Associations and Dissociations between Oculomotor Readiness and Covert Attention
Abstract
1. Introduction
2. The Case for OMRH/PMT
3. The Case against OMRH/PMT
3.1. Pre-Saccadic Attention Is Not Equivalent to Covert Attention
3.2. Association Is Not Causation
3.3. Saccade Programming Does Not Necessarily Produce a Shift of Attention
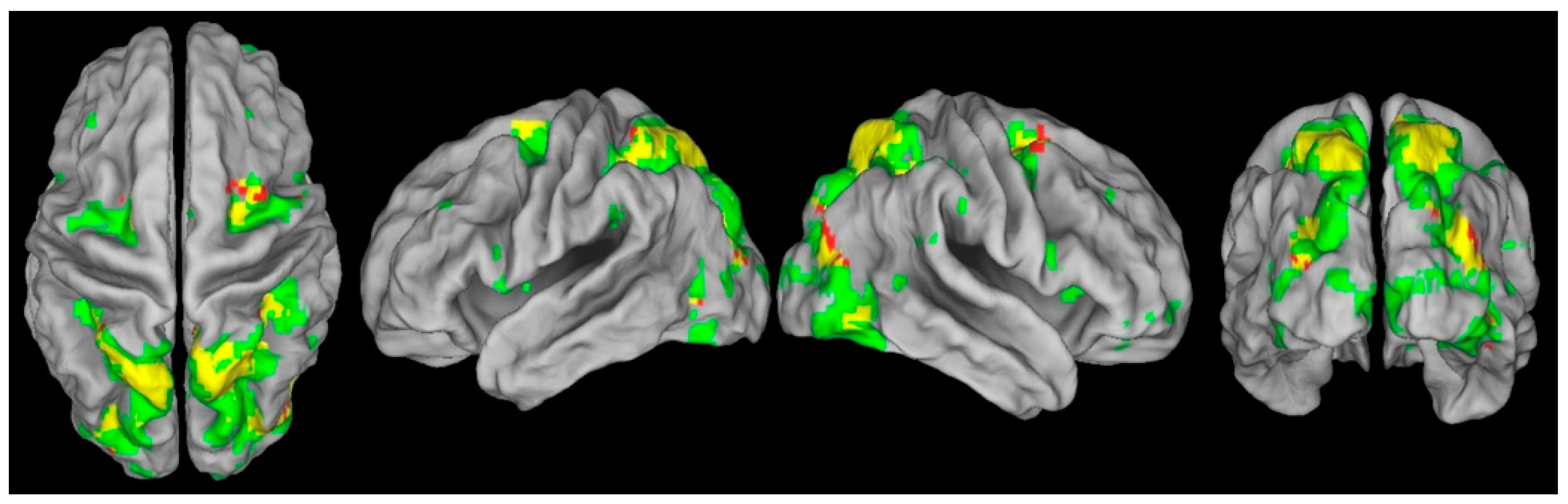
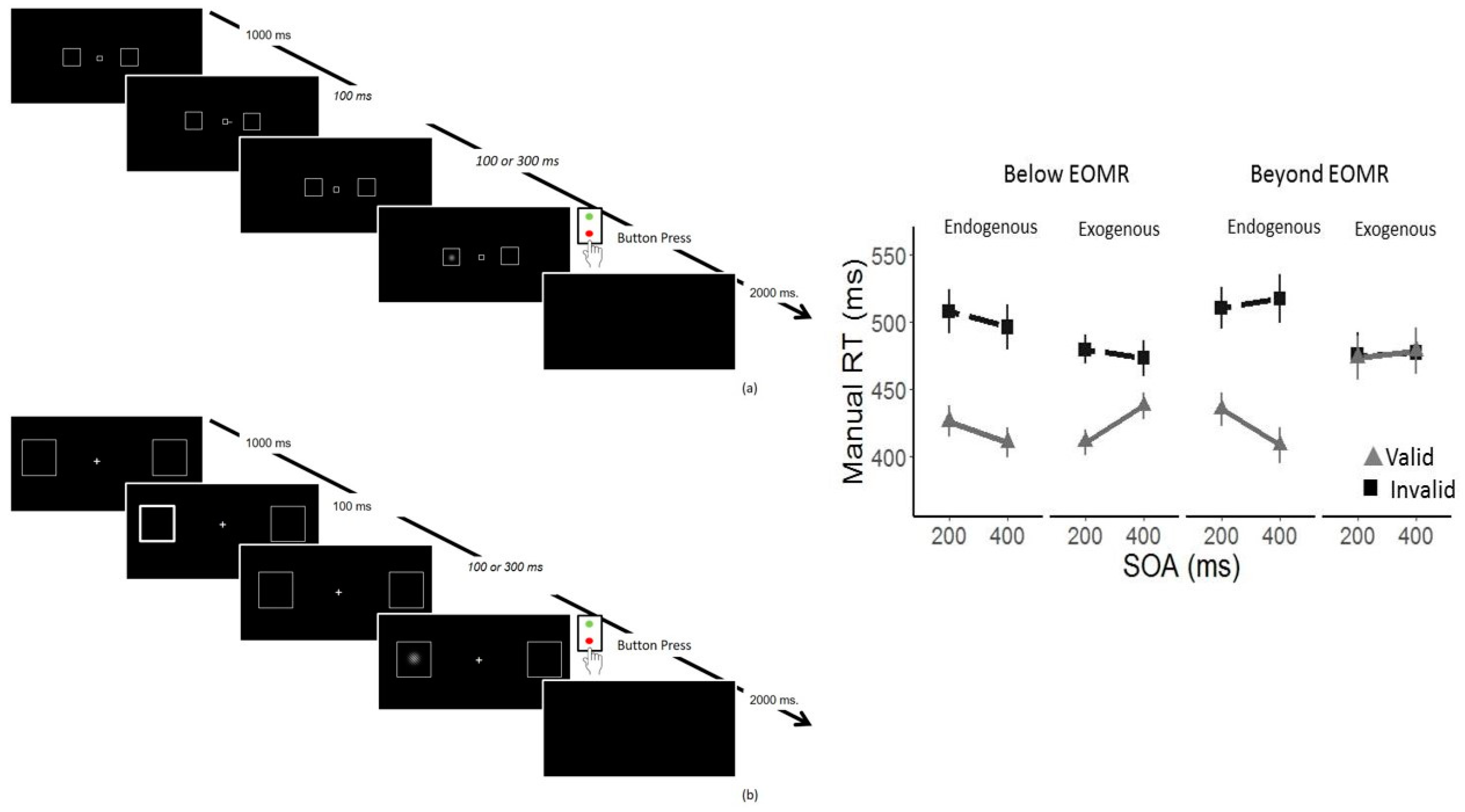
3.4. Impaired Oculomotor Control Disrupts Exogenous but Not Endogenous Covert Attention
3.5. Saccades Curve away from Attended Locations
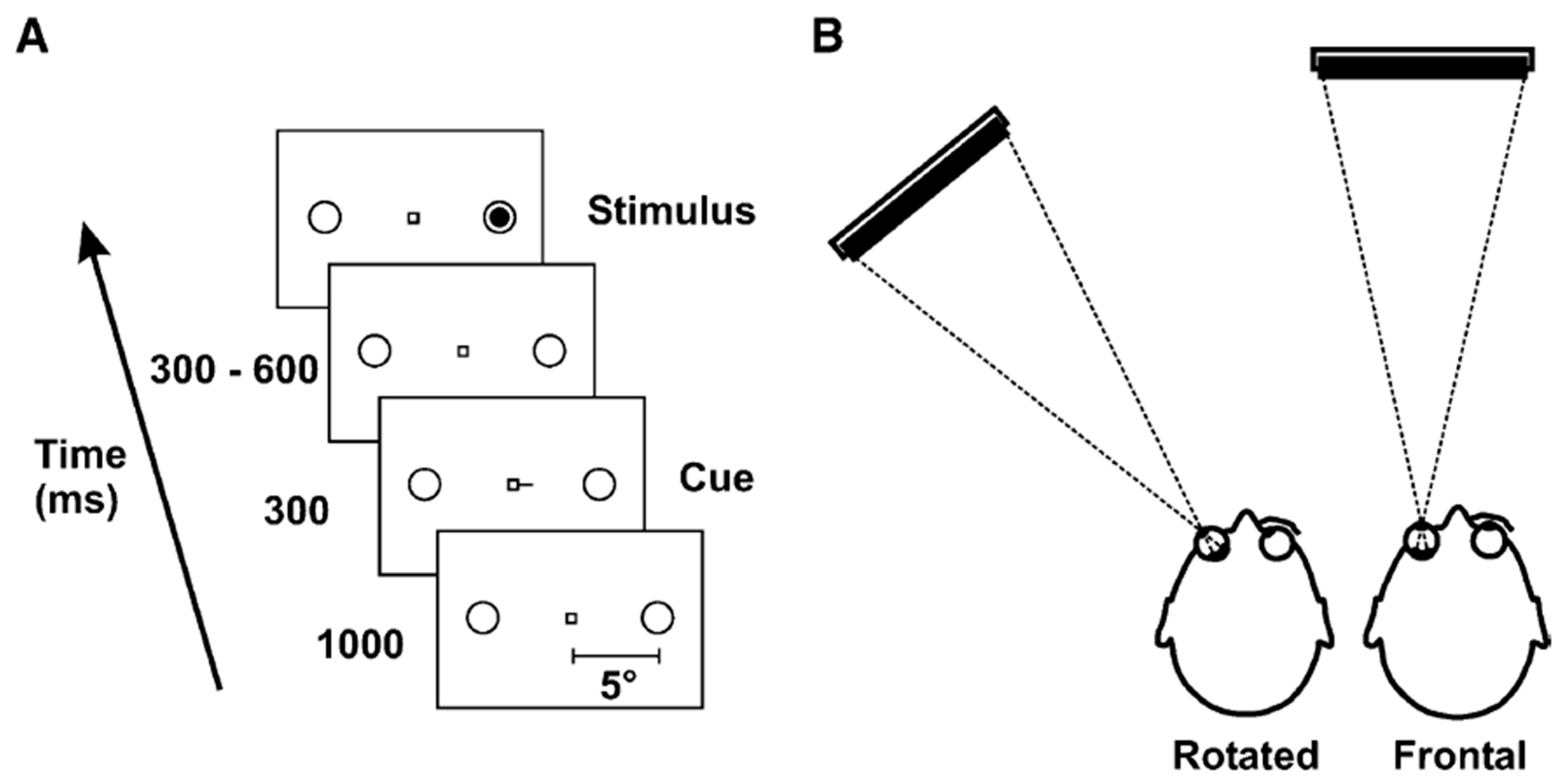
4. An Oculomotor Readiness Hypothesis of Exogenous Orienting (OREO)
5. Summary and Conclusions
Funding
Conflicts of Interest
References
- Carrasco, M. Visual attention: The past 25 years. Vis. Res. 2011, 51, 1484–1525. [Google Scholar] [CrossRef]
- Posner, M.I.; Cohen, Y. Attention and the Control of Movements. Tutor. Mot. Behav. 1980, 1, 243–258. [Google Scholar] [CrossRef]
- Chica, A.B.; Bartolomeo, P.; Lupianez, J. Two cognitive and neural systems for endogenous and exogenous spatial attention. Behav. Brain Res. 2013, 237, 107–123. [Google Scholar] [CrossRef]
- Smith, D.T.; Schenk, T. The Premotor theory of attention: Time to move on? Neuropsychologia 2012, 50, 1104–1114. [Google Scholar] [CrossRef]
- Klein, R.M. Does oculomotor readiness mediate cognitive control of visual attention? Atten. Perform. 1980, 8, 259–276. [Google Scholar]
- Rizzolatti, G.; Riggio, L.; Dascola, I.; Umilta, C. Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 1987, 25, 31–40. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Riggio, L.; Sheliga, B.M. Space and Selective Attention. In Attention and Performance Series. Attention and Performance 15: Conscious and Nonconscious Information Processing; Umiltà, C., Moscovitch, M., Eds.; The MIT Press: Cambridge, MA, USA, 1994; Vol. 15, pp. 231–265. [Google Scholar]
- Klein, R.M.; Pontefract, A. Does Oculomotor Readiness Mediate Cognitive Control of Visual Attention? Revisited! In Attention and performance XV: Conscious and Nonconscious Information Processing; Umiltà, C., Moscovitch, M., Eds.; The MIT Press: Cambrige, MA, USA, 1994; pp. 333–350. [Google Scholar]
- Belopolsky, A.V.; Theeuwes, J. Updating the premotor theory: The allocation of attention is not always accompanied by saccade preparation. J. Exp. Psychol: Hum. Percept. Perform. 2012, 38, 902. [Google Scholar] [CrossRef]
- Schneider, W.X.; Deubel, H. Selection-for-perception and selection-for-spatial-motor-action are coupled by visual attention: A review of recent findings and new evidence from stimulus-driven saccade control. Atten. Perform. XIX 2002, 19, 609–627. [Google Scholar]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Hoffman, J.E.; Subramaniam, B. The role of visual attention in saccadic eye movements. Percept. Psychophys. 1995, 57, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, M.; Findlay, J.M.; Hockey, R.J. The relationship between eye movements and spatial attention. Q. J. Exp. Psychol. A 1986, 38, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Deubel, H.; Schneider, W.X. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vis. Res. 1996, 36, 1827–1837. [Google Scholar] [CrossRef]
