Optical Coherence Tomography Reveals Sigmoidal Crystalline Lens Changes during Accommodation
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koretz, J.F.; Cook, C.A.; Kaufman, P.L. Accommodation and presbyopia in the human eye. Changes in the anterior segment and crystalline lens with focus. Investig. Ophthalmol. Vis. Sci. 1997, 38, 569–578. [Google Scholar]
- Koretz, J.F.; Cook, C.A.; Kaufman, P.L. Aging of the human lens: Changes in lens shape upon accommodation and with accommodative loss. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2002, 19, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Rosales, P.; Dubbelman, M.; Marcos, S.; van der Heijde, R. Crystalline lens radii of curvature from Purkinje and Scheimpflug imaging. J. Vis. 2006, 6, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Garner, L.F.; Yap, M.K. Changes in ocular dimensions and refraction with accommodation. Ophthalmic Physiol. Opt. 1997, 17, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Kirschkamp, T.; Dunne, M.; Barry, J.C. Phakometric measurement of ocular surface radii of curvature, axial separations and alignment in relaxed and accommodated human eyes. Ophthalmic Physiol. Opt. 2004, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, K.J. The Glenn, A. Fry invited lecture. Accommodation to gratings and more naturalistic stimuli. Optom. Vis. Sci. 1991, 68, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Glasser, A.; Kaufman, P.L. The mechanism of accommodation in primates. Ophthalmology 1999, 106, 863–872. [Google Scholar] [CrossRef]
- Strenk, S.A.; Semmlow, J.L.; Strenk, L.M.; Munoz, P.; Gronlund-Jacob, J.; DeMarco, J.K. Age-related changes in human ciliary muscle and lens: A Magnetic Resonance Imaging study. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1162–1169. [Google Scholar]
- Richdale, K.; Sinnott, L.T.; Bullimore, M.A.; Wassenaar, P.A.; Schmalbrock, P.; Kao, C.Y.; Patz, S.; Mutti, D.O.; Glasser, A.; Zadnik, K. Quantification of age-related and per diopter accommodative changes of the lens and ciliary muscle in the emmetropic human eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Richdale, K.; Bullimore, M.A.; Sinnott, L.T.; Zadnik, K. The effect of age, accommodation, and refractive error on the adult human eye. Optom. Vis. Sci. 2016, 93, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kasthurirangan, S.; Markwell, E.L.; Atchison, D.A.; Pope, J.M. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J. Vis. 2011, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.E.; Atchison, D.A.; Pope, J.M. Changes in lens dimensions and refractive index with age and accommodation. Optom. Vis. Sci. 2007, 84, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Pope, J.M.; Verkicharla, P.K.; Suheimat, M.; Atchison, D.A. Change in human lens dimensions, lens refractive index distribution and ciliary body ring diameter with accommodation. Biomed. Opt. Express 2018, 9, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.L.; Evans, C.J.; Singh, K.D.; Wolffsohn, J.S.; Dunne, M.C.; Davies, L.N. Three-dimensional Magnetic Resonance Imaging of the phakic crystalline lens during accommodation. Investig. Ophthalmol. Vis. 2011, 52, 3689–3697. