Effects of Explosive vs. Strength Resistance Training on Plantar Flexor Neuromuscular and Functional Capacities in Institutionalized Older Adults: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Recruitment
- -
- They were aged 65 years or older;
- -
- They could walk independently without technical assistance (e.g., canes, walkers) or help from another person;
- -
- They were able to communicate verbally effectively with the research team.
- -
- They had neurological or cognitive disorders.
- -
- They suffered severe cardiovascular diseases.
- -
- They experienced significant musculoskeletal issues in the lower limbs.
- -
- They were taking medications that could affect the neuromuscular or functional tests.
2.3. Evaluation Protocol
2.3.1. Anthropometric Parameters
2.3.2. Gait Speed
2.3.3. Neuromuscular Parameters
2.4. Resistance Training Programs
2.5. Statistical Analysis
3. Results
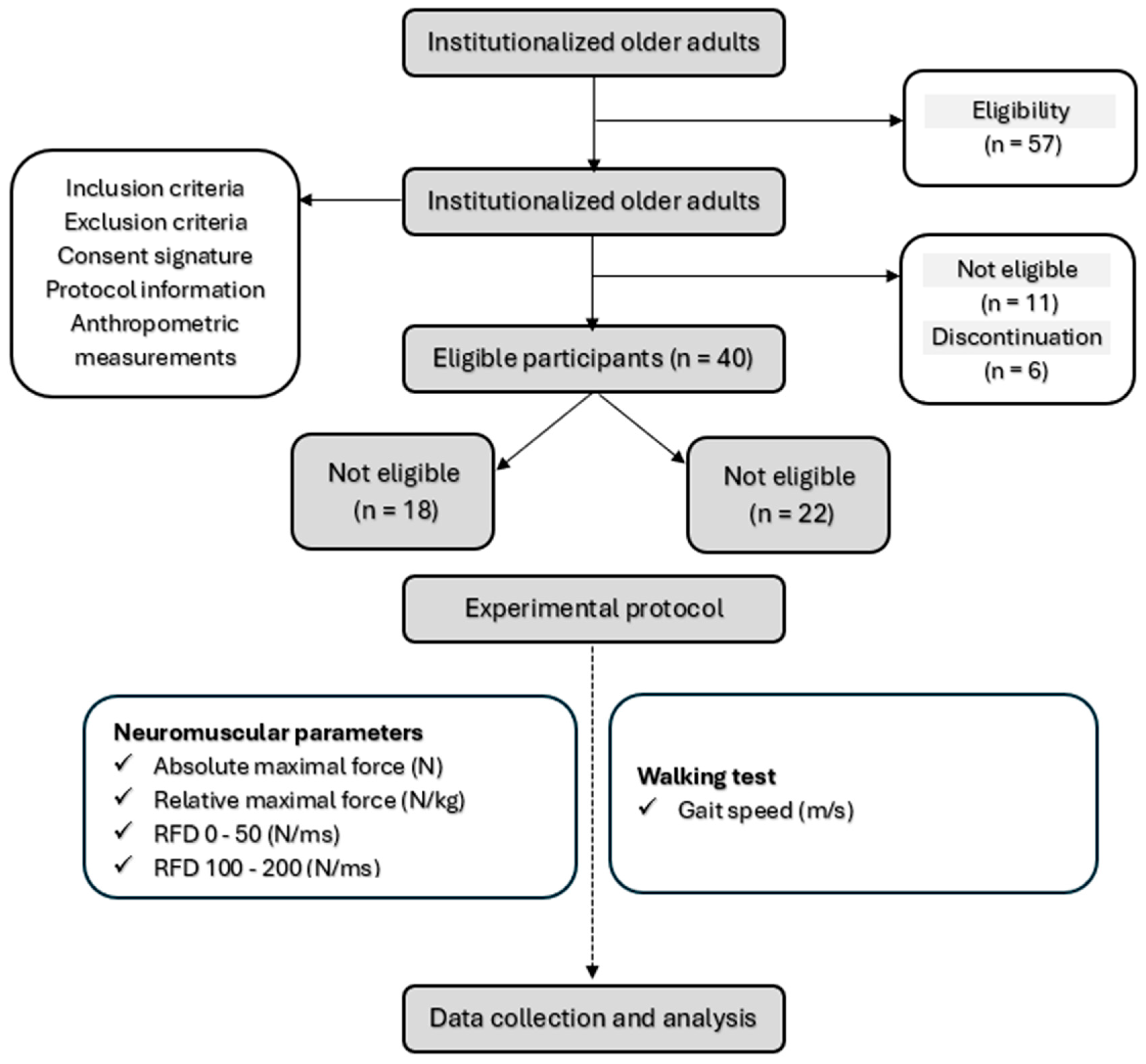
3.1. Participants
3.2. Training Programs
3.3. Anthropometric Parameters
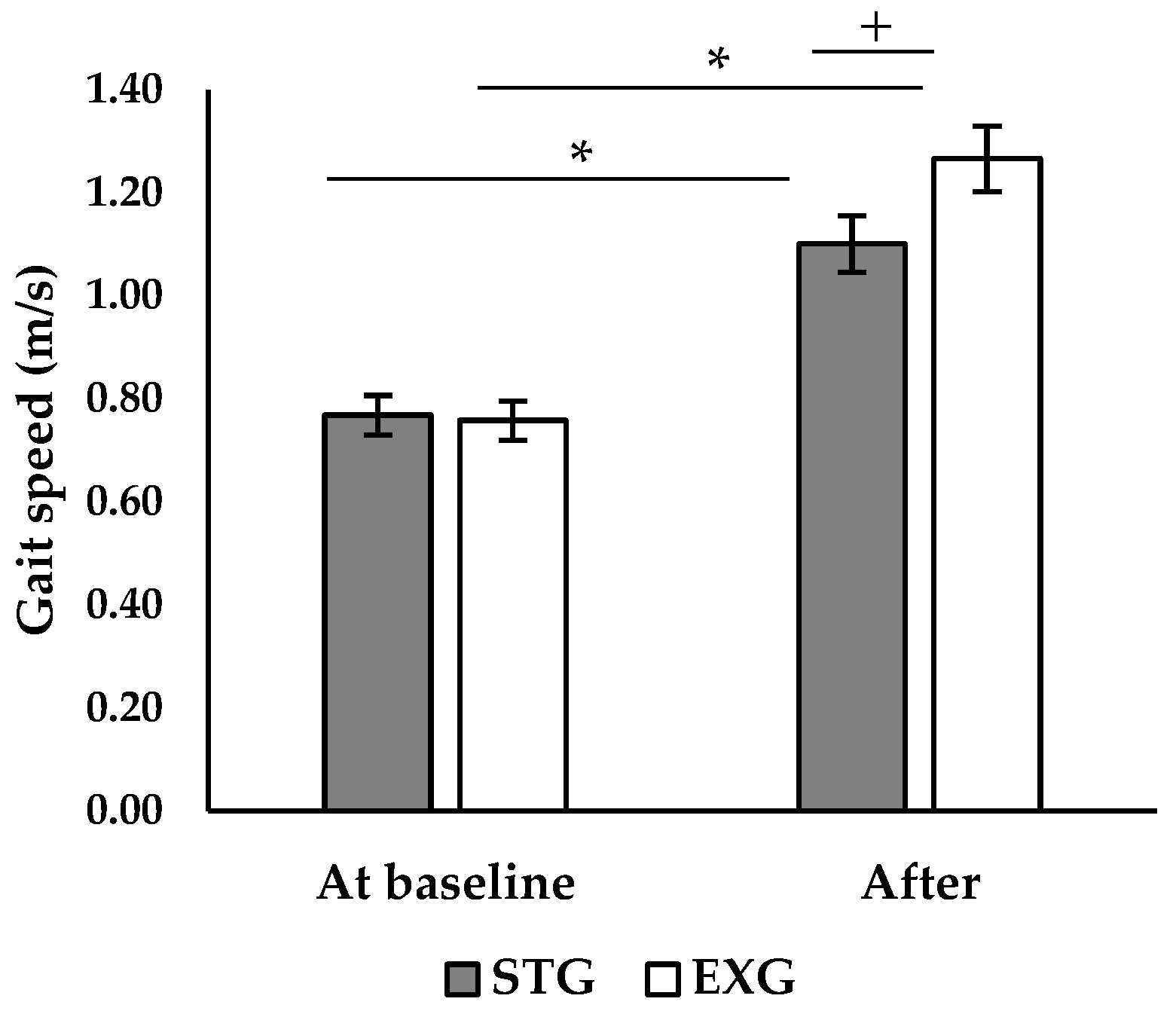
3.4. Walking Speed
3.5. Neuromuscular Parameters
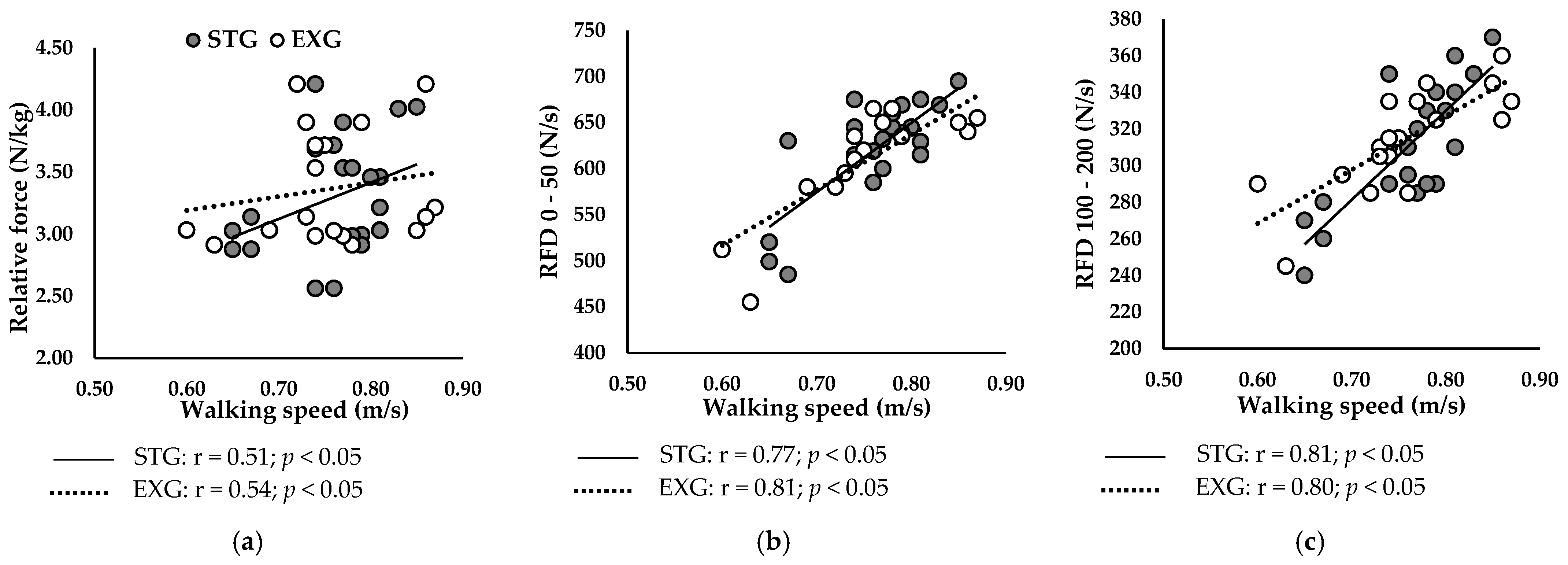
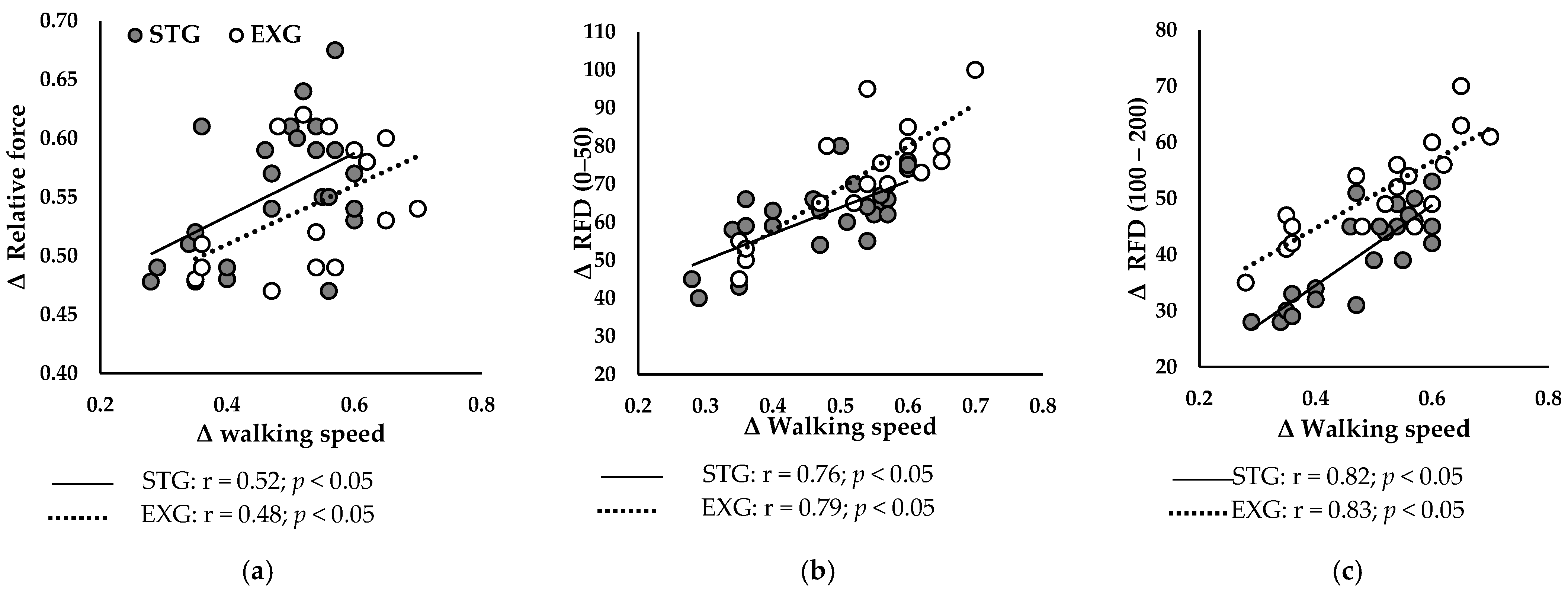
3.6. Relationship Between Neuromuscular Parameters of the PF and Walking Speed
3.6.1. At Baseline
3.6.2. After Training Programs
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-Mediated Reinnervation of Skeletal Muscle in Elderly People: An Update. Eur. J. Transl. Myol. 2022, 32, 10416. [Google Scholar] [CrossRef] [PubMed]
- Bellumori, M.; Jaric, S.; Knight, C.A. Age-Related Decline in the Rate of Force Development Scaling Factor. Motor Control 2013, 17, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J.; Ryan, E.D.; Herda, T.J.; Costa, P.B.; Herda, A.A.; Cramer, J.T. Age-Related Changes in the Rate of Muscle Activation and Rapid Force Characteristics. Age 2014, 36, 839–849. [Google Scholar] [CrossRef]
- Cattagni, T.; Scaglioni, G.; Laroche, D.; Van Hoecke, J.; Gremeaux, V.; Martin, A. Ankle Muscle Strength Discriminates Fallers from Non-Fallers. Front. Aging Neurosci. 2014, 6, 336. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, R.C.D.; Pereira, G.; Beck, J.K.; Lodovico, A.; Rodacki, A.L.F. Multicomponent Training Program with High-Speed Movement Execution of Ankle Muscles Reduces Risk of Falls in Older Adults. Rejuvenation Res. 2019, 22, 43–50. [Google Scholar] [CrossRef]