- Kowler, E.; Anderson, E.; Dosher, B.; Blaser, E. The Role of Attention in the Programming of Saccades. Vis. Res. 1995, 35, 1897–1916. [Google Scholar] [CrossRef]
- Van der Stigchel, S.; Theeuwes, J. The influence of attending to multiple locations on eye movements. Vis. Res. 2005, 45, 1921–1927. [Google Scholar] [CrossRef]
- Crovitz, H.F.; Daves, W. Tendencies to eye movement and perceptual accuracy. J. Exp. Psychol. 1962, 63, 495–498. [Google Scholar] [CrossRef]
- Bryden, M.P. The Role of Post-Exposural Eye-Movements in Tachistoscopic Perception. Can. J. Psychol. 1961, 15, 220–225. [Google Scholar] [CrossRef]
- Khan, A.Z.; Blohm, G.; Pisella, L.; Munoz, D.P. Saccade execution suppresses discrimination at distractor locations rather than enhancing the saccade goal location. Eur. J. Neurosci. 2015, 41, 1624–1634. [Google Scholar] [CrossRef]
- Deubel, H. The time course of presaccadic attention shifts. Psychol. Res. 2008, 72, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Castet, E.; Jeanjean, S.; Montagnini, A.; Laugier, D.; Masson, G.S. Dynamics of attentional deployment during saccadic programming. J. Vis. 2006, 6, 196–212. [Google Scholar] [CrossRef]
- Sheliga, B.M.; Riggio, L.; Rizzolatti, G. Orienting of attention and eye movements. Exp. Brain Res. 1994, 98, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Moehler, T.; Fiehler, K. Effects of spatial congruency on saccade and visual discrimination performance in a dual-task paradigm. Vis. Res. 2014, 105, 100–111. [Google Scholar] [CrossRef]
- de Haan, B.; Morgan, P.S.; Rorden, C. Covert orienting of attention and overt eye movements activate identical brain regions. Brain Res. 2008, 1204, 102–111. [Google Scholar] [CrossRef]
- Perry, R.J.; Zeki, S. The neurology of saccades and covert shifts in spatial attention: An event-related fMRI study. Brain 2000, 123, 2273–2288. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.S.; Petit, L.; Ellmore, T.M.; Ingeholm, J.; Haxby, J.V. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage 2001, 14, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Nobre, A.C.; Gitelman, D.R.; Dias, E.C.; Mesulam, M.M. Covert visual spatial orienting and saccades: Overlapping neural systems. Neuroimage 2000, 11, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Akbudak, E.; Conturo, T.E.; Snyder, A.Z.; Ollinger, J.M.; Drury, H.A.; Linenweber, M.R.; Petersen, S.E.; Raichle, M.E.; Van Essen, D.C.; et al. A common network of functional areas for attention and eye movements. Neuron 1998, 21, 761–773. [Google Scholar] [CrossRef]
- Andersen, R.A. Visual and eye movement functions of the posterior parietal cortex. Annu. Rev. Neurosci. 1989, 12, 377–403. [Google Scholar] [CrossRef] [PubMed]