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Enriquez, E.; Perez-Merino, P.; Velasco-Ocana, M.; Marcos, S. OCT-based full crystalline lens shape change during accommodation in vivo. Biomed. Opt. Express 2017, 8, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Shum, P.J.; Ko, L.S.; Ng, C.L.; Lin, S.L. A biometric study of ocular changes during accommodation. Am. J. Ophthalmol. 1993, 115, 76–81. [Google Scholar] [CrossRef]
- Drexler, W.; Baumgartner, A.; Findl, O.; Hitzenberger, C.K.; Fercher, A.F. Biometric investigation of changes in the anterior eye segment during accommodation. Vis. Res. 1997, 37, 2789–2800. [Google Scholar] [CrossRef]
- Dubbelman, M.; van der Heijde, G.L.; Weeber, H.A.; Vrensen, G.F. Changes in the internal structure of the human crystalline lens with age and accommodation. Vis. Res. 2003, 43, 2363–2375. [Google Scholar] [CrossRef]
- Dubbelman, M.; van der Heijde, G.L.; Weeber, H.A. Change in shape of the aging human crystalline lens with accommodation. Vis. Res. 2005, 45, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Ostrin, L.; Kasthurirangan, S.; Win-Hall, D.; Glasser, A. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom. Vis. Sci. 2006, 83, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Tsorbatzoglou, A.; Nemeth, G.; Szell, N.; Biro, Z.; Berta, A. Anterior segment changes with age and during accommodation measured with Partial Coherence Interferometry. J. Cataract. Refract. Surg. 2007, 33, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Collins, M.J.; Woodman, E.C.; Cheong, S.H. Axial length changes during accommodation in myopes and emmetropes. Optom. Vis. Sci. 2010, 87, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Strenk, S.A.; Strenk, L.M.; Semmlow, J.L.; DeMarco, J.K. Magnetic Resonance Imaging study of the effects of age and accommodation on the human lens cross-sectional area. Investig. Ophthalmol. Vis. Sci. 2004, 45, 539–545. [Google Scholar] [CrossRef]
- Strenk, S.A.; Strenk, L.M.; Koretz, J.F. The mechanism of presbyopia. Prog. Retin. Eye. Res. 2005, 24, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.; Dubbelman, M.; van der Heijde, R.; Heethaar, R. The shape of the human lens nucleus with accommodation. J. Vis. 2007, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Richdale, K.; Bullimore, M.A.; Zadnik, K. Lens thickness with age and accommodation by Optical Coherence Tomography. Ophthalmic Physiol. Opt. 2008, 28, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Little, J.A.; Saunders, K.J. Repeatability of OCT lens thickness measures with age and accommodation. Optom. Vis. Sci. 2013, 90, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.N.; Dunne, M.C.; Gibson, G.A.; Wolffsohn, J.S. Vergence analysis reveals the influence of axial distances on accommodation with age and axial ametropia. Ophthalmic Physiol. Opt. 2010, 30, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Vilupuru, A.S.; Glasser, A. The