Abstract
Background/Objectives: Low-level laser therapy (LLLT) has gained traction in sports and exercise medicine as a non-invasive therapeutic for preconditioning the body, exertion recovery, repair and injury rehabilitation. LLLT is hypothesized to modulate cellular metabolism, tissue microenvironment(s) and to decrease inflammation while posing few adverse risks. This review critically examines the evidence-base for LLLT effectiveness focusing on immediate care settings and acute/subacute applications (<6 months post-injury). Methods: A comprehensive literature search was conducted, prioritizing systematic reviews, meta-analyses and their primary research papers. Results: Findings are relevant to trainers and athletes as they manage a wide range of issues from superficial abrasions to deeper tissue concerns. LLLT parameters in the research literature include wide ranges. For body surface structures, studies show that LLLT holds promise in accelerating wound healing. In sport performance studies, LLLT is typically delivered pre-exercise and reveals beneficial effects on exertion recovery, improvements in muscle strength, endurance and reduced fatigue. Evidence is less convincing for acute, deep tissue injury models, where most studies do not report significant benefits for functional outcomes over conventional therapeutic modalities. Conclusions: Variability in LLLT delivery parameters and findings across studies underscores a need for clear treatment guidelines for the profession. Technical properties of laser light delivery to the body also differ materially from LED devices. Sport physiotherapists, team physicians, trainers and athletes should understand limitations in the current evidence-base informing photobiomodulation use in high-performance sport settings and weigh potential benefits versus shortcomings of LLLT use in the mentioned therapeutic contexts.
1. Introduction
Low level laser therapy (LLLT) is a clinical application of laser light to the body with aims to modulate tissue recovery and repair, decrease inflammation and/or reduce pain. LLLT stimulates cellular repair mechanisms and is calibrated such that intended therapeutic effects are in response to wavelengths of light and not due to heat. Introduced as a Medical Subject Heading (MeSH) term in 2002 [1], LLLT is used interchangeably with other terms, including photobiomodulation (PBM), laser biostimulation, cold laser and laser phototherapy [2]. LLLT’s therapeutic applications are wide-ranging and include managing musculoskeletal conditions, sports injuries, dental surgery, dermatologic procedures, general surgery, oncology and veterinary medicine [3]. Given such sweeping use of LLLT as a non-invasive and medication-free therapeutic, it is clinically relevant to ask probing questions of LLLT’s supportive research and experimental data. For instance, is LLLT use in immediate care settings, such as a “recovery window” therapeutic in sport performance or as a treatment for acute musculoskeletal injury, anecdotal, evidence informed, or evidence-based? In this paper, findings from the LLLT clinical literature are assessed to address this question. Is laser therapy’s widespread therapeutic use informed by high quality clinical evidence in acute settings?
Endre Mester is widely acknowledged as the “godfather” of LLLT by researchers and clinicians working in this space. This is in recognition of his unexpected finding in 1967 that low-power laser application to tissues could lead to hair growth and improved wound healing in mouse models [4]. Mester and colleagues went on to evaluate 15 biological systems, including human wound models with difficult to treat ulcers [5], and hypothesized that LLLT may have broad application and utility in human health and healing.
The FDA categorizes four classes of lasers (I to IV), plus three subclasses (IIa, IIIa, IIIb) [6], according to a laser’s power and risks with use. Class IIIb and IV are used in clinical and therapeutic settings. There are a growing number of FDA-, EU-, and UK-approved photobiomodulation devices, although not all devices sold on the market are necessarily approved for therapeutic use with human subjects. Under FDA regulations, a 510(k) application is required for new LLLT device clearance [7]. Market authorization by regulators includes biocompatibility data versus an established device, as well as clinical performance testing, software performance, and electrical and thermal safety testing. Not all marketed devices adhere to such standards.
Photobiomodulation or PBM is used as an umbrella term for laser light clinical treatments, as well as LED-based applications. There is an ongoing debate in both clinical (among practitioners) and commercial settings (manufacturers and distributors) about the bioequivalence or non-equivalence of functional outcomes with laser or LED devices [8]. The FDA classifies LEDs differently from laser diodes and therefore LED devices are not subject to Federal laser product performance standards [7].
In clinical practice settings, LLLT dosing is a key variable. The World Association for Photobiomodulation Therapy (WALT) is a leading organization supporting research, education, clinical application and treatment standards [9]. WALT publishes LLLT dose and administration recommendations for Class IIIb laser use in chronic arthritic and inflammatory conditions (780–860 nm or 904 nm GaAs nanosecond pulse lasers) [9].
The aim of this review is to assess the available data on LLLT effectiveness and safety, in immediate or acute settings of tissue recovery and repair. Findings are evaluated for laser use in settings relevant to athletes and trainers ranging from wound healing, athletic performance/recovery and acute musculoskeletal injury rehabilitation.
2. Literature Search Strategy
Therapeutic topics of interest and in-scope literature ranged from management of: (1) surface conditions such as wound healing (pre-, post-, and non-surgical), (2) sport performance/recovery models, to more complex sequelae including (3) acute/subacute soft tissue injuries (<6 months onset). The aims were to identify clear LLLT parameters and outcome measures to inform nonpharmacologic approaches that may support musculoskeletal health, functional recovery and to identify areas for future research. This review considers the breadth of scientific evidence from the existing published literature rather than depth with aims to identify gaps in the overall state of the research. By its nature, a review paper addresses broad questions and synthesizes concepts.