- Thickbroom, G.W.; Stell, R.; Mastaglia, F.L. Transcranial magnetic stimulation of the human frontal eye field. J. Neurol. Sci. 1996, 144, 114–118. [Google Scholar] [CrossRef]
- Grosbras, M.H.; Paus, T. Transcranial magnetic stimulation of the human frontal eye field: Effects on visual perception and attention. J. Cogn. Neurosci. 2002, 14, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Muggleton, N.G.; Juan, C.H.; Cowey, A.; Walsh, V. Human frontal eye fields and visual search. J. Neurophysiol. 2003, 89, 3340–3343. [Google Scholar] [CrossRef] [PubMed]
- Muri, R.M.; Hess, C.W.; Meienberg, O. Transcranial Stimulation of the Human Frontal Eye Field by Magnetic Pulses. Exp. Brain Res. 1991, 86, 219–223. [Google Scholar] [CrossRef]
- Muri, R.M.; Vermersch, A.I.; Rivaud, S.; Gaymard, B.; Pierrot-Deseilligny, C. Effects of single-pulse transcranial magnetic stimulation over the prefrontal and posterior parietal cortices during memory-guided saccades in humans. J. Neurophysiol. 1996, 76, 2102–2106. [Google Scholar] [CrossRef]
- Smith, D.T.; Jackson, S.R.; Rorden, C. Transcranial magnetic stimulation of the left human frontal eye fields eliminates the cost of invalid endogenous cues. Neuropsychologia 2005, 43, 1288–1296. [Google Scholar] [CrossRef]
- Smith, D.T.; Jackson, S.R.; Rorden, C. An intact eye-movement system is not required to generate Inhibition of Return. J. Neuropsychol. 2009, 3, 267–271. [Google Scholar] [CrossRef]
- Moore, T.; Armstrong, K.M.; Fallah, M. Visuomotor origins of covert spatial attention. Neuron 2003, 40, 671–683. [Google Scholar] [CrossRef]
- Moore, T.; Fallah, M. Control of eye movements and spatial attention. Proc. Natl. Acad. Sci. USA 2001, 98, 1273–1276. [Google Scholar] [CrossRef]
- Armstrong, K.M.; Fitzgerald, J.K.; Moore, T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron 2006, 50, 791–798. [Google Scholar] [CrossRef]
- Moore, T.; Armstrong, K.M. Selective gating of visual signals by microstimulation of frontal cortex. Nature 2003, 421, 370–373. [Google Scholar] [CrossRef]
- Craighero, L.; Carta, A.; Fadiga, L. Peripheral oculomotor palsy affects orienting of visuospatial attention. Neuroreport 2001, 12, 3283–3286. [Google Scholar] [CrossRef]
- Craighero, L.; Nascimben, M.; Fadiga, L. Eye position affects orienting of visuospatial attention. Curr. Biol. 2004, 14, 331–333. [Google Scholar] [CrossRef]
- Mclaughlin, S.C. Parametric Adjustment in Saccadic Eye Movements. Percep. Psychophys. 1967, 2, 359–362. [Google Scholar] [CrossRef]
- Pelisson, D.; Alahyane, N.; Panouilleres, M.; Tilikete, C. Sensorimotor adaptation of saccadic eye movements. Neurosci. Biobehav. Rev. 2010, 34, 1103–1120. [Google Scholar] [CrossRef] [PubMed]