relationship between refractive and biometric changes during Edinger-Westphal stimulated accommodation in Rhesus monkeys. Exp. Eye Res. 2005, 80, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.K.; Rabie, E.P. Ultrasound-A research tool in the study of accommodation. Ophthalmic Physiol. Opt. 1983, 3, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.; Mitchell, B. Ultrasound measures of vitreous chamber depth during ocular accommodation. Am. J. Optom. Physiol. Opt. 1985, 62, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Koretz, J.F.; Bertasso, A.M.; Neider, M.W.; True-Gabelt, B.A.; Kaufman, P.L. Slit-lamp studies of the Rhesus monkey eye: II. Changes in crystalline lens shape, thickness and position during accommodation and aging. Exp. Eye Res. 1987, 45, 317–326. [Google Scholar] [CrossRef]
- Wendt, M.; Croft, M.A.; McDonald, J.; Kaufman, P.L.; Glasser, A. Lens diameter and thickness as a function of age and pharmacologically stimulated accommodation in rhesus monkeys. Exp. Eye Res. 2008, 86, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijde, G.L.; Weber, J. Accommodation used to determine ultrasound velocity in the human lens. Optom. Vis. Sci. 1989, 66, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijde, G.L.; Beers, A.P.; Dubbelman, M. Microfluctuations of steady-state accommodation measured with Ultrasonography. Ophthalmic Physiol. Opt. 1996, 16, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Beers, A.P.; van der Heijde, G.L. In vivo determination of the biomechanical properties of the component elements of the accommodation mechanism. Vis. Res. 1994, 34, 2897–2905. [Google Scholar] [CrossRef]
- Beers, A.P.; van der Heijde, G.L. Presbyopia and velocity of sound in the lens. Optom. Vis. Sci. 1994, 71, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Beers, A.P.; van der Heijde, G.L. Age-related changes in the accommodation mechanism. Optom. Vis. Sci. 1996, 73, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.A.; McDonald, J.P.; Nadkarni, N.V.; Lin, T.L.; Kaufman, P.L. Age-related changes in centripetal ciliary body movement relative to centripetal lens movement in monkeys. Exp. Eye Res. 2009, 89, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.A.; Nork, T.M.; McDonald, J.P.; Katz, A.; Lutjen-Drecoll, E.; Kaufman, P.L. Accommodative movements of the vitreous membrane, choroid, and sclera in young and presbyopic human and nonhuman primate eyes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5049–5058. [Google Scholar] [CrossRef] [PubMed]
- Croft, M.A.; Heatley, G.; McDonald, J.P.; Katz, A.; Kaufman, P.L. Accommodative movements of the lens/capsule and the strand that extends between the posterior vitreous zonule insertion zone & the lens equator, in relation to the vitreous face and aging. Ophthalmic Physiol. Opt. 2016, 36, 21–32. [Google Scholar] [PubMed]