- Hester, G.M.; Ha, P.L.; Dalton, B.E.; VanDusseldorp, T.A.; Olmos, A.A.; Stratton, M.T.; Bailly, A.R.; Vroman, T.M. Rate of Force Development as a Predictor of Mobility in Community-Dwelling Older Adults. J. Geriatr. Phys. Ther. 2021, 44, 74–81. [Google Scholar] [CrossRef]
- Olmos, A.A.; Stratton, M.T.; Ha, P.L.; Dalton, B.E.; VanDusseldorp, T.A.; Mangine, G.T.; Feito, Y.; Poisal, M.J.; Jones, J.A.; Smith, T.M.; et al. Early and Late Rapid Torque Characteristics and Select Physiological Correlates in Middle-Aged and Older Males. PLoS ONE 2020, 15, e0231907. [Google Scholar] [CrossRef]
- Gerstner, G.R.; Thompson, B.J.; Rosenberg, J.G.; Sobolewski, E.J.; Scharville, M.J.; Ryan, E.D. Neural and Muscular Contributions to the Age-Related Reductions in Rapid Strength. Med. Sci. Sports Exerc. 2017, 49, 1331–1339. [Google Scholar] [CrossRef]
- Klass, M.; Baudry, S.; Duchateau, J. Age-Related Decline in Rate of Torque Development Is Accompanied by Lower Maximal Motor Unit Discharge Frequency during Fast Contractions. J. Appl. Physiol. 2008, 104, 739–746. [Google Scholar] [CrossRef]
- Andersen, L.L.; Aagaard, P. Influence of Maximal Muscle Strength and Intrinsic Muscle Contractile Properties on Contractile Rate of Force Development. Eur. J. Appl. Physiol. 2006, 96, 46–52. [Google Scholar] [CrossRef]
- Clark, D.J.; Manini, T.M.; Fielding, R.A.; Patten, C. Neuromuscular Determinants of Maximum Walking Speed in Well-Functioning Older Adults. Exp. Gerontol. 2013, 48, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Hughes, L.; Lewis, H. Walking Speed and Peak Plantar Pressure Distribution during Barefoot Walking in Persons with Diabetes. Physiother. Res. Int. 2012, 17, 29–35. [Google Scholar] [CrossRef]
- Holmes, S.J.; Mudge, A.J.; Wojciechowski, E.A.; Axt, M.W.; Burns, J. Impact of Multilevel Joint Contractures of the Hips, Knees and Ankles on the Gait Profile Score in Children with Cerebral Palsy. Clin. Biomech. 2018, 59, 8–14. [Google Scholar] [CrossRef]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodriguez-Mañas, L.; Izquierdo, M. Multicomponent Exercises Including Muscle Power Training Enhance Muscle Mass, Power Output, and Functional Outcomes in Institutionalized Frail Nonagenarians. Age 2014, 36, 773–785. [Google Scholar] [CrossRef]