- Ditterich, J.; Eggert, T.; Straube, A. Relation Between the Metrics of the Presaccadic Attention Shift and of the Saccade Before and After Saccadic Adaptation. J. Neurophysiol. 2000, 84, 1809–1813. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Dore-Mazars, K. Eye movement signals influence perception: Evidence from the adaptation of reactive and volitional saccades. Vis. Res. 2006, 46, 3659–3673. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dore-Mazars, K.; Collins, T. Saccadic adaptation shifts the pre-saccadic attention focus. Exp. Brain Res. 2005, 162, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Heed, T.; Röder, B. Visual target selection and motor planning define attentional enhancement at perceptual processing stages. Front. Hum. Neurosci. 2010, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Habchi, O.; Rey, E.; Mathieu, R.; Urquizar, C.; Farne, A.; Pelisson, D. Deployment of spatial attention without moving the eyes is boosted by oculomotor adaptation. Front. Hum. Neurosci. 2015, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Yarbus, A.L. Eye movements during perception of complex objects. In Eye Movements and Vision; Springer: Boston, MA, USA, 1967; pp. 171–211. [Google Scholar]
- Viviani, P.; Berthoz, A.; Tracey, D. The curvature of oblique saccades. Vis. Res. 1977, 17, 661–664. [Google Scholar] [CrossRef]
- Smit, A.C.; Van Gisbergen, J.A.M. An analysis of curvature in fast and slow human saccades. Exp. Brain Res. 1990, 81, 335–345. [Google Scholar] [CrossRef]
- Doyle, M.; Walker, R. Curved saccade trajectories: Voluntary and reflexive saccades curve away from irrelevant distractors. Exp. Brain Res. 2001, 139, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Van der Stigchel, S.; Theeuwes, J. Our eyes deviate away from a location where a distractor is expected to appear. Exp. Brain Res. 2006, 169, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; McSorley, E.; Haggard, P. The control of saccade trajectories: Direction of curvature depends on prior knowledge of target location and saccade latency. Percept. Psychophys. 2006, 68, 129–138. [Google Scholar] [CrossRef] [PubMed]
- McPeek, R.M.; Skavenski, A.A.; Nakayama, K. Concurrent processing of saccades in visual search. Vis. Res. 2000, 40, 2499–2516. [Google Scholar] [CrossRef]
- Walker, R.; McSorley, E. The parallel programming of voluntary and reflexive saccades. Vis. Res. 2006, 46, 2082–2093. [Google Scholar] [CrossRef]
- Sheliga, B.M.; Riggio, L.; Craighero, L.; Rizzolatti, G. Spatial attention-determined modifications in saccade trajectories. Neuroreport 1995, 6, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Sheliga, B.M.; Riggio, L.; Rizzolatti, G. Spatial attention and eye movements. Exp. Brain Res. 1995, 105, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Van der Stigchel, S.; Theeuwes, J. The relationship between covert and overt attention in endogenous cuing. Percept. Psychophys. 2007, 69, 719–731. [Google Scholar] [CrossRef]