Out-of-scope topics included LLLT management of neurological conditions, dermatologic procedures, ophthalmology interventions, chronic pain, behavioural or mental health conditions, research with animal models and chronic injuries (>6 months onset). Other exclusion criteria for LLLT research included no mention of injury timelines, LED-only device research, non-musculoskeletal injuries and non-English language publications (Table 1). Retrieved papers were assessed to document alignment with the literature search inclusion and exclusion criteria.

Table 1.
In scope and out-of-scope criteria for literature assessment.
Literature search criteria included meta-analyses, systematic and scoping reviews and primary studies informing on therapeutic laser use, specifically LLLT, in performance/recovery and acute tissue repair. Aims of the literature synthesis included informing therapeutic choice and shared decision-making for practitioners and their clients. Literature databases included PubMed, Google Scholar and Mendeley, with no timeline restriction up to 2023, although prioritizing publications within the previous 5 years. Research focused on laser device interventions or combined laser treatment clustered with LED technology. Studies using LED therapeutic protocols as the sole light intervention were excluded due to potential heterogeneity introduced across research studies with lower power devices and uncertain effects of LED on cellular constituents or deeper tissue structures. The topic of LED therapeutic use is evaluated elsewhere [10,11].
The quality of evidence described in the literature was categorized as follows: evidence was moderate-to-high quality if there were clear, prospective analysis plans with adequate sample size, treatment controls, statistical power, clear LLLT dosing and treatment parameters, and with clear data reporting. Evidence was deemed low quality when studies were constrained by unclear research methods, vague study population(s), small sample sizes and low statistical power.
3. Findings from the Clinical Literature
LLLT uses beams of light at specific wavelengths, typically red and near-infrared wavelengths, to stimulate cellular metabolism and modulate tissue microenvironments [12]. Class IV lasers are among the most powerful used in light therapy, while LED devices are considered to have shallower penetration depth compared to LLLT (Figure 1).
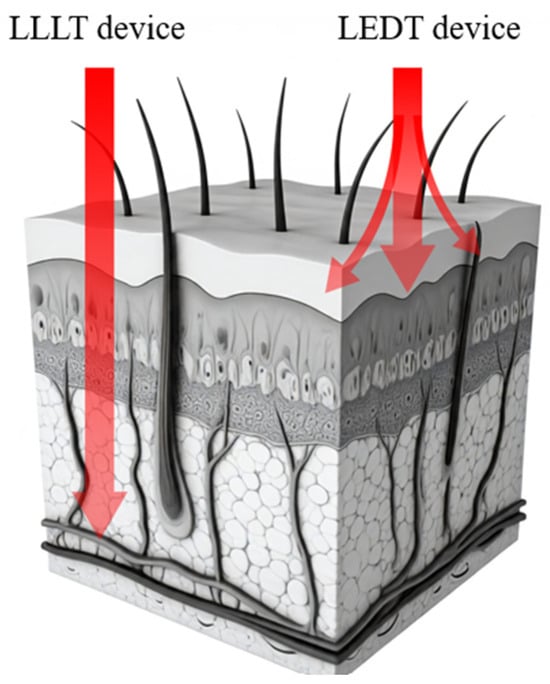
Figure 1.
Low-level laser therapy (LLLT) and light-emitting diode therapy (LEDT) are both forms of red-light therapy used for various medical purposes, including the treatment of superficial wounds and sore or injured connective tissues. Not to scale; for illustrative purposes only.
3.1. Wound Healing
Wound healing processes are relevant to trainers and athletes as they manage a wide range of issues from superficial abrasions to deeper tissue concerns that impact performance. Wound healing requires coordination of cellular and molecular events including cell migration, proliferation and extracellular matrix deposition (e.g., collagen). Scar formation is a natural stage of the healing process; however, excessive scarring may also result in functional impairments and impede tissue range of motion. Already 50 years ago, Mester et al. [5] proposed that laser light stimulates cells at the lips of a wound and in the wound bed accelerating healing. Clinically, this remains an unmet need today. Novel and adjunctive treatments are needed to reduce scarring, accelerate wound closure, improve wound esthetics, and improve outcomes. Does LLLT as a nonpharmacologic approach facilitate wound healing?