- Tavoian, D.; Clark, B.C.; Clark, L.A.; Wages, N.P.; Russ, D.W. Comparison of Strategies for Assessment of Rate of Torque Development in Older and Younger Adults. Eur. J. Appl. Physiol. 2024, 124, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Foster-Burns, S.B. Sarcopenia and Decreased Muscle Strength in the Elderly Woman: Resistance Training as a Safe and Effective Intervention. J. Women Aging 1999, 11, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Peltonen, H.; Häkkinen, K. Medium-Intensity, High-Volume “Hypertrophic” Resistance Training Did Not Induce Improvements in Rapid Force Production in Healthy Older Men. Age 2015, 37, 41. [Google Scholar] [CrossRef]
- Cadore, E.L.; Moneo, A.B.B.; Mensat, M.M.; Muñoz, A.R.; Casas-Herrero, A.; Rodriguez-Mañas, L.; Izquierdo, M. Positive Effects of Resistance Training in Frail Elderly Patients with Dementia after Long-Term Physical Restraint. Age 2014, 36, 801–811. [Google Scholar] [CrossRef]
- Cadore, E.L.; Pinto, R.S.; Reischak-Oliveira, Á.; Izquierdo, M. Explosive Type of Contractions Should Not Be Avoided during Resistance Training in Elderly. Exp. Gerontol. 2018, 102, 81–83. [Google Scholar] [CrossRef]
- Magtouf, E.; Chortane, S.G.; Chortane, O.G.; Boyas, S.; Beaune, B.; Durand, S.; Maktouf, W. Influence of Concurrent Exercise Training on Ankle Muscle Activation during Static and Proactive Postural Control on Older Adults with Sarcopenic Obesity: A Multicenter, Randomized, and Controlled Trial. Eur. J. Investig. Health Psychol. Educ. 2023, 13, 2779–2794. [Google Scholar] [CrossRef]
- Ferhi, H.; Chortane, S.G.; Durand, S.; Beaune, B.; Boyas, S.; Maktouf, W. Effects of Physical Activity Program on Body Composition, Physical Performance, and Neuromuscular Strategies during Walking in Older Adults with Sarcopenic Obesity: Randomized Controlled Trial. Healthcare 2023, 11, 2294. [Google Scholar] [CrossRef] [PubMed]