- Craighero, L.; Rizzolatti, G. The premotor theory of attention. In Neurobiology of Attention; Itti, L., Rees, G., Tsotsos, J.K., Eds.; Academic Press: Burlington, MA. USA, 2005; pp. 181–186. [Google Scholar]
- Duhamel, J.R.; Colby, C.L.; Goldberg, M.E. The updating of the representation of visual space in parietal cortex by intended eye movements. Science 1992, 255, 90–92. [Google Scholar] [CrossRef]
- Colby, C.L. A neurophysiological distinction between attention and intention. In Attention and Performance XVI: Information Integration in Perception and Communication; Inui, T., McClelland, J.L., Eds.; MIT Press: Cambridge, MA, USA, 1996; pp. 157–177. [Google Scholar]
- Ladavas, E.; Zeloni, G.; Zaccara, G.; Gangemi, P. Eye movements and orienting of attention in patients with visual neglect. J. Cogn. Neurosci. 1997, 9, 67–74. [Google Scholar] [CrossRef]
- Benson, V.; Ietswaart, M.; Milner, D. Eye Movements and Verbal Report in a Single Case of Visual Neglect. PLoS ONE 2012, 7, 11. [Google Scholar] [CrossRef]
- Blangero, A.; Khan, A.Z.; Salemme, R.; Deubel, H.; Schneider, W.X.; Rode, G.; Vighetto, A.; Rossetti, Y.; Pisella, L. Pre-saccadic perceptual facilitation can occur without covert orienting of attention. Cortex 2010, 46, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.R.; Schall, J.D. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron 2003, 38, 637–648. [Google Scholar] [CrossRef]
- Thompson, K.G.; Biscoe, K.L.; Sato, T.R. Neuronal basis of covert spatial attention in the frontal eye field. J. Neurosci. 2005, 25, 9479–9487. [Google Scholar] [CrossRef]
- Thompson, K.G.; Bichot, N.P.; Schall, J.D. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J. Neurophysiol. 1997, 77, 1046–1050. [Google Scholar] [CrossRef]
- Tehovnik, E.J. Electrical stimulation of neural tissue to evoke behavioral responses. J. Neurosci. Methods 1996, 65, 1–17. [Google Scholar] [CrossRef]
- Juan, C.H.; Muggleton, N.G.; Tzeng, O.J.; Hung, D.L.; Cowey, A.; Walsh, V. Segregation of visual selection and saccades in human frontal eye fields. Cereb. Cortex 2008, 18, 2410–2415. [Google Scholar] [CrossRef] [PubMed]
- Deubel, H.; Wolf, W.; Hauske, G. The evaluation of the oculomotor error signal. Adv. Psychol. 1984, 22, 55–62. [Google Scholar]
- Weaver, M.D.; van Zoest, W.; Hickey, C. A temporal dependency account of attentional inhibition in oculomotor control. NeuroImage 2016, 147, 880–894. [Google Scholar] [CrossRef]
- Remington, R.W. Attention and saccadic eye movements. J. Exp. Psychol. 1980, 6, 726–744. [Google Scholar] [CrossRef]
- Stelmach, L.B.; Campsall, J.M.; Herdman, C.M. Attentional and ocular movements. J. Exp. Psychol. 1997, 23, 823–844. [Google Scholar] [CrossRef]
- Born, S.; Mottet, I.; Kerzel, D. Presaccadic perceptual facilitation effects depend on saccade execution: Evidence from the stop-signal paradigm. J. Vis. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Findlay, J.M. Global visual processing for saccadic eye movements. Vis. Res. 1982, 22, 1033–1045. [Google Scholar] [CrossRef]