3.1.1. Post Surgery
LLLT has been studied and is used clinically to accelerate healing of postsurgical (“fresh”) wounds and to minimize functional or traumatic sequelae. For the purposes of this paper, the focus is on clinical use of non-ablative lasers. Artzi et al. (2020) [13] published a systematic review on the utility of laser treatment on wound healing and scar mitigation in post-operative settings. Fourteen clinical studies met inclusion criteria, and included scar treatment following plastic surgery, breast, thyroid, or hernia surgeries. Pre- and post-operative laser treatment scores based on validated scales included the (1) Patient and Observer Scar Assessment Scale, (2) Vancouver Scar Scale and (3) Global Assessment Scale. Pulsed dye laser (PDL), carbon dioxide and diode lasers were the most frequently used devices in the studies followed by potassium titanyl phosphate (KTP) and erbium glass (Er-Glass) lasers. Various treatment protocols were used: most studies utilized three to four laser treatments at intervals of 2–4 weeks with variable wavelengths applied from 585–596 nm or 810–830 nm with select studies utilizing much longer wavelength light. In these post-operative settings, LLLT facilitated healing and improved scar appearance versus no laser controls. Artzi (2020) [13] recommend early (upon suture removal), post-op use of laser to support the wound healing process. Diode lasers, PDL and carbon dioxide lasers were reported to have the best effects relative to controls. During LLLT, wounds are monitored for side effects including redness, discoloration, pain and infection. Seago et al. (2019) [14] in an International Consensus Guideline on postsurgical and traumatic scarring, state laser treatment improves wound appearance, pliability and tissue range of motion, and that laser therapy should be considered a primary therapeutic option for anticipatory scar mitigation, as well as scar related pain and pruritus. A recently published systematic review and meta-analysis of 12 randomized, controlled trials further informs this topic [15]. Future controlled studies should continue to guide athletes and sports medicine specialists and inform standardized treatment protocols in these settings.
3.1.2. Pre-Conditioning of Wounds
In non-sport settings, pre-conditioning physical therapy is performed before orthopedic surgeries to strengthen muscles and improve range of motion around an affected joint and post-operatively to help reduce pain, swelling, and accelerate overall functional improvements and mobility. Similarly, LLLT pre-treatment of a surgical site may be beneficial to patients’ healing. Of note, Friedman 2020 [16] studied laser pre-treatment of surgical incision sites for individuals undergoing planned lipoma removal. Subjects served as their own controls. Treatment included one pass with an Er-Glass laser (50% overlap between adjacent laser grids) with energy level 40 mJ, 15 ms pulse duration at wavelength 1540 nm, 24 h before surgery. This “conditioning” approach of the skin resulted in significant improvement of final scar appearance 12 months post-procedure. Further, high quality research is needed to generate guidelines for practice.
3.2. Athletic Training and Performance
Innovation in sport technologies aim to optimize performance and recovery for amateur and professional athletes. LLLT is used in select athletic settings across various treatment parameters and timelines. Studies with healthy volunteers (uninjured ≥ 3 months) were chosen to limit interference/confounding factors of other training regimens. Twelve systematic reviews and meta-analyses published 2019–2023 were retrieved in the literature search (Table 2) [17,18,19,20,21,22,23,24,25,26,27,28]. Across the 12 papers, 108 studies were assessed with 64 studies of interest on recovery and performance measures in athletic settings. Fifty-two of the 64 studies concluded benefits in performance with LLLT regimens when accompanying training for younger, healthy participants/athletes. Studies were interpreted with positive outcomes based on conclusions by each research group as published via peer review process. The most often used LLLT parameters included 808–850, or 905 nm wavelength of light, less than 30 s exposure per point, and <10 J of energy over 17 or fewer exposure points. Beyond the most used ranges, LLLT parameters spanned 632.8–980 nm, up to 10 min exposure per point, and <60 J over at most 85 exposure points.

Table 2.
Summary of the technical features of LLLT in athletic performance-related research studies.
Outside a sports context and not listed in Table 2, four studies were assessed that asked questions on LLLT use and aging and included older participants (women 60–70 years) [29,30,31], or people with heart failure (35–65 years of age) [32]. Across the four studies, improvements in functional capacities were reported with LLLT (e.g., increased repetitions of exercises such as leg flexion/extensions) [29,30,31], and delayed fatigue onset [30,32].
González-Muñoz et al., (2023) [17] evaluated 15 RCTs published 2017–2022, assessing LLLT in sports performance (Table 2). From the 15 studies, 9 included LLLT [33,34,35,36,37,38,39,40,41], 8 reported positive functional outcomes such as increased time on the soccer pitch, longer time to reach exhaustion, and accelerated muscle recovery [33,34,35,36,37,38,39,40]. Variable laser parameters were used, with energy density ranging from 0.285–30 J per treated point (2–17 or 85 exposure points), with treatment immediately to 40 min before [35,36,37,38,40], after [33] or a combination of before and after [34,39] exertion. There is one exception of immediate treatment with performing LLLT at 3-, 6- and 24-h post exertion timepoints [38]. González-Muñoz et al. [17] suggest future research is needed to specify precise LLLT parameters, as well as conducting RCTs that evaluate professional athletes and untrained healthy individuals to further understand the utility of LLLT for both high-performance sport versus “weekend warriors”.
Oliveira et al. (2023) [18] assessed 15 RCTs published 2012–2018 on LLLT’s impact on skeletal muscle performance and recovery with all 13 studies [34,36,42,43,44,45,46,47,48,49,50,51,52] using LLLT reporting favorable results for athletic performance, and inflammatory marker expression (Table 2). LLLT promoted maximum voluntary contraction, peak strength, isometric capacity and longer time to achieve fatigue/exhaustion. Nine studies utilized LLLT before exercise [36,43,44,45,46,48,49,51,52], two after exercise [34,42], and two using a combination of before and after exercise [47,50] with 640–670 nm, 800–880 nm or 905–980 nm, 0.095–50 J per point (3–17 or 42 exposure points) for a total of 10–480 J total energy per limb, and application times ranging < 30 s up to 6 min. Oliveira et al. [18] concluded that LLLT had favorable results in muscle regardless of treatment timeline, although better results were reported when applying LLLT prior to exercise.