- Maktouf, W.; Durand, S.; Boyas, S.; Pouliquen, C.; Beaune, B. Combined Effects of Aging and Obesity on Postural Control, Muscle Activity and Maximal Voluntary Force of Muscles Mobilizing Ankle Joint. J. Biomech. 2018, 79, 198–206. [Google Scholar] [CrossRef]
- Henwood, T.R.; Riek, S.; Taaffe, D.R. Strength versus Muscle Power-Specific Resistance Training in Community-Dwelling Older Adults. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2008, 63, 83–91. [Google Scholar] [CrossRef]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose–Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef]
- Pruitt, L.A.; Taaffe, D.R.; Marcus, R. Effects of a One-Year High-Intensity versus Low-Intensity Resistance Training Program on Bone Mineral Density in Older Women. J. Bone Miner. Res. 1995, 10, 1788–1795. [Google Scholar] [CrossRef]
- Ramírez-Campillo, R.; Castillo, A.; de la Fuente, C.I.; Campos-Jara, C.; Andrade, D.C.; Álvarez, C.; Martínez, C.; Castro-Sepúlveda, M.; Pereira, A.; Marques, M.C.; et al. High-Speed Resistance Training Is More Effective than Low-Speed Resistance Training to Increase Functional Capacity and Muscle Performance in Older Women. Exp. Gerontol. 2014, 58, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Stylianides, G.; Djaoui, L.; Dellal, A.; Chamari, K. Session-RPE Method for Training Load Monitoring: Validity, Ecological Usefulness, and Influencing Factors. Front. Neurosci. 2017, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Foster, C. Monitoring Training in Athletes with Reference to Overtraining Syndrome. Med. Sci. Sports Exerc. 1998, 30, 1164–1168. [Google Scholar] [CrossRef]
- Pereira, A.; Izquierdo, M.; Silva, A.J.; Costa, A.M.; González-Badillo, J.J.; Marques, M.C. Muscle performance and functional capacity retention in older women after high-speed power training cessation. Exp. Gerontol. 2012, 47, 620–624. [Google Scholar] [CrossRef]
- Pereira, A.; Izquierdo, M.; Silva, A.J.; Costa, A.M.; Bastos, E.; González-Badillo, J.J.; Marques, M.C. Effects of High-Speed Power Training on Functional Capacity and Muscle Performance in Older Women. Exp. Gerontol. 2012, 47, 250–255. [Google Scholar] [CrossRef]