- Coren, S.; Hoenig, P. Effect of Non-Target Stimuli Upon Length of Voluntary Saccades. Percept. Mot. Skills 1972, 34, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Van der Stigchel, S.; de Vries, J.P. There is no attentional global effect: Attentional shifts are independent of the saccade endpoint. J. Vis. 2015, 15, 12. [Google Scholar] [CrossRef]
- Wollenberg, L.; Deubel, H.; Szinte, M. Visual attention is not deployed at the endpoint of averaging saccades. PLoS Biol. 2018, 16, e2006548. [Google Scholar] [CrossRef]
- Van der Stigchel, S.; de Vries, J.P. Commentary: Visual attention is not deployed at the endpoint of averaging saccades. Front. Psychol. 2018, 9. [Google Scholar] [CrossRef]
- Bedard, P.; Song, J.H. Attention modulates generalization of visuomotor adaptation. J. Vis. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.R.; Kingstone, A. Inhibition of return: Dissociating attentional and oculomotor components. J. Exp. Psychol. Hum. Percept. Perform. 2003, 29, 1068–1074. [Google Scholar] [CrossRef]
- Belopolsky, A.V.; Theeuwes, J. When are attention and saccade preparation dissociated? Psychol. Sci. 2009, 20, 1340–1347. [Google Scholar] [CrossRef]
- Smith, D.T.; Casteau, S. The effect of offset cues on saccade programming and covert attention. Q. J. Exp. Psychol. 2019, 72, 481–490. [Google Scholar] [CrossRef]
- Smith, D.T.; Rorden, C.; Jackson, S.R. Exogenous orienting of attention depends upon the ability to execute eye movements. Curr. Biol. 2004, 14, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Gabay, S.; Henik, A.; Gradstein, L. Ocular motor ability and covert attention in patients with Duane Retraction Syndrome. Neuropsychologia 2010, 48, 3102–3109. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.C.; Richardson, J.C.; Olszewski, J. Progressive Supranuclear Palsy. A Heterogeneous Degeneration Involving the Brain Stem, Basal Ganglia and Cerebellum with Vertical Gaze and Pseudobulbar Palsy, Nuchal Dystonia and Dementia. Arch. Neurol. 1964, 10, 333–359. [Google Scholar] [CrossRef]
- Posner, M.I.; Cohen, Y.; Rafal, R.D. Neural systems control of spatial orienting. Philos. Trans. R. Soc. Lond. B 1982, 298, 187–198. [Google Scholar] [CrossRef]
- Rafal, R.D.; Posner, M.I.; Friedman, J.H.; Inhoff, A.W.; Bernstein, E. Orienting of Visual-Attention in Progressive Supranuclear Palsy. Brain 1988, 111, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.T.; Archibald, N. Visual Search in Progressive Supranuclear Palsy. Curr. Top. Behav. Neurosci. 2018. [Google Scholar] [CrossRef]
- Smith, D.T.; Schenk, T.; Rorden, C. Saccade preparation is required for exogenous attention but not endogenous attention or IOR. J. Exp. Psychol Hum. Percept. Perform. 2012, 38, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.T.; Ball, K.; Ellison, A. Covert visual search within and beyond the effective oculomotor range. Vis. Res. 2014, 95, 11–17. [Google Scholar] [CrossRef][Green Version]
