Evidence Transcranial Direct Current Stimulation Can Improve Saccadic Eye Movement Control in Older Adults
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Design
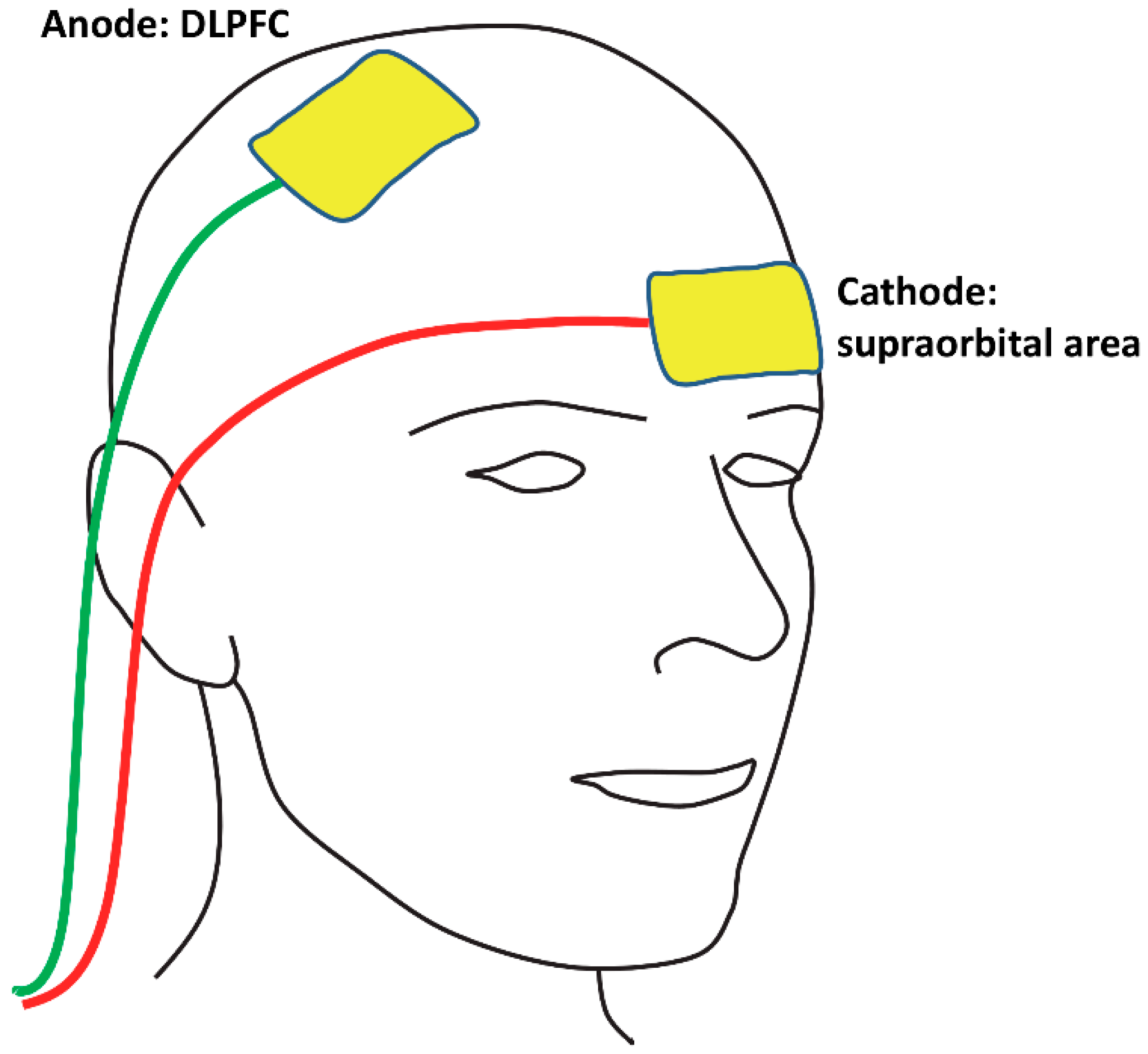
2.3. tDCS Protocol
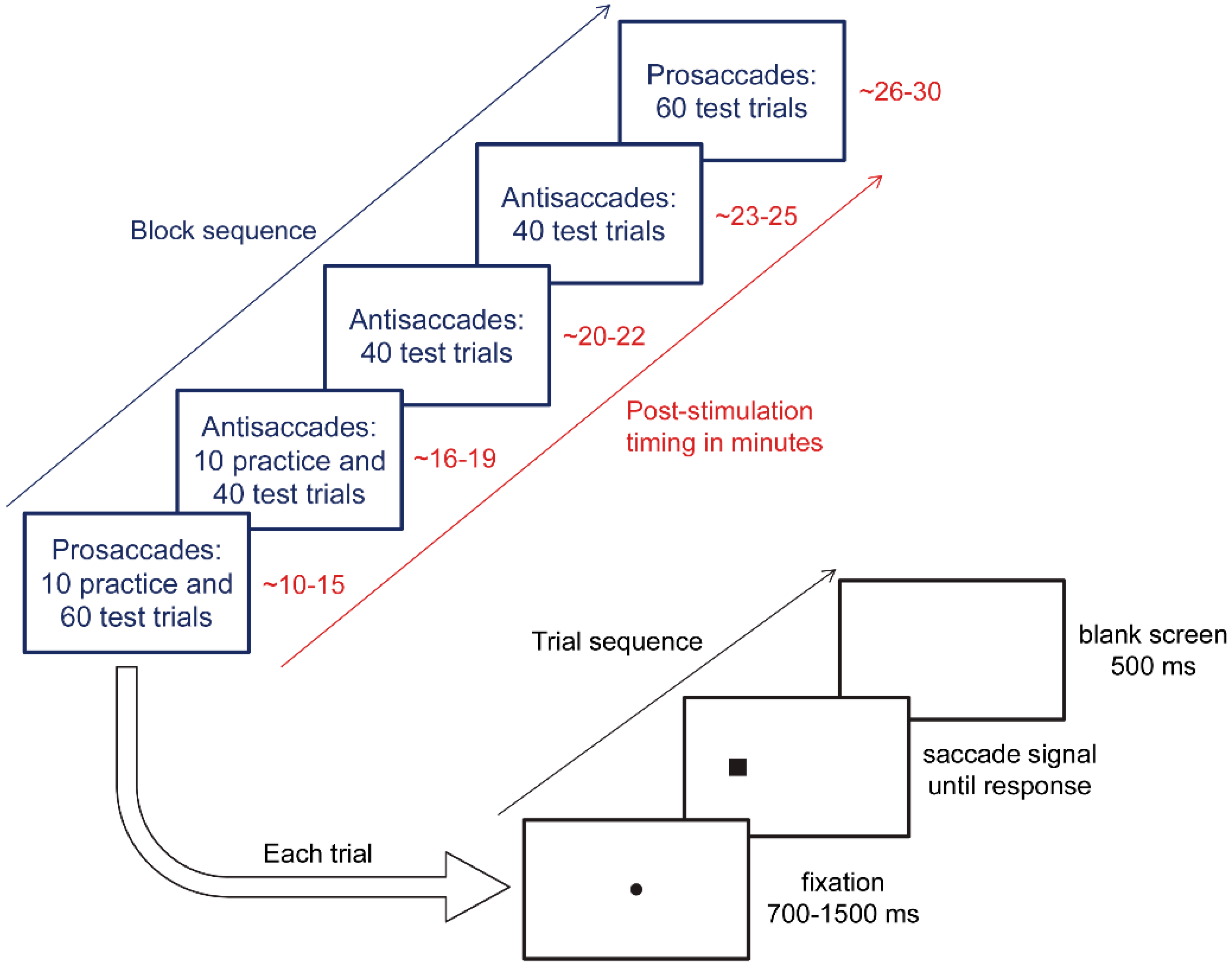
2.4. Eye Tracking Protocol
2.5. Statistical Analyses
3. Results
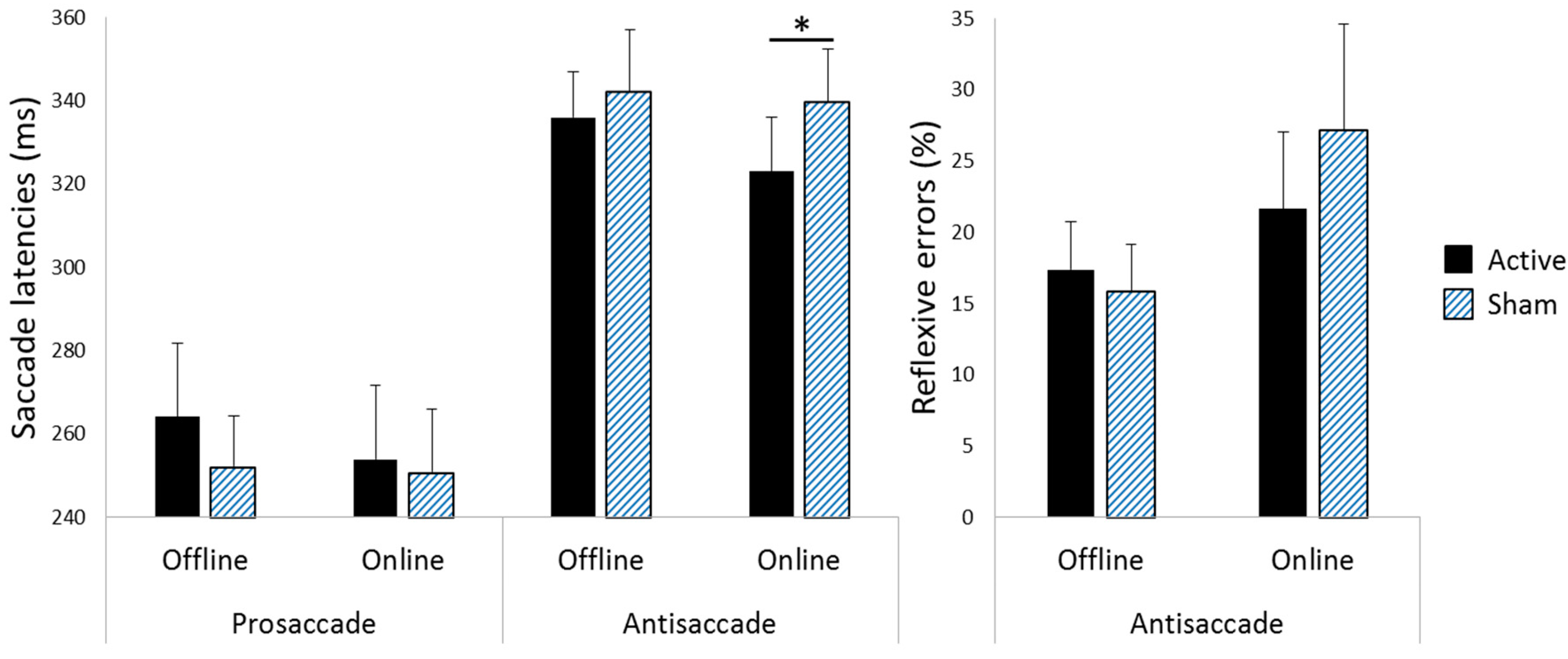
3.1. Saccade Latencies
3.2. Reflexive Error Rates during Antisaccade Blocks
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Peltsch, A.; Hemraj, A.; Garcia, A.; Munoz, D.P. Age-related trends in saccade characteristics among the elderly. Neurobiol. Aging 2011, 32, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.L.; Machado, L. Age-related deficits in voluntary control over saccadic eye movements: Consideration of electrical brain stimulation as a therapeutic strategy. Neurobiol. Aging 2016, 41, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bos, H.; Machado, L. Aging delays strategic modulation of the fixation reflex. Psychol. Aging 2013, 28, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.H.R.; Machado, L. Manual versus saccadic assessment of cognitive inhibition and switching in young and older adults. Psychol. Assess. 2017, 29, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Abel, L.A.; Douglas, J. Effects of age on latency and error generation in internally mediated saccades. Neurobiol. Aging 2007, 28, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Doroudgar, S.; Chuang, H.M.; Perry, P.J.; Thomas, K.; Bohnert, K.; Canedo, J. Driving performance comparing older versus younger drivers. Traffic Inj. Prev. 2017, 18, 41–46. [Google Scholar] [CrossRef]
- Bédard, M.; Leonard, E.; McAuliffe, J.; Weaver, B.; Gibbons, C.; Dubois, S. Visual attention and older drivers: The contribution of inhibition of return to safe driving. Exp. Aging Res. 2006, 32, 119–135. [Google Scholar] [CrossRef]
- Zito, G.A.; Cazzoli, D.; Scheffler, L.; Jager, M.; Muri, R.M.; Mosimann, U.P.; Nyffeler, T.; Mast, F.W.; Nef, T. Street crossing behavior in younger and older pedestrians: An eye- and head-tracking study. BMC Geriatr. 2015, 15, 176. [Google Scholar] [CrossRef]