- He, L.; Wendt, M.; Glasser, A. Pharmacologically and Edinger-Westphal stimulated accommodation in Rhesus monkeys does not rely on changes in anterior chamber pressure. Exp. Eye Res. 2014, 125, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Lutjen-Drecoll, E.; Kaufman, P.L.; Wasielewski, R.; Ting-Li, l.; Croft, M.A. Morphology and accommodative function of the vitreous zonule in human and monkey eyes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Brown, N. The change in shape and internal form of the lens of the eye on accommodation. Exp. Eye Res. 1973, 15, 441–459. [Google Scholar] [CrossRef]
- Dubbelman, M.; van der Heijde, G.L. The shape of the aging human lens: Curvature, equivalent refractive index and the lens paradox. Vis. Res. 2001, 41, 1867–1877. [Google Scholar] [CrossRef]
- Dubbelman, M.; van der Heijde, G.L.; Weeber, H.A. The thickness of the aging human lens obtained from corrected Scheimpflug images. Optom. Vis. Sci. 2001, 78, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dubbelman, M.; Weeber, H.A.; van der Heijde, R.G.; Volker-Dieben, H.J. Radius and asphericity of the posterior corneal surface determined by corrected Scheimpflug photography. Acta Ophthalmol. Scand. 2002, 80, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Mallen, E.A.; Kashyap, P.; Hampson, K.M. Transient axial length change during the accommodation response in young adults. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Baikoff, G.; Lutun, E.; Ferraz, C.; Wei, J. Static and dynamic analysis of the anterior segment with Optical Coherence Tomography. J. Cataract. Refract. Surg. 2004, 30, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Baikoff, G.; Lutun, E.; Ferraz, C.; Wei, J. Analysis of the eye’s anterior segment with Optical Coherence Tomography. Static and dynamic study. J. Fr. Ophtalmol. 2005, 28, 343–352. [Google Scholar] [CrossRef]
- Baikoff, G.; Lutun, E.; Wei, J.; Ferraz, C. An in vivo OCT study of human natural accommodation in a 19-year-old albino. J. Fr. Ophtalmol. 2005, 28, 514–519. [Google Scholar] [CrossRef]
- Goldsmith, J.A.; Li, Y.; Chalita, M.R.; Westphal, V.; Patil, C.A.; Rollins, A.M.; Izatt, J.A.; Huang, D. Anterior chamber width measurement by high-speed Optical Coherence Tomography. Ophthalmology 2005, 112, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, R.; Teo, L.; Friedman, D.S.; Aung, H.T.; Baskaran, M.; Gao, H.; Alfred, T.; Seah, S.K.; Kashiwagi, K.; Foster, P.J.; et al. Comparison of anterior chamber depth measurements using the IOLMaster, scanning peripheral anterior chamber depth analyser, and anterior segment Optical Coherence Tomography. Br. J. Ophthalmol. 2007, 91, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, G.; Vajas, A.; Tsorbatzoglou, A.; Kolozsvari, B.; Modis, L., Jr.; Berta, A. Assessment and reproducibility of anterior chamber depth measurement with anterior segment Optical Coherence Tomography compared with immersion Ultrasonography. J. Cataract. Refract. Surg. 2007, 33, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.K.; Li, H.; Weinreb, R.N.; Liu, J.; Cheung, C.Y.; Lai, R.Y.; Pang, C.P.; Lam, D.S. Anterior chamber angle measurement with anterior segment Optical Coherence Tomography: A comparison between slit lamp OCT and Visante OCT. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3469–3474. [Google Scholar] [CrossRef] [PubMed]