- Smith, D.T.; Ball, K.; Ellison, A.; Schenk, T. Deficits of reflexive attention induced by abduction of the eye. Neuropsychologia 2010, 48, 1269–1276. [Google Scholar] [CrossRef]
- Ball, K.; Pearson, D.G.; Smith, D.T. Oculomotor involvement in spatial working memory is task-specific. Cognition 2013, 129, 439–446. [Google Scholar] [CrossRef]
- Pearson, D.G.; Ball, K.; Smith, D.T. Oculomotor preparation as a rehearsal mechanism in spatial working memory. Cognition 2014, 132, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Boon, P.J.; Theeuwes, J.; Belopolsky, A.V. Eye abduction reduces but does not eliminate competition in the oculomotor system. J. Vis. 2017, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.J.; Ball, K.; Smith, D.T. The role of the oculomotor system in covert social attention. Atten. Percept. Psychophys. 2014, 76, 1265–1270. [Google Scholar] [CrossRef][Green Version]
- Kuhn, G.; Tatler, B.W.; Cole, G.G. You look where I look! Effect of gaze cues on overt and covert attention in misdirection. Vis. Cogn. 2009, 17, 925–944. [Google Scholar] [CrossRef]
- Ricciardelli, P.; Bricolo, E.; Aglioti, S.M.; Chelazzi, L. My eyes want to look where your eyes are looking: Exploring the tendency to imitate another individual’s gaze. Neuroreport 2002, 13, 2259–2264. [Google Scholar] [CrossRef]
- Michalczyk, L.; Paszulewicz, J.; Bielas, J.; Wolski, P. Is saccade preparation required for inhibition of return (IOR)? Neurosci. Lett. 2018, 665, 13–17. [Google Scholar] [CrossRef] [PubMed]
- MacLean, G.H.; Klein, R.M.; Hilchey, M.D. Does oculomotor readiness mediate exogenous capture of visual attention? J. Exp. Psychol. Hum. Percept. Perform. 2015, 41, 1260–1270. [Google Scholar] [CrossRef]
- Balslev, D.; Newman, W.; Knox, P.C. Extraocular Muscle Afferent Signals Modulate Visual AttentionEye Proprioception and Visual Attention. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7004–7009. [Google Scholar] [CrossRef]
- Casteau, S.; Smith, D.T. Covert attention beyond the range of eye-movements: Evidence for a dissociation between exogenous and endogenous orienting. Cortex 2018. [Google Scholar] [CrossRef]
- Paap, K.R.; Ebenholtz, M. Perceptual consequences of potentiation in the extraocular muscles: An alternative explanation for adaptation to wedge prisms. J. Exp. Psychol. Hum. Percept. Perform. 1976, 2, 457–468. [Google Scholar] [CrossRef]
- Gilligan, T.M.; Cristino, F.; Bultitude, J.H.; Rafal, R.D. The effect of prism adaptation on state estimates of eye position in the orbit. Cortex 2019, 115, 246–263. [Google Scholar] [CrossRef]
- Casteau, S.; Smith, D.T. Is pre-attentive search restricted to the range of eye-movements? Under Review. 2018. [Google Scholar]
- Godijn, R.; Theeuwes, J. Programming of endogenous and exogenous saccades: Evidence for a competitive integration model. J. Exp. Psychol. 2002, 28, 1039–1054. [Google Scholar] [CrossRef]