- Sweeney, J.A.; Rosano, C.; Berman, R.A.; Luna, B. Inhibitory control of attention declines more than working memory during normal aging. Neurobiol. Aging 2001, 22, 39–47. [Google Scholar] [CrossRef]
- Kanai, R.; Muggleton, N.; Walsh, V. Transcranial direct current stimulation of the frontal eye fields during pro- and antisaccade tasks. Front. Psychiatry 2012, 3, 45. [Google Scholar] [CrossRef]
- Chen, P.L.; Machado, L. Developing clinically practical transcranial direct current stimulation protocols to improve saccadic eye movement control. J. Eye Mov. Res. 2017, 10, 5. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Ku, Y.; Zanto, T.P.; Gazzaley, A. Effects of noninvasive brain stimulation on cognitive function in healthy aging and Alzheimer's disease: A systematic review and meta-analysis. Neurobiol. Aging 2015, 36, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Dennis, N.A. Frontal lobes and aging: Deterioration and compensation. In Principles of Frontal Lobe Function; Stuss, D.T., Knight, R.T., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 628–652. [Google Scholar]
- Bierre, K.L.; Lucas, S.J.; Guiney, H.; Cotter, J.D.; Machado, L. Cognitive difficulty intensifies age-related changes in anterior frontal hemodynamics: Novel evidence from near-infrared spectroscopy. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T.; Stephens, J.A.; Alam, M.; Bikson, M.; Berryhill, M.E. Longitudinal neurostimulation in older adults improves working memory. PLoS ONE 2015, 10, e0121904. [Google Scholar] [CrossRef] [PubMed]
- Dayan, E.; Censor, N.; Buch, E.R.; Sandrini, M.; Cohen, L.G. Noninvasive brain stimulation: From physiology to network dynamics and back. Nat. Neurosci. 2013, 16, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Prehn, K.; Flöel, A. Potentials and limits to enhance cognitive functions in healthy and pathological aging by tDCS. Front. Cell. Neurosci. 2015, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, A.C.; Nafee, T.; Sampaio, A.; Leite, J.; Carvalho, S. Effects of transcranial direct current stimulation on working memory in healthy older adults: A systematic review. Princ. Pract. Clin. Res. 2015, 1, 73–81. [Google Scholar]