- Dawczynski, J.; Koenigsdoerffer, E.; Augsten, R.; Strobel, J. Anterior segment Optical Coherence Tomography for evaluation of changes in anterior chamber angle and depth after intraocular lens implantation in eyes with glaucoma. Eur. J. Ophthalmol. 2007, 17, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.P.; Cottet, L.; Dosso, A.A. Evaluation of the anterior chamber depth after cataract surgery with OCT Visante. Klin. Monbl. Augenheilkd. 2008, 225, 438–440. [Google Scholar]
- Baikoff, G. Anterior segment OCT and phakic intraocular lenses: A perspective. J. Cataract. Refract. Surg. 2006, 32, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Koivula, A.; Kugelberg, M. Optical Coherence Tomography of the anterior segment in eyes with phakic refractive lenses. Ophthalmology 2007, 114, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Guell, J.L.; Morral, M.; Gris, O.; Gaytan, J.; Sisquella, M.; Manero, F. Evaluation of Verisyse and Artiflex phakic intraocular lenses during accommodation using Visante optical coherence tomography. J. Cataract. Refract. Surg. 2007, 33, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Kaiserman, I.; Bahar, I.; Rootman, D.S. Corneal wound malapposition after penetrating keratoplasty: An optical coherence tomography study. Br. J. Ophthalmol. 2008, 92, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, A.; Hossain, P.; Anderson, D.F. Recent advances in ophthalmic anterior segment imaging: A new era for ophthalmic diagnosis? Br. J. Ophthalmol. 2007, 91, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Rao, S.K.; Lau, S.; Leung, C.K.; Lam, D.S. Central corneal thickness measurements by Ultrasound, Orbscan II, and Visante OCT after LASIK for myopia. J. Refract. Surg. 2008, 24, 361–365. [Google Scholar] [PubMed]
- Bolz, M.; Prinz, A.; Drexler, W.; Findl, O. Linear relationship of refractive and biometric lenticular changes during accommodation in emmetropic and myopic eyes. Br. J. Ophthalmol. 2007, 91, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S. The Dubbelman Eye Model analysed by ray tracing through aspheric surfaces. Ophthalmic Physiol. Opt. 2005, 25, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.N.; Mallen, E.A.; Wolffsohn, J.S.; Gilmartin, B. Clinical evaluation of the Shin-Nippon Nvision-K 5001/Grand Seiko WR-5100k autorefractor. Optom. Vis. Sci. 2003, 80, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Stark, L.R.; Atchison, D.A. Subject instructions and methods of target presentation in accommodation research. Investig. Ophthalmol. Vis. Sci. 1994, 35, 528–537. [Google Scholar]