- Maktouf, W.; Durand, S.; Beaune, B.; Boyas, S. Influence of Obesity and Impact of a Physical Activity Program on Postural Control and Functional and Physical Capacities in Institutionalized Older Adults: A Pilot Study. J. Phys. Act. Health 2019, 17, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kryger, A.I.; Andersen, J.L. Resistance Training in the Oldest Old: Consequences for Muscle Strength, Fiber Types, Fiber Size, and MHC Isoforms. Scand. J. Med. Sci. Sports 2007, 17, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Pinto, R.S.; Radaelli, R.; Rech, A.; Grazioli, R.; Izquierdo, M.; Cadore, E.L. Benefits of Resistance Training in Physically Frail Elderly: A Systematic Review. Aging Clin. Exp. Res. 2018, 30, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.; Caldow, M.K.; Watts, R.; Levinger, P.; Cameron-Smith, D.; Levinger, I. Age and Sex Differences in Human Skeletal Muscle Fibrosis Markers and Transforming Growth Factor-β Signaling. Eur. J. Appl. Physiol. 2017, 117, 1463–1472. [Google Scholar] [CrossRef]
- Han, X.; Møller, L.L.V.; De Groote, E.; Bojsen-Møller, K.N.; Davey, J.; Henríquez-Olguin, C.; Li, Z.; Knudsen, J.R.; Jensen, T.E.; Madsbad, S.; et al. Mechanisms Involved in Follistatin-induced Hypertrophy and Increased Insulin Action in Skeletal Muscle. J. Cachexia Sarcopenia Muscle 2019, 10, 1241–1257. [Google Scholar] [CrossRef]
- Seo, M.W.; Jung, S.W.; Kim, S.W.; Lee, J.M.; Jung, H.C.; Song, J.K. Effects of 16 Weeks of Resistance Training on Muscle Quality and Muscle Growth Factors in Older Adult Women with Sarcopenia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 6762. [Google Scholar] [CrossRef]
- Bårdstu, H.B.; Andersen, V.; Fimland, M.S.; Raastad, T.; Saeterbakken, A.H. Muscle Strength Is Associated with Physical Function in Community-Dwelling Older Adults Receiving Home Care. A Cross-Sectional Study. Front. Public. Health 2022, 10, 856632. [Google Scholar] [CrossRef]
- Unhjem, R.; Lundestad, R.; Fimland, M.S.; Mosti, M.P.; Wang, E. Strength Training-Induced Responses in Older Adults: Attenuation of Descending Neural Drive with Age. Age 2015, 37, 47. [Google Scholar] [CrossRef]
- Marsh, A.P.; Miller, M.E.; Rejeski, W.J.; Hutton, S.L.; Kritchevsky, S.B. Lower Extremity Muscle Function after Strength or Power Training in Older Adults. J. Aging Phys. Act. 2009, 17, 416–443. [Google Scholar] [CrossRef]