- Homan, R.W.; Herman, J.; Purdy, P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 376–382. [Google Scholar] [CrossRef]
- Stagg, C.J.; Jayaram, G.; Pastor, D.; Kincses, Z.T.; Matthews, P.M.; Johansen-Berg, H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011, 49, 800–804. [Google Scholar] [CrossRef]
- Martin, D.M.; Liu, R.; Alonzo, A.; Green, M.; Loo, C.K. Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: Effect of timing of stimulation. Exp. Brain Res. 2014, 232, 3345–3351. [Google Scholar] [CrossRef]
- Oldrati, V.; Colombo, B.; Antonietti, A. Combination of a short cognitive training and tDCS to enhance visuospatial skills: A comparison between online and offline neuromodulation. Brain Res. 2018, 1678, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, L.E.; Ilieva, I.P.; Hamilton, R.H.; Farah, M.J. Does transcranial direct current stimulation improve healthy working memory?: A meta-analytic review. J. Cogn. Neurosci. 2016, 28, 1063–1089. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.; Machado, L. Using transcranial direct current stimulation to improve verbal working memory: A detailed review of the methodology. J. Clin. Exp. Neuropsychol. 2018, 40, 790–804. [Google Scholar] [CrossRef]
- Bikson, M.; Rahman, A. Origins of specificity during tDCS: Anatomical, activity-selective, and input-bias mechanisms. Front. Hum. Neurosci. 2013, 7, 688. [Google Scholar] [CrossRef] [PubMed]
- Fertonani, A.; Miniussi, C. Transcranial Electrical Stimulation: What We Know and do Not Know about Mechanisms. Neuroscientist 2017, 23, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Chapman, L.J.; Chapman, J.P. The measurement of handedness. Brain Cogn. 1987, 6, 175–183. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D Scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. "Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Hallett, M. Induction of errors in a delayed response task by repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuroreport 1994, 5, 2517–2520. [Google Scholar] [CrossRef]
- Antoniades, C.; Ettinger, U.; Gaymard, B.; Gilchrist, I.; Kristjansson, A.; Kennard, C.; John Leigh, R.; Noorani, I.; Pouget, P.; Smyrnis, N.; et al. An internationally standardised antisaccade protocol. Vis. Res. 2013, 84, 1–5. [Google Scholar] [CrossRef]