- Francis, E.L.; Jiang, B.C.; Owens, D.A.; Tyrrell, R.A. Accommodation and vergence require effort-to-see. Optom. Vis. Sci. 2003, 80, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.D.; Sinnott, L.T.; Mutti, D.O. Ciliary body thickness and refractive error in children. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4353–4360. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R. A.; Eperjesi, F.; Gilmartin, B. The application of analysis of variance (ANOVA) to different experimental designs in optometry. Ophthalmic Physiol. Opt. 2002, 22, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.C.; Davies, L. N.; Wolffsohn, J.S. Accuracy of cornea and lens biometry using anterior segment optical coherence tomography. J. Biomed. Opt. 2007, 12. [Google Scholar] [CrossRef] [PubMed]
- Sebag, J. Anatomy and pathology of the vitreo-retinal interface. Eye 1992, 6, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Buehren, T.; Collins, M.J. Influence of accommodation on the anterior and posterior cornea. J. Cataract. Refract. Surg. 2007, 33, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Bayramlar, H.; Sadigov, F.; Yildirim, A. Effect of accommodation on corneal topography. Cornea 2013, 32, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.; Pérez-Merino, P.; Gambra, E.; de Castro, A.; Marcos, S. In vivo human crystalline lens topography. Biomed. Opt. Express 2012, 3, 2471–2488. [Google Scholar] [CrossRef] [PubMed]
- Garner, L.F.; Smith, G. Changes in equivalent and gradient refractive index of the crystalline lens with accommodation. Optom. Vis. Sci. 1997, 74, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Glasser, A.; Campbell, M.C. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vis. Res. 1999, 39, 1991–2015. [Google Scholar] [CrossRef]
- Borja, D.; Siedlecki, D.; de Castro, A.; Uhlhorn, S.; Ortiz, S.; Arrieta, E.; Parel, J.M.; Marcos, S.; Manns, F. Distortions of the posterior surface in optical coherence tomography images of the isolated crystalline lens: Effect of the lens index gradient. Biomed. Opt. Express 2010, 1, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, D.; de Castro, A.; Gambra, E.; Ortiz, S.; Borja, D.; Uhlhorn, S.; Manns, F.; Marcos, S.; Parel, J.M. Distortion correction of OCT images of the crystalline lens: Gradient index approach. Optom. Vis. Sci. 2012, 89, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Uhlhorn, S.R.; Borja, D.; Manns, F.; Parel, J.M. Refractive index measurement of the isolated crystalline lens using optical coherence tomography. Vis. Res. 2008, 27, 2732–2738. [Google Scholar] [CrossRef] [PubMed]
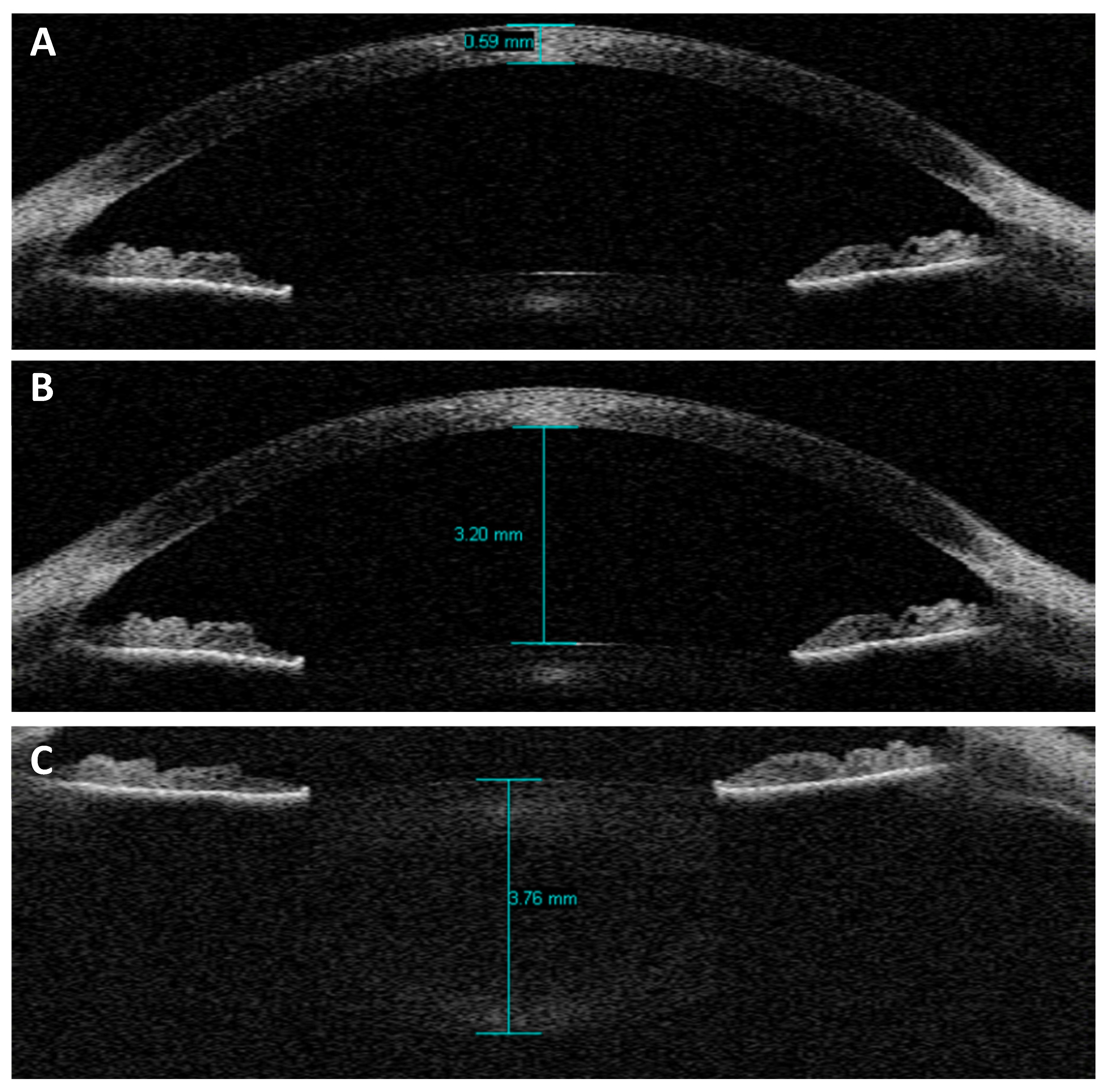
| Component | Parameter |
|---|---|
| Cornea | |
| Anterior radius (mm) | 7.87 |
| Thickness (mm) | 0.574 |
| Refractive index | 1.376 |
| Posterior radius (mm) | 6.40 |
| Anterior Chamber | |
| Depth (mm) | 3.87 − 0.010A − D (0.048 − 0.0004A) |
| Refractive index | 1.336 |
| Crystalline Lens | |
| Anterior radius (mm) | 1/[1/(12.7 − 0.058A) + 0.0077D] |
| Thickness (mm) | 2.93 + 0.0236A + D (0.058 − 0.0005A) |
| Refractive index | 1.441 − 0.00039A + 0.0013D |
| Posterior radius (mm) | 1/[1/(5.9 − 0.0013A) + 0.0043D] |
| Vitreous | |
| Depth (mm) | Variable (see text) |
| Refractive index | 1.376 |
| Accommodation Demand (D) | Dubbelman Model (mm) | Dubbelman Model (mm) Adjusted for CT | AS-OCT (mm ± SD) | AS-OCT (mm ± SD) Adjusted for CT |
|---|---|---|---|---|
| Corneal Thickness (CT) | ||||