- Sayers, S.P.; Gibson, K. A Comparison of High-Speed Power Training and Traditional Slow-Speed Resistance Training in Older Men and Women. J. Strength Cond. Res. 2010, 24, 3369–3380. [Google Scholar] [CrossRef]
- Gabriel, D.A.; Kamen, G.; Frost, G. Neural Adaptations to Resistive Exercise. Sports Med. 2006, 36, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Talar, K.; Hernández-Belmonte, A.; Vetrovsky, T.; Steffl, M.; Kałamacka, E.; Courel-Ibáñez, J. Benefits of Resistance Training in Early and Late Stages of Frailty and Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Clin. Med. 2021, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-B.; Kang, K.-Y. The Effects of Isokinetic Eccentric Resistance Exercise for the Hip Joint on Functional Gait of Stroke Patients. J. Phys. Ther. Sci. 2013, 25, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.L.; Kautz, S.A.; Neptune, R.R. Braking and Propulsive Impulses Increase with Speed during Accelerated and Decelerated Walking. Gait Posture 2011, 33, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Jaque, C.; Véliz, P.; Ramirez-Campillo, R.; Moran, J.; Gentil, P.; Cancino, J. High-Speed Bodyweight Resistance Training Improves Functional Performance Through Maximal Velocity in Older Females. J. Aging Phys. Act. 2021, 29, 659–669. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Uchida, M.C. Effects of Low-Speed and High-Speed Resistance Training Programs on Frailty Status, Physical Performance, Cognitive Function, and Blood Pressure in Prefrail and Frail Older Adults. Front. Med. 2021, 8, 702436. [Google Scholar] [CrossRef]
- Clark, D.J.; Reid, K.F.; Patten, C.; Phillips, E.M.; Ring, S.A.; Wu, S.S.; Fielding, R.A. Does Quadriceps Neuromuscular Activation Capability Explain Walking Speed in Older Men and Women? Exp. Gerontol. 2014, 55, 49–53. [Google Scholar] [CrossRef]


| Explosive Resistance Training | Strength Resistance Training | |
|---|---|---|
| Sets | 3 to 5 sets | 3 to 4 sets |
| Repetitions | 12 to 14 repetitions | 6 to 7 repetitions |
| Intensity | 40% to 45% of 1–1-maximal repetition | 80% to 85% of 1–1-maximal repetition |