- Pelli, D.G. The VideoToolbox transforming software numbers for visual into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.C.; Abe, S. Is two-tailed testing for directional research hypotheses tests legitimate? J. Bus. Res. 2013, 66, 1261–1266. [Google Scholar] [CrossRef]
- Jones, L.V. Test of hypotheses: One-sided vs. two-sided alternatives. Psychol. Bull. 1952, 49, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Wagenmakers, E.J.; Love, J.; Marsman, M.; Jamil, T.; Ly, A.; Verhagen, J.; Selker, R.; Gronau, Q.F.; Dropmann, D.; Boutin, B.; et al. Bayesian inference for psychology. Part II: Example applications with JASP. Psychon. Bull. Rev. 2018, 25, 58–76. [Google Scholar] [CrossRef]
- Rouder, J.N.; Speckman, P.L.; Sun, D.; Morey, R.D.; Iverson, G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 2009, 16, 225–237. [Google Scholar] [CrossRef]
- Andraszewicz, S.; Scheibehenne, B.; Rieskamp, J.; Grasman, R.; Verhagen, J.; Wagenmakers, E.J. An Introduction to Bayesian Hypothesis Testing for Management Research. J. Manag. 2015, 41, 521–543. [Google Scholar] [CrossRef]
- Ro, T.; Henik, A.; Machado, L.; Rafal, R.D. Transcranial magnetic stimulation of the prefrontal cortex delays contralateral endogenous saccades. J. Cogn. Neurosci. 1997, 9, 433–440. [Google Scholar] [CrossRef]
- Machado, L.; Rafal, R.D. Control of fixation and saccades during an anti-saccade task: An investigation in humans with chronic lesions of oculomotor cortex. Exp. Brain Res. 2004, 156, 55–63. [Google Scholar] [CrossRef]
- Lane, A.R.; Smith, D.T.; Schenk, T.; Ellison, A. The involvement of posterior parietal cortex and frontal eye fields in spatially primed visual search. Brain Stimul. 2012, 5, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, H.; Hyde, J.; Hinder, M.R.; Kim, S.J.; McCormack, G.H.; Vickers, J.C.; Summers, J.J. Delayed plastic responses to anodal tDCS in older adults. Front. Aging Neurosci. 2014, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Tanaka, S.; Koyama, S.; De Santis, V.; Hirata, A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul. 2015, 8, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Utz, K.S.; Dimova, V.; Oppenlander, K.; Kerkhoff, G. Electrified minds: Transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—A review of current data and future implications. Neuropsychologia 2010, 48, 2789–2810. [Google Scholar] [CrossRef] [PubMed]
