, P.C. Age changes in the visual field using the Humphrey visual field analyser. Clin. Exp. Optom. 1988, 71, 174–178. [Google Scholar] [CrossRef]
- Croft, M.A.; Glasser, A.; Kaufman, P.L. Accommodation and presbyopia. Int. Ophthalmol. Clin. 2001, 41, 33–46. [Google Scholar] [CrossRef]
- Mercer, R.N.; Milliken, C.M.; V, G.O.W.I.; Rocha, K.M. Future Trends in Presbyopia Correction. J. Refract. Surg. 2021, 37, S28–S34. [Google Scholar] [CrossRef]
- Palomino-Bautista, C.; Sánchez-Jean, R.; Carmona-Gonzalez, D.; Piñero, D.P.; Molina-Martín, A. Depth of field measures in pseudophakic eyes implanted with different type of presbyopia-correcting IOLS. Sci. Rep. 2021, 11, 1–8. [Google Scholar]
- McDonald, M.B.; Mychajlyszyn, A.; Mychajlyszyn, D.; Klyce, S.D. Advances in Corneal Surgical and Pharmacological Approaches to the Treatment of Presbyopia. J. Refract. Surg. 2021, 37, S20–S27. [Google Scholar] [CrossRef]
- Thompson, W.B.; Legge, G.E.; Kersten, D.J.; Shakespeare, R.A.; Lei, Q. Simulating visibility under reduced acuity and contrast sensitivity. JOSA A 2017, 34, 583–593. [Google Scholar] [CrossRef]
- Elliott, D.B. Contrast sensitivity decline with ageing: A neural or optical phenomenon? Ophthalmic Physiol. Opt. 1987, 7, 415–419. [Google Scholar] [CrossRef]
- Wright, C.E.; Drasdo, N. The influence of age on the spatial and temporal contrast sensitivity function. Doc. Ophthalmol. 1985, 59, 385–395. [Google Scholar] [CrossRef]
- Owsley, C.; Sekuler, R.; Siemsen, D. Contrast sensitivity throughout adulthood. Vis. Res. 1983, 23, 689–699. [Google Scholar] [CrossRef]
- Ross, J.; Clarke, D.; Bron, A. Effect of age on contrast sensitivity function: Uniocular and binocular findings. Br. J. Ophthalmol. 1985, 69, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.E.; Gelfand, J.M.; Lui, L.Y.; Ou, Y.; Green, A.J.; Stone, K.; Pedula, K.L.; Cummings, S.R.; Yaffe, K. Reduced contrast sensitivity among older women is associated with increased risk of cognitive impairment. Ann. Neurol. 2018, 83, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Majid, N.S.; Badarudin, N.E.; Yahaya, N.A. The awareness of visual function deterioration in the elderly: A review. J. Optom. Eye Health Res. 2019, 1, 18–33. [Google Scholar]
- Burton, K.B.; Owsley, C.; Sloane, M.E. Aging and neural spatial contrast sensitivity: Photopic vision. Vis. Res. 1993, 33, 939–946. [Google Scholar] [CrossRef]
- Owsley, C.; Sekuler, R.; Bold, C. Aging and low-contrast vision: Face perception. Investig. Ophthalmol. Vis. Sci. 1981, 21, 362–365. [Google Scholar]
- Li, Z.; Hu, Y.; Yu, H.; Li, J.; Yang, X. Effect of age and refractive error on quick contrast sensitivity function in Chinese adults: A pilot study. Eye 2021, 35, 966–972. [Google Scholar] [CrossRef]
- Chen, K.G.; Alvarez, J.A.; Yazdanie, M.; Papudesu, C.; Wong, W.T.; Wiley, H.E.; Keenan, T.D.; Chew, E.Y.; Ferris, F.L., III; Cukras, C.A. Longitudinal study of dark adaptation as a functional outcome measure for age-related macular degeneration. Ophthalmology 2019, 126, 856–865. [Google Scholar] [CrossRef]
- Messerlin, A.; Greth, M.; Bourcier, T.; Sauer, A.; Speeg-Schatz, C.; Gaucher, D. Dark adaptation changes in highly myopic patients. Eur. J. Ophthalmol. 2019, 29, 287–294. [Google Scholar] [CrossRef]
- Jackson, G.R.; Owsley, C.G.M., Jr. Aging and dark adaptation. Vis. Res. 1999, 39, 3975–3982. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Rosa, A.M.; Miranda, Â.; Costa, J.F.; Almeida, E.A.; Silva, F.; Castelo-Branco, M.; Murta, J.N. Functional magnetic resonance imaging as an innovative tool to assess neuroadaptation after cataract surgery. Invest. Ophthalmol. Vis. Sci 2016, 57, 22–28. [Google Scholar]
- Hwang, A.D.; Tuccar-Burak, M.; Goldstein, R.; Peli, E. Impact of oncoming headlight glare with cataracts: A pilot study. Front. Psychol. 2018, 9, 164. [Google Scholar] [CrossRef]