- Van der Stigchel, S.; Meeter, M.; Theeuwes, J. Eye movement trajectories and what they tell us. Neurosci. Biobehav. Rev. 2006, 30, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Van der Stigchel, S. Recent advances in the study of saccade trajectory deviations. Vis. Res. 2010, 50, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- McSorley, E.; Haggard, P.; Walker, R. Time course of oculomotor inhibition revealed by saccade trajectory modulation. J. Neurophysiol. 2006, 96, 1420–1424. [Google Scholar] [CrossRef]
- Desimone, R. Visual attention mediated by biased competition in extrastriate visual cortex. Philos. Trans. R. Soc. Lond. B 1998, 353, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Bisley, J.W.; Goldberg, M.E. Attention, intention, and priority in the parietal lobe. Annu. Rev. Neurosci. 2010, 33, 1–21. [Google Scholar] [CrossRef]
- Paré, M.; Dorris, M.C. The role of posterior parietal cortex in the regulation of saccadic eye movements. In The Oxford Handbook of Eye Movements; Liversedge, S.P., Gilchrist, I., Everling, S., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 257–278. [Google Scholar]
- Bisley, J.W.; Mirpour, K.; Arcizet, F.; Ong, W.S. The role of the lateral intraparietal area in orienting attention and its implications for visual search. Eur. J. Neurosci. 2011, 33, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; VanRullen, R.; Koch, C.; Perona, P. Rapid natural scene categorization in the near absence of attention. Proc. Nat. Acad. Sci. USA 2002, 99, 9596–9601. [Google Scholar] [CrossRef]
- Dunne, S.; Ellison, A.; Smith, D.T. Rewards modulate saccade latency but not exogenous spatial attention. Front. Psychol. 2015, 6, 1080. [Google Scholar] [CrossRef] [PubMed]
- McCoy, B.; Theeuwes, J. Overt and covert attention to location-based reward. Vis. Res. 2018, 142, 27–39. [Google Scholar] [CrossRef]
- McFadden, S.A.; Khan, A.; Wallman, J. Gain adaptation of exogenous shifts of visual attention. Vis. Res. 2002, 42, 2709–2726. [Google Scholar] [CrossRef]
- Schneider, W.X. VAM: A neuro-cognitive model for visual attention control of segmentation, object recognition, and space-based motor action. Vis. Sel. Atten. 1995, 2, 331–376. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casteau, S.; Smith, D.T. Associations and Dissociations between Oculomotor Readiness and Covert Attention. Vision 2019, 3, 17. https://doi.org/10.3390/vision3020017
Casteau S, Smith DT. Associations and Dissociations between Oculomotor Readiness and Covert Attention. Vision. 2019; 3(2):17. https://doi.org/10.3390/vision3020017
Chicago/Turabian StyleCasteau, Soazig, and Daniel T. Smith. 2019. "Associations and Dissociations between Oculomotor Readiness and Covert Attention" Vision 3, no. 2: 17. https://doi.org/10.3390/vision3020017
APA StyleCasteau, S., & Smith, D. T. (2019). Associations and Dissociations between Oculomotor Readiness and Covert Attention. Vision, 3(2), 17. https://doi.org/10.3390/vision3020017