Dutra et al. (2022) [28] performed a systematic review and meta-analysis on the ergogenic effects of LLLT in sport specific exercises. From 37 included studies, 24 involved laser therapy [33,42,43,44,46,49,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69], 20 with positive results [33,42,43,44,46,49,52,53,54,55,56,59,60,61,62,63,64,67,68,69] (Table 2). Dutra et al. (2022) were specifically interested in preconditioning LLLT in sport. The LLLT parameters across the assessed studies ranged from 600, 780–905 nm, 0.81–60 J per point across 2–29 points of exposure for a total of 12–540 J over 13–300 s of exposure. The findings suggest LLLT increases muscle endurance in single-joint exercises, and time to exhaustion in cycling. However, no significant effects in muscle strength in single-joint exercises, running or swimming performance scores were reported.
Luo et al. (2022) [19] conducted a meta-analysis on performance and soreness recovery for athletes. From 24 included studies, 16 involved laser therapy [33,36,42,43,45,50,58,59,60,61,70,71,72,73,74,75], 14 with positive results [33,36,42,43,45,50,59,60,61,70,71,72,73,74] (Table 2). All but three used pre-exercise LLLT, one applied LLLT after exercise [50], and one used pre- and post-exercise LLLT [71]. Parameters were wide ranging, with wavelengths of 633, 655, 810–905 nm, 0.285–45 J energy per treated point (2–9, 17, 42, 85 points) and 15–600 s exposure. Overall, LLLT was found to improve muscular performance and enhance soreness recovery. Luo et al. [19] recommend LLLT use before or after competition to regain muscle capacity between strenuous work.
De Marchi et al. (2022) [20] evaluated eight RCTs published 2011–2022 to assess whether LLLT impacts exercise-induced oxidative stress among healthy individuals. Five in-scope studies [33,36,42,49,76] evaluated lower body LLLT application, three treating pre-exercise [36,42,49], one post-exercise [33], and one using a combination [76]. The parameter ranges were 810, 830 or 905 nm, 0.285–30 J per point (6–17 or 85 points), and 25–228 s exposure. Outcomes of 5 studies [33,36,42,49,76] showed LLLT was beneficial in both performance and recovery (Table 2).
In a 2021 narrative review by Ailioaie et al. [21], 39 studies evaluated LLLT’s impact on sports performance; 23 included LLLT, and the remaining 16 were LED-only studies. Eighteen of the 23 LLLT studies reported “valuable protective and ergogenic effects” of laser treatment for clinically healthy and physically active individuals [33,36,37,38,39,42,43,45,48,50,62,63,64,70,77,78,79,80] (Table 2). Treatment timelines varied across LLLT with 12 studies investigating LLLT use before exertion [33,36,38,42,43,45,48,62,63,64,70,80], 3 after [37,50,77], and 3 used a combination of before and after exertion [39,78,79]. LLLT parameter ranges were 640 nm, 810–905 nm, < 60 s or 180–381 s, and 0.095–50 J per treated point (3–9, 17, 85 points). Taken together, LLLT was reported to be a key companion therapeuetic for high-performance sport and recovery. Ailioaie et al. [21], also commented on studies that were inconclusive demonstrating no effect of LLLT. Technological limitations (devices, techniques and parameters used), heterogeneous study participants, or unclear protocols for physical activity were cited as some of the challenges [21].
In a scoping review, Alves et al. 2019 [22] evaluated the “immediate” effects (within 40 min of exertion) of LLLT on muscle performance across 27 studies with healthy individuals. A total of 25 studies used laser [29,30,31,32,36,45,47,49,50,52,58,59,60,61,67,68,69,73,79,81,82,83,84,85,86], of which 21 showed improved markers of muscle performance and endurance (assessments: best task performance, cardiorespiratory assessment, increased exercise load or number of repetitions studies) [29,30,31,36,45,47,49,50,52,59,60,61,67,68,69,73,79,81,82,83,85] (Table 2). From these studies, 16 studies treated subjects prior to exertion (immediately to 10 min prior) [30,31,45,49,52,59,60,61,67,68,69,73,78,81,83,85], 4 studies treated post-exercise (immediately to 40 min after) [29,47,50,82], and 1 treated individuals between exercise sets [79]. Parameter ranges were 655, 660, 808–950 nm, 8–300 s exposure, and 0.6–50 J per treated point (2–12, 29, 30, 42 points). The doses per point were 7 J and 30 J. Alves et al. [22] concluded that LLLT optimizes muscle performance and reduces fatigue, especially when administered pre-exertion.
Vanin et al. (2018) [23] retrieved 39 RCTs published prior to March 2017 with the aim of determining optimal LLLT dosages impacting athletic performance and muscular fatigue. A total of 27 included laser [43,45,47,48,49,50,52,58,59,60,61,64,67,68,71,73,77,78,79,81,82,83,85,86,87,88,89], and 24 reported positive results [43,45,47,48,49,50,52,59,60,61,64,67,68,71,73,77,78,79,81,82,83,85,87,89]. Vanin et al. [23] revealed positive results for small muscle groups within the LLLT energy dose range of 20–60 J total, and 60–300 J total for larger muscles. From the studies with favorable outcome, 16 studies treated participants pre-exertion [43,45,48,49,52,59,60,61,64,67,68,73,81,83,85,89], 4 treating post-exertion [47,50,77,82], and 4 using a combination of pre- or post-, or in-between exercise sets [71,78,79,87] (Table 2). The results suggest the use of LLLT may improve athletic performance and reduce muscle fatigue, with best effects occurring when used before exertion.