- Skalicky, S.E.; Martin, K.R.; Fenwick, E.; Crowston, J.G.; Goldberg, I.; McCluskey, P. Cataract and quality of life in patients with glaucoma. Clin. Exp. Ophthalmol. 2015, 43, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Gutstein, W.; Sinclair, S.H.; Presti, P.; North, R.V. Computer measurement of central visual acuity under Mesopic and glare conditions in eyes with nuclear cataract. J. Comput. Sci. Syst. Biol. 2015, 8, 354–364. [Google Scholar] [CrossRef]
- Ichikawa, K.; Yokoyama, S.; Tanaka, Y.; Nakamura, H.; Smith, R.T.; Tanabe, S. The Change in Color Vision with Normal Aging Evaluated on Standard Pseudoisochromatic Plates Part-3. Curr. Eye Res. 2020, 46, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, H.B.; Cagil, N.; Simavlı, H.; Duzen, B.; Simsek, S. Refractive error may influence mesopic pupil size. Curr. eye Res. 2010, 35, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Bécu, M.; Sheynikhovich, D.; Tatur, G.; Agathos, C.P.; Bologna, L.L.; Sahel, J.-A.; Arleo, A. Age-related preference for geometric spatial cues during real-world navigation. Nat. Hum. Behav. 2020, 4, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Caffò, A.O.; Bosco, A. Topographical disorientation in aging. Familiarity with the environment does matter. Neurol. Sci. 2018, 39, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Heffner, K.L.; Ren, P.; Tivarus, M.E.; Brasch, J.; Chen, D.G.; Mapstone, M.; Porsteinsson, A.P.; Tadin, D. Cognitive and neural effects of vision-based speed-of-processing training in older adults with amnestic mild cognitive impairment: A pilot study. J. Am. Geriatr. Soc. 2016, 64, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.; Petersen, A.H.; Bundesen, C.; Vangkilde, S.; Habekost, T. The effect of phasic auditory alerting on visual perception. Cognition 2017, 165, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Gögler, N.; Willacker, L.; Funk, J.; Strube, W.; Langgartner, S.; Napiórkowski, N.; Hasan, A.; Finke, K. Single-session transcranial direct current stimulation induces enduring enhancement of visual processing speed in patients with major depression. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 671–686. [Google Scholar] [CrossRef]
- Haupt, M.; Sorg, C.; Napiórkowski, N.; Finke, K. Phasic alertness cues modulate visual processing speed in healthy aging. Neurobiol. Aging 2018, 70, 30–39. [Google Scholar] [CrossRef]
- Owsley, C. Aging and vision. Vis. Res. 2011, 51, 1610–1622. [Google Scholar] [CrossRef]
- Penning, M.D.; Ruiz-Rizzo, A.L.; Redel, P.; Müller, H.J.; Salminen, T.; Strobach, T.; Behrens, S.; Schubert, T.; Sorg, C.; Finke, K. Alertness training increases visual processing speed in healthy older adults. Psychol. Sci. 2021, 32, 340–353. [Google Scholar] [CrossRef]
- Elhakeem, A.; Hannam, K.; Deere, K.C.; Wong, A.; Gaysin, T.; Kuh, D.; Cooper, R.; Richards, M.; Tobias, J.H. Day-to-day physical activity producing low gravitational impacts is associated with faster visual processing speed at age 69: Cross-sectional study. Eur. Rev. Aging Phys. Act. 2019, 16, 1–8. [Google Scholar] [CrossRef]
- Mkrtchyan, G.V.; Abdelmohsen, K.; Andreux, P.; Bagdonaite, I.; Barzilai, N.; Brunak, S.; Cabreiro, F.; Cabo, R.d.; Campisi, J.; Cuervo, A.M.; et al. ARDD 2020: From aging mechanisms to interventions. Aging 2020, 12, 24484–24503. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdinest, N.; London, N.; Lavy, I.; Morad, Y.; Levinger, N. Vision through Healthy Aging Eyes. Vision 2021, 5, 46. https://doi.org/10.3390/vision5040046
Erdinest N, London N, Lavy I, Morad Y, Levinger N. Vision through Healthy Aging Eyes. Vision. 2021; 5(4):46. https://doi.org/10.3390/vision5040046
Chicago/Turabian StyleErdinest, Nir, Naomi London, Itay Lavy, Yair Morad, and Nadav Levinger. 2021. "Vision through Healthy Aging Eyes" Vision 5, no. 4: 46. https://doi.org/10.3390/vision5040046
APA StyleErdinest, N., London, N., Lavy, I., Morad, Y., & Levinger, N. (2021). Vision through Healthy Aging Eyes. Vision, 5(4), 46. https://doi.org/10.3390/vision5040046