| Concentric Phase Execution | rapidly | ≈3 s |
| Eccentric Phase Execution | ≈3 s | ≈3 s |
| Recovery Between Repetitions | 1 s | 1 s |
| Recovery Between Sets | 5 min between sets | 5 min between sets |
| Week | Micro-Cycle | Explosive Resistance Training | Strength Resistance Training | Volume Load (kg) |
|---|---|---|---|---|
| 1–2 | 1 | conditioning phase | conditioning phase | - |
| 3–4 | 2 | 4 sets × 12 reps @ 40% 1-RM (8.8 kg) | 3 sets × 8 reps @ 80% 1-RM (17.6 kg) | 422.4 kg each |
| 5–6 | 3 | 4 sets × 13 reps @ 40% 1-RM (8.8 kg) | 3 sets × 9 reps @ 80% 1-RM (17.6 kg) | 457.6 kg each |
| 7–8 | 4 | 4 sets × 14 reps @ 45% 1-RM (9.9 kg) | 4 sets × 7 reps @ 85% 1-RM (18.7 kg) | 554.4 kg each |
| 9–10 | 5 | 5 sets × 12 reps @ 45% 1-RM (9.9 kg) | 4 sets × 8 reps @ 85% 1-RM (18.7 kg) | 594.0 kg each |
| 11–12 | 6 | 5 sets × 14 reps @ 50% 1-RM (11.0 kg) | 4 sets × 9 reps @ 85% 1-RM (18.7 kg) | 770.0 kg each |
| Mean ± SD | Mean ± SD | p (F) | ||||
|---|---|---|---|---|---|---|
| Parameters | Groups | At Baseline | After | Time | Time × Group | Group |
| Age (years) | STG | 82.89 ± 5.32 | 82.89 ± 5.32 | 0.92 (0.48) | 0.91 (0.01) | 0.76 (0.09) |
| EXG | 80.41 ± 10.12 | 80.41 ± 10.12 | ||||
| Height (m) | STG | 1.70 ± 0.13 | 1.70 ± 0.13 | 0.84 (0.69) | 0.792 (0.07) | 0.92 (0.16) |
| EXG | 1.64 ± 0.16 | 1.64 ± 0.16 | ||||
| Weight (kg) | STG | 68.81 ± 5.60 | 70.43 ± 7.60 | 0.76 (0.85) | 0.746 (0.03) | 0.81 (0.02) |
| EXG | 61.62 ± 9.45 | 64.19 ± 4.45 | ||||
| Body mass index (kg/m2) | STG | 23.81 ± 3.45 | 24.39 ± 3.34 | 0.71 (0.85) | 0.746 (0.32) | 0.87 (0.24) |
| EXG | 22.89 ± 2.77 | 23.78 ± 3.23 | ||||
| Lean mass (kg) | STG | 44.90 ± 4.74 | 48.34 ± 2.71 | 0.54 (0.48) | 0.908 (0.01) | 0.76 (0.09) |
| EXG | 41.65 ± 3.65 | 44.94 ± 5.64 | ||||
| Fat mass (kg) | STG | 23.91 ± 9.26 | 22.09 ± 7.28 | 0.95 (0.69) | 0.792 (0.07) | 0.90 (0.02) |
| EXG | 19.97 ± 4.60 | 19.25 ± 6.55 | ||||
| Fat mass (%) | STG | 34.76 ± 3.04 | 31.38 ± 5.10 | 0.79 (0.75) | 0.75 (0.03) | 0.87 (0.02) |
| EXG | 32.40 ± 6.15 | 29.99 ± 7.16 | ||||
| Mean ± SD | Mean ± SD | p (F) | ||||
|---|---|---|---|---|---|---|
| Parameters | Groups | At Baseline | After | Time | Time × Group | Group |