Ferraresi et al. (2016) [24] retrieved 46 RCTs published prior to 2016. A total of 34 included LLLT intervention [29,30,31,43,45,47,48,49,50,51,52,58,59,60,61,64,67,68,69,71,73,77,78,79,81,82,83,84,86,87,88,89,90,91] and 28 reported positive results [29,30,31,43,45,47,48,49,50,51,52,59,60,61,64,67,68,69,71,73,77,78,79,81,82,83,87,89] (Table 2). Across healthy, athletically trained or untrained individuals and elite athletes, the results showed LLLT favorably affected muscle performance and recovery. Dose recommendations were categorized by activity, and treatment timeline. A therapeutic window for smaller muscle groups (e.g., biceps brachii) was identified as 20–80 J energy dose. Larger muscles (e.g., quadriceps femoris) responded favorably when treated with total energy dose range of 56–315 J. From these results, there appears to be favorability of using LLLT pre-exertion with 19 included studies pre-exertion [30,31,43,48,49,51,52,59,60,61,64,67,68,69,73,78,81,83,89], 5 post-exertion [29,47,50,77,82], and 4 either incorporating both pre- and post-exertion or in between sets [71,78,79,87]. Further research on how different muscle types, groups and sizes respond to LLLT will provide clinicians with data on how to customize treatments.
Three additional systematic reviews or meta-analyses published earlier than those discussed above [25,26,27] had similar research questions and with overlapping coverage of the primary research so are not re-reviewed here.
Across all 12 systematic reviews, LLLT results in beneficial outcomes for exertion and recovery although the quality of evidence of the primary papers is considered low (Table 2; Figure 2). LLLT administration in sport performance settings requires additional high-quality evidence. Reproducible results are required to gain better insight on how to specify treatments for optimal muscle endurance and performance. LLLT shows promise to impact sport performance and improve recovery. LLLT as an adjunctive therapeutic is gaining attention with athletes, sport franchises and federations, in particular on the use of LLLT for pain management in sport by the International Olympic Committee [92]. As of the date of this literature synthesis, no peer-reviewed, evidence-based guidelines of laser use in athletic performance, support or recovery were found.
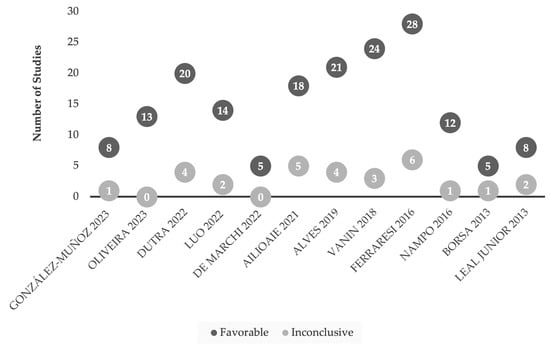
Figure 2.
Comparison of favorable and inconclusive research studies of LLLT for sports-related models [17,18,19,20,21,22,23,24,25,26,27,28]. Research findings are considered favorable based on the conclusions drawn from the authors of LLLT use in sport performance settings (i.e., number of repetitions, time on field), or recovery (i.e., delayed onset of muscle soreness (DOMS)).
3.3. Management of Acute Musculoskeletal Injury
Here, the focus is on acute/subacute (<6 month) injuries, where the literature included lateral epicondylitis (LE), rotator cuff/bicep tendinitis or subacromial impingement syndrome (SIS) (Table 3). Functional outcomes across studies included: Disabilities of the Arm, Shoulder, and Hand (DASH), Quick DASH, the Shoulder Pain and Disability Index (SPADI), and the Shoulder Disability Questionnaire (SDQ), as well as pain measurement scales (Visual Analog Scale, VAS). From the analysis of the literature, there are many fewer publications focusing on LLLT utility in acute/subacute injuries (Table 3) versus those mentioned above in sport performance models (Table 2).

Table 3.
Summary of the main features of LLLT and acute injury review papers.
Tripodi et al.’s., (2021) systematic review [93] evaluated 17 injury studies, with 5 acute/subacute studies for individuals with LE [98,99,100], tendinitis [101], or SIS [102] (Table 3). Emanet et al. (2010) [98] used WALT guidelines [9] to inform their treatment protocols for LE (1 J/cm2 for 2 min at 100 W peak power). Significant improvements in DASH and patient-reported questionnaires were reported at 3- and 12-weeks. Kaydok et al. (2020) [99] used LLLT (904 nm, 2.4 J/cm2 for 30 s) as an active control versus high level laser therapy (protocol 1: 1064 nm, 6 J/cm2, 8 W for 75 s; protocol 2: 1064 nm, 120–150 J/cm2, 6 W for 30 s). Both intensities significantly improved LE functional assessments (Q-DASH and hand grip strength), as well as VAS after 3 weeks of treatment. Lam et al. [100] randomly assigned 39 LE patients to receive either LLLT (904 nm, 2.4 J/cm2, for 11 s) or a sham laser. Their results found LLLT effective in improving functional assessments (DASH and maximum grip strength). For rotator cuff tendinitis, Eslamian [101] found significant improvements in function (based on SDQ) at a 3-week assessment, with no noted advantages for range of motion. Bal et al.,’s (2009) [102] SIS study reported no benefit of LLLT plus a home exercise program versus a home exercise program alone. They used an energy dose 20% below WALT recommendations (1.6 J/cm2 versus minimum 2–3 J/cm2), which may explain the study reporting no benefit of LLLT versus exercise [102]. Taken together, the five studies are largely using subjective test measurements (e.g., DASH, Q-DASH, SDQ). Objective measurements (e.g., hand grip strength, and range of motion) showed statistically significant improvements in two of the five studies [99,100].