- van Koningsbruggen, M.G.; Pender, T.; Machado, L.; Rafal, R.D. Impaired control of the oculomotor reflexes in Parkinson’s disease. Neuropsychologia 2009, 47, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Rafal, R.D. Control of fixation and saccades in humans with chronic lesions of oculomotor cortex. Neuropsychology 2004, 18, 115–123. [Google Scholar] [CrossRef]
| Offline Experiment (n = 16) | Online Experiment (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|
| Active | Sham | Active | Sham | |||||
| Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | |
| Prosaccade RTs (ms) | 258 (73) | 270 (79) | 246 (49) | 258 (54) | 260 (59) | 248 (56) | 256 (59) | 246 (40) |
| Antisaccade RTs (ms) | 335 (46) | 337 (45) | 340 (53) | 344 (71) | 329 (53) | 317 (33) | 342 (53) | 337 (35) |
| Reflexive Errors (%) | 18.3 (14.8) | 16.4 (16.3) | 15.5 (13.4) | 16.1 (18.1) | 20.2 (19.7) | 23.0 (15.8) | 24.0 (25.0) | 30.3 (23.8) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.L.; Stenling, A.; Machado, L. Evidence Transcranial Direct Current Stimulation Can Improve Saccadic Eye Movement Control in Older Adults. Vision 2018, 2, 42. https://doi.org/10.3390/vision2040042
Chen PL, Stenling A, Machado L. Evidence Transcranial Direct Current Stimulation Can Improve Saccadic Eye Movement Control in Older Adults. Vision. 2018; 2(4):42. https://doi.org/10.3390/vision2040042
Chicago/Turabian StyleChen, Po Ling, Andreas Stenling, and Liana Machado. 2018. "Evidence Transcranial Direct Current Stimulation Can Improve Saccadic Eye Movement Control in Older Adults" Vision 2, no. 4: 42. https://doi.org/10.3390/vision2040042
APA StyleChen, P. L., Stenling, A., & Machado, L. (2018). Evidence Transcranial Direct Current Stimulation Can Improve Saccadic Eye Movement Control in Older Adults. Vision, 2(4), 42. https://doi.org/10.3390/vision2040042