| Absolute Fmax (N) | STG | 220.00 ± 13.69 | 265.00 ± 13.69 * | <0.001 (113.00) | <0.001 (81.77) | 0.130 (2.53) |
| EXG | 216.50 ± 9.44 | 251.50 ± 9.44 *+ | ||||
| Relative Fmax (N/kg) | STG | 3.45 ± 0.53 | 4.177 ± 0.61 * | <0.001 (973.71) | 0.001 (14.62) | 0.376 (0.83) |
| EXG | 3.31 ± 0.45 | 3.875 ± 0.53 *+ | ||||
| RFD 0–50 (N/s) | STG | 630.00 ± 41.07 | 693.22 ± 45.23 * | <0.001 (625.51) | 0.07 (8.71) | 0.106 (2.92) |
| EXG | 627.50 ± 27.00 | 753.40 ± 32.87 *+ | ||||
| RFD 100–200 (N/s) | STG | 320.00 ± 27.38 | 352.00 ± 30.12 | <0.001 (908.95) | <0.001 (107.70) | 0.202 (1.76) |
| EXG | 318.00 ± 18.88 | 383.60 ± 19.99 *+ | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magtouf, E.; Peyrot, N.; Cherni, Y.; Chortane, O.G.; Jolibois, J.; Rahmani, A.; Maktouf, W. Effects of Explosive vs. Strength Resistance Training on Plantar Flexor Neuromuscular and Functional Capacities in Institutionalized Older Adults: A Randomized Controlled Trial. J. Funct. Morphol. Kinesiol. 2024, 9, 261. https://doi.org/10.3390/jfmk9040261
Magtouf E, Peyrot N, Cherni Y, Chortane OG, Jolibois J, Rahmani A, Maktouf W. Effects of Explosive vs. Strength Resistance Training on Plantar Flexor Neuromuscular and Functional Capacities in Institutionalized Older Adults: A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology. 2024; 9(4):261. https://doi.org/10.3390/jfmk9040261
Chicago/Turabian StyleMagtouf, Elmoetez, Nicolas Peyrot, Yosra Cherni, Oussema Gaied Chortane, Jonathan Jolibois, Abderrahmane Rahmani, and Wael Maktouf. 2024. "Effects of Explosive vs. Strength Resistance Training on Plantar Flexor Neuromuscular and Functional Capacities in Institutionalized Older Adults: A Randomized Controlled Trial" Journal of Functional Morphology and Kinesiology 9, no. 4: 261. https://doi.org/10.3390/jfmk9040261
APA StyleMagtouf, E., Peyrot, N., Cherni, Y., Chortane, O. G., Jolibois, J., Rahmani, A., & Maktouf, W. (2024). Effects of Explosive vs. Strength Resistance Training on Plantar Flexor Neuromuscular and Functional Capacities in Institutionalized Older Adults: A Randomized Controlled Trial. Journal of Functional Morphology and Kinesiology, 9(4), 261. https://doi.org/10.3390/jfmk9040261