Awotidebe et al. (2019) [95] evaluated 11 RCTs with clinical or radiological diagnoses of various shoulder disorders (SIS and rotator cuff tendinitis RCTs within the acute/subacute injury timeline). The study selection criteria required the intervention group to receive exercise and either class IIIb (780–806 nm with 5–500 mW) or class IIIb (904 nm with >5 mW) with the 2010 WALT [103] recommendations. Four studies [101,102,104,105] recruited subjects with acute/subacute injuries (<6 months), three of those studies [102,104,105] found no advantage to incorporating LLLT while one [101] reported LLLT superior to routine physiotherapy (solely based on subjective measures). Awotidebe et al. [95] concluded that LLLT combined with an exercise program was no more effective than exercise alone.
Taylor et al. (2020) [94] set out to identify LLLT dosing variables for neuromusculoskeletal conditions. From 86 included studies, 4 [100,101,102,105] covered acute/subacute injuries, with 3 [100,101,102] previously referenced by Tripodi [93], and 1 [105] by Awotidebe [95]. Taylor’s scoping review synthesized findings from individual trials across various performance measures, acute and chronic injuries, neuromuscular and neurological conditions. While general treatment approaches are summarized in their review with LLLT’s clinical value being noted, the heterogeneity of study participants and treatment modalities make it challenging to draw firm conclusions on recommendations for acute injury settings.
Two other review articles assessed acute injury models [96,97]. Haslerud [97] focused on shoulder injuries, and from four in-scope studies [101,102,104,105], it was reported that LLLT does not contribute meaningful benefits as an adjunct to conventional therapies. For Clijsen [96], while predominantly a review on LLLT’s impact on pain, they did note two studies using functional measures, one study in LE [98] and another in carpal tunnel syndrome [106]. However, neither found benefit in implementing LLLT with functional assessments.
Overall, there are few published works focusing on LLLT’s clinical impact on acute/subacute injuries (Table 3, Figure 3). Across the five review articles [93,94,95,96,97] published 2015–2021, there was minimal to no additional benefit when incorporating LLLT with conventional treatment plans (e.g., heat therapy or physiotherapy; Figure 3). Across the 7 primary studies [98,99,100,101,102,105,106], all use Class IIIb lasers as opposed to Class IV. An argument could be made that the Class IIIb laser might deliver dosages below what is required for therapeutic impact on acute injuries. Eslamian study [101], was included in four of the review papers [93,94,95,97], and while concluding LLLT had beneficial results, it is important to note the numerous interventions used in combination with laser including: heat, ultrasound, electrotherapy, or trans-cutaneous electrical nerve stimulation (TENS). These multimodal therapeutics likely recruit healing pathways, obscuring potential treatment impact of LLLT alone. Statistically significant (p < 0.05) beneficial results were noted solely in subjective measures. As the literature stands, LLLT does not appear to offer meaningful therapeutic benefit(s) in acute injury tissue rehabilitation, other than perhaps recruiting analgesic pathways.
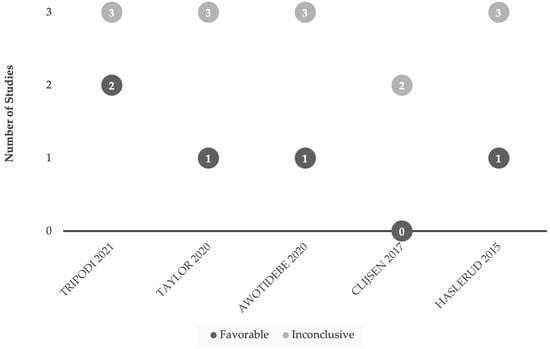
Figure 3.
Comparison of favorable and inconclusive results when applying LLLT (Class IIIb) for acute or subacute (<6 month onset) injuries. Research findings are considered favorable based on the conclusions drawn from the authors [93,94,95,96,97]. Our interest was in studies emphasizing functional outcomes versus studies focusing primarily on subjective measures or pain management.
3.4. LLLT at a Cellular Level
The light-sensitive photoreceptor or “chromophore” for LLLT (activated by red to near-infrared light; Figure 1) is proposed to be mitochondrial cytochrome C or, alternatively, mitochondrial bound water [107,108]. When tissue is damaged, overexerted or otherwise dysfunctional, ATP production decreases. In the presence of red or infrared light, mitochondrial function increases, ATP production increases, and the release of reactive oxygen species goes up which may act in molecular signaling, as well as nitric oxide to tip cellular signaling pathways in favor of anti-inflammatory mediators [12,109] (see Figure 4). The activation of transcription factors, such as NFkB, regulate cell migration, proliferation, inflammatory and stress-induced responses, and hypoxia-induced factor (HIF) activation upregulates several genes and glycolysis enzymes, which allows ATP synthesis in hypoxic conditions in an oxygen-independent manner.