| 0 | 0.574 | - | 0.551 ± 0.030 | - |
| 1 | 0.574 | - | 0.552 ± 0.033 | - |
| 2 | 0.574 | - | 0.553 ± 0.029 | - |
| 3 | 0.574 | - | 0.554 ± 0.029 | - |
| 4 | 0.574 | - | 0.552 ± 0.033 | - |
| Anterior Chamber Depth (ACD) | ||||
| 0 | 3.096 | 3.670 | 3.102 ± 0.280 | 3.653 ± 0.277 |
| 1 | 3.056 | 3.630 | 3.066 ± 0.287 | 3.618 ± 0.285 |
| 2 | 3.016 | 3.590 | 3.021 ± 0.287 | 3.574 ± 0.286 |
| 3 | 2.976 | 3.550 | 2.970 ± 0.283 | 3.524 ± 0.278 |
| 4 | 2.936 | 3.510 | 2.928 ± 0.282 | 3.480 ± 0.280 |
| Lens Thickness (LT) | ||||
| 0 | 3.402 | - | 3.632 ± 0.205 | - |
| 1 | 3.450 | - | 3.669 ± 0.200 | - |
| 2 | 3.498 | - | 3.719 ± 0.210 | - |
| 3 | 3.546 | - | 3.802 ± 0.226 | - |
| 4 | 3.594 | - | 3.867 ± 0.219 | - |
| Lens Centroid (ACD + LT/2) | ||||
| 0 | 4.797 | 5.371 | 4.918 ± 0.235 | 5.469 ± 0.232 |
| 1 | 4.781 | 5.355 | 4.900 ± 0.235 | 5.452 ± 0.233 |
| 2 | 4.765 | 5.339 | 4.881 ± 0.239 | 5.434 ± 0.237 |
| 3 | 4.749 | 5.323 | 4.871 ± 0.232 | 5.425 ± 0.229 |
| 4 | 4.733 | 5.307 | 4.861 ± 0.228 | 5.413 ± 0.226 |
| Anterior segment Length (ACD + LT) | ||||
| 0 | 6.498 | 7.072 | 6.734 ± 0.230 | 7.285 ± 0.229 |
| 1 | 6.506 | 7.080 | 6.735 ± 0.220 | 7.287 ± 0.218 |
| 2 | 6.514 | 7.088 | 6.740 ± 0.231 | 7.294 ± 0.230 |
| 3 | 6.522 | 7.096 | 6.772 ± 0.232 | 7.326 ± 0.231 |
| 4 | 6.530 | 7.104 | 6.795 ± 0.221 | 7.347 ± 0.218 |
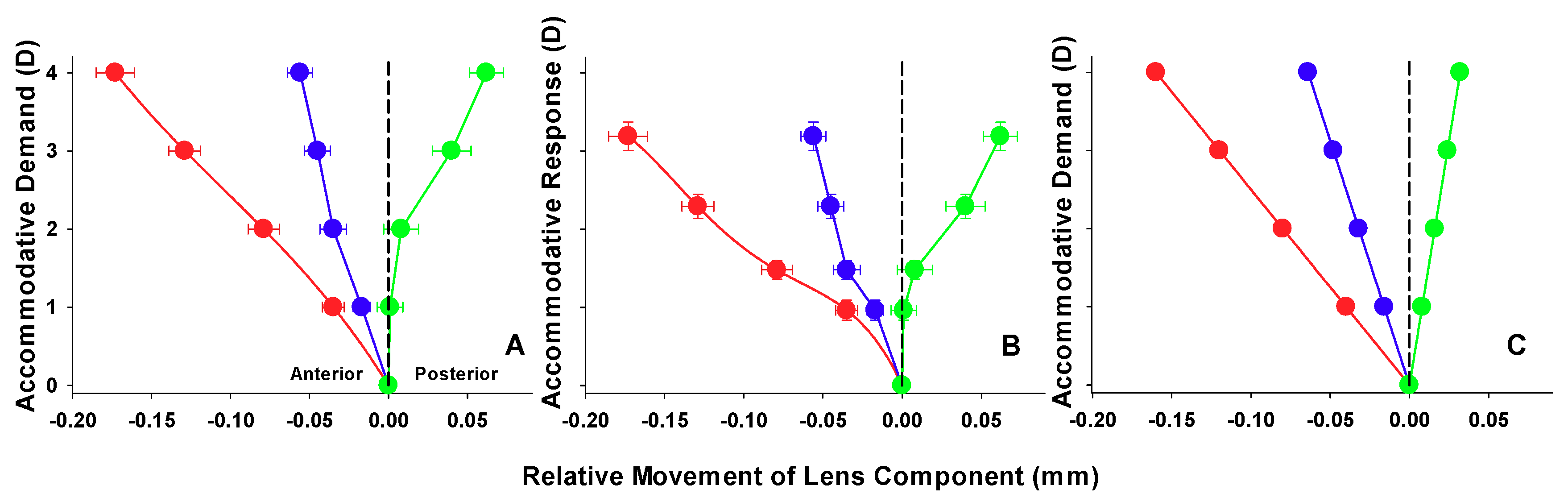
| Accommodation Demand (D) | Dubbelman Model (mm) | AS-OCT (mm ± SD) |
|---|---|---|
| Anterior chamber depth (ACD) | ||
| 1 | −0.040 | −0.035 ± 0.038 |
| 2 | −0.080 | −0.079 ± 0.054 |
| 3 | −0.120 | −0.129 ± 0.055 |
| 4 | −0.160 | −0.173 ± 0.067 |
| Lens Thickness (LT) | ||
| 1 | 0.048 | 0.037 ± 0.059 |
| 2 | 0.096 | 0.087 ± 0.070 |
| 3 | 0.144 | 0.170 ± 0.083 |
| 4 | 0.192 | 0.235 ± 0.091 |
| Lens Centroid (ACD + LT/2) | ||
| 1 | −0.016 | −0.017 ± 0.029 |
| 2 | −0.032 | −0.035 ± 0.046 |
| 3 | −0.048 | −0.045 ± 0.045 |
| 4 | −0.064 | −0.056 ± 0.043 |
| Anterior segment Length (ACD + LT) | ||
| 1 | 0.008 | 0.001 ± 0.044 |
| 2 | 0.016 | 0.008 ± 0.061 |
| 3 | 0.024 | 0.040 ± 0.067 |
| 4 | 0.032 | 0.062 ± 0.059 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibson, G.A.; Cruickshank, F.E.; Wolffsohn, J.S.; Davies, L.N. Optical Coherence Tomography Reveals Sigmoidal Crystalline Lens Changes during Accommodation. Vision 2018, 2, 33. https://doi.org/10.3390/vision2030033
Gibson GA, Cruickshank FE, Wolffsohn JS, Davies LN. Optical Coherence Tomography Reveals Sigmoidal Crystalline Lens Changes during Accommodation. Vision. 2018; 2(3):33. https://doi.org/10.3390/vision2030033
Chicago/Turabian StyleGibson, George A., Fiona E. Cruickshank, James S. Wolffsohn, and Leon N. Davies. 2018. "Optical Coherence Tomography Reveals Sigmoidal Crystalline Lens Changes during Accommodation" Vision 2, no. 3: 33. https://doi.org/10.3390/vision2030033
APA StyleGibson, G. A., Cruickshank, F. E., Wolffsohn, J. S., & Davies, L. N. (2018). Optical Coherence Tomography Reveals Sigmoidal Crystalline Lens Changes during Accommodation. Vision, 2(3), 33. https://doi.org/10.3390/vision2030033