Figure 4.
Delivery of red or infrared light to body structures may involve an interplay of biological processes that lead to decreased inflammation and accelerated recovery of strained or damaged tissues [12].
Collagen is the major protein in the extracellular matrix that constitutes most of the connective tissue during wound healing. In vitro studies show that LLLT increases cell viability, cell migration, proliferation and collagen synthesis [12,109]. LLLT induces macrophages to release factors that stimulate fibroblast proliferation. In addition, LLLT promotes the production of interleukin-1 alpha (IL-1α) and interleukin-8 (IL-8), which stimulate the migration of keratinocytes. LLLT also increases the expression of platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β). PDGF stimulates mitogenicity and chemotaxis of fibroblasts and smooth muscle cells and chemotaxis of neutrophils and macrophages.
LLLT thereby recruits tissue recovery, healing and/or repair mechanisms [12] as ATP levels and related metabolic processes are activated. The cellular microenvironment tips in favor for recovery and repair.
4. Discussion
The objective of this review was to critically assess the LLLT literature in immediate care settings, for sport performance exertion or acute injury rehabilitation. Across these distinct therapeutic settings, each have their own complexities associated with the recovery process, spanning from superficial trauma to recovery of deeper tissue targets. Differences exist in technical specifications between Class IIIb or Class IV laser LLLT and LED devices (Table 4). The therapeutic choice between laser or LED use will depend on specific therapeutic goals, target tissue depth and desired treatment outcomes for the light therapy.

Table 4.
Comparative parameters of low-level laser therapy (LLLT) and light emitting diode (LED) devices for musculoskeletal therapeutic use.
PBM parameters reported in the literature show wide ranges as summarized in Table 4. For sport training contexts, there is no standardization of total light energy delivered to muscles, energy density (J/cm2), ideal power output (mW) of the laser device or time/duration of light exposure to target tissue(s) across research studies. One reason contributing to these inconsistencies is a wide range of PBM devices available on the market both regulated and unregulated. Laser devices, LED and mixed array devices are available to practitioners and researchers all contributing variation to the understanding of efficacious treatment protocols.
Wound healing, however, is an injury model that avails itself to assess healing time course with LLLT using objective outcomes such as visual tracking and direct measures of healing. In research studies, wound healing has the unique benefit of being able to use the same incision or ulcer as a control and treatment area [13]. This eliminates confounding factors that arise when attempting to match control subjects and experimental subject conditions (e.g., wound depth, body composition, skin color, etc.). Advanced therapeutic options for large or complex wound closures remain limited resulting in prolonged healing times and increased risk of infection. However, the available data suggest that laser treatment improves wound appearance and tissue integrity, and that laser therapy should be considered a primary therapeutic option. The most recent International Consensus (2020) deems first-line laser treatment for traumatic scars and contractures as the best available care [14]. Skin color and body composition affects the properties by which light interacts with tissue [112]. Thus, additional consideration is required for treatment protocols to calibrate LLLT approaches according to skin color and/or adiposity. In the future, laser therapy could be tested as an adjunctive treatment to facilitate engraftment of bioengineered skin substitutes with advanced dressings, or stem cells to accelerate skin and tissue repair.
In sports performance and recovery models, LLLT demonstrated predominantly positive outcomes across individual research studies (see Figure 2). For studies that were inconclusive, short treatment cycle or significantly lower energy administered may have impacted the outcomes. From the included reviews [17,18,19,20,21,22,23,24,25,26,27,28], there is a wide range of LLLT parameters applied across various therapeutic settings (Table 2). One of the inconclusive studies [88] referenced WALT in their choice of LLLT dose, delivering 2.5–3.5 J/cm2 at 5 and 10 min, respectively. While WALT is a consensus guideline on Class IIIb laser use for management of chronic conditions, the guidelines do not provide evidence-based guidance on LLLT use to manage exertion or acute muscular strain. Interestingly, a 2008 pilot study [88] of muscular fatigue in healthy male volunteers applied WALT’s recommendations as treatment protocols. Many studies [29,30,32,33,34,36,42,43,45,48,49,50,58,59,60,61,67,69,71,73,74,76,77,78,81,86,113], both favorable and inconclusive, explored LLLT’s impact on muscle damaging mediators such as creatine kinase and blood lactate. Such measurements allow for more objective, biomarker-based testing, and the findings suggest possible benefits of LLLT use for performance athlete recovery. Bettleyon and Kaminski [114] note micro-CK level elevations hinder the recovery process via cellular membrane damage, localized hypoxia, and electrolyte imbalances. One hypothesis is LLLT may reduce these mediators. Clearly, more translational research is needed on correlatives between muscle biomarkers and performance measures in the context of LLLT.
For other tissue types, few human studies are published on bone repair and LLLT to inform the athletic setting. At present, the bone literature is seemingly limited to mechanistic and animal models. A 2023 systematic review [115] was published evaluating human studies and the effect of LLLT on bone repair by assessing in vitro targets. To date, the underlying mechanisms by which LLLT might modulate bone formation remain unknown, though would serve to inform orthopedics in sports in the instance of fractures. Factors such as wavelength, energy density, exposure and frequency of LLLT might influence calcium signaling and the cellular mechanisms of bone repair and requires further research.
When comparing studies of LLLT in sport performance models (Figure 2) with those in acute injury settings (Figure 3), the number of LLLT research papers was far fewer in the setting of acute injuries. This could be due to challenges with funding and conducting studies in acute injury settings (e.g., subject recruitment) or may also reflect publication bias in which studies of LLLT in acute settings with inconclusive or negative results for LLLT intervention are not submitted or accepted for publication.
The effectiveness of introducing LLLT across all aspects of an athlete’s training and performance routine remains an open question. There are still limitations in the current evidence-base informing LLLT in sports settings. Additional variables such as athlete body composition, skin color, age and gender may all influence the therapeutic index of LLLT and desired outcomes. With laser therapy continuing to make headway in the sporting world, those planning to integrate this modality into their current routine should investigate how current limitations balance against potential benefits. Continued interest and research in this non-invasive therapeutic will continue to grow the database of knowledge and fine tune parameters for optimal results.
5. Conclusions
LLLT has emerged as a promising technology across the various aspects of an athlete’s training, performance, and recovery. From our investigation, LLLT interventions, both alone and as adjunctive therapy, yield variable outcomes in immediate care settings with beneficial effects for surface lesions/wound healing and athletic exertion/recovery. In contrast, the research suggests LLLT is of questionable utility for accelerating repair of deeper musculoskeletal structures in the setting of acute/sub-acute injury in the near-term post-injury period. LLLT’s effectiveness in supporting preconditioning, exertion and recovery is relevant for high performance sport This non-invasive modality may support quicker return-to-play times for athletes by mitigating fatigue and strain, allowing for more consistent training and performance. While subjective testing in the research studies is an important element of participants’ experiences, quantitative objective testing is needed to advance the field. Understanding how body composition, skin color, age and gender impact LLLT clinical outcomes is also needed. High-quality research must continue to inform the science and practice parameters of LLLT’s effects on functional outcomes. A call to action is the publication of guidelines for the profession to inform laser care alongside traditional therapeutic or conditioning protocols in sport and could be modeled after guidelines in other fields such as the International Consensus Guideline on Postsurgical and Traumatic Scarring [14] or WALT [9]. Such steps will serve to advance effective, safe and ethical use of PBM devices and advocate for improved regulations and standardization in this field for sport physiotherapists, team physicians, trainers and athletes.
Author Contributions
Conceptualization, J.L. and K.S.; investigation, J.L. and K.S.; data curation, J.L. and K.S.; writing—draft preparation, J.L. and K.S.; writing—final review and editing, J.L. and K.S.; visualization, J.L. and K.S.; supervision, K.S.; project administration, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest. The authors declare that this study received no outside funding. Both authors are employees of Arroscience Inc., a research and health sciences consulting company, and were involved in the study design, collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication.
References
- National Library of Medicine. MeSH Descriptor Data. 2023. Available online: https://meshb.nlm.nih.gov/record/ui?ui=D028022 (accessed on 31 July 2024).
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-Level Light/Laser Therapy Versus Photobiomodulation Therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Mussttaf, R.A.; Jenkins, D.F.L.; Jha, A.N. Assessing the impact of low level laser therapy (LLLT) on biological systems: A review. Int. J. Radiat. Biol. 2019, 95, 120–143. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photobiomodulation or low-level laser therapy. J. Biophotonics 2016, 9, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Mester, E.; Mester, A.F.; Mester, A. The biomedical effects of laser application. Lasers Surg. Med. 1985, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Laser Products and Instruments. 2023. Available online: https://www.fda.gov/radiation-emitting-products/home-business-and-entertainment-products/laser-products-and-instruments (accessed on 31 July 2024).
- Regulatory Affairs Professionals Society. FDA Draft Guidance Covers Low-Level Light Therapy Devices. 2023. Available online: https://www.raps.org/News-and-Articles/News-Articles/2023/1/FDA-draft-guidance-covers-low-level-light-therapy (accessed on 31 July 2024).
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- World Association for Photobiomodulation Therapy. WALT Recommendations—Dosage Recommendations 2022. 2022. Available online: https://waltpbm.org/documentation-links/recommendations/ (accessed on 31 July 2024).
- Gayton, J.; Monga, A. Goal setting in physiotherapy-led adult musculoskeletal care: A scoping review. Musculoskelet. Care 2023, 21, 1315–1340. [Google Scholar] [CrossRef]
- Opel, D.R.; Hagstrom, E.; Pace, A.K.; Sisto, K.; Hirano-Ali, S.A.; Desai, S.; Swan, J. Light-emitting Diodes: A Brief Review and Clinical Experience. J. Clin. Aesthet. Dermatol. 2015, 8, 36–44. [Google Scholar]
- Rola, P.; Wlodarczk, S.; Lesiak, M.; Doroszko, A.; Wlodarczk, A. Changes in Cell Biology under the Influence of Low-LevelLaser Therapy. Photonics 2022, 9, 502. [Google Scholar] [CrossRef]
- Artzi, O.; Friedman, O.; Al-niaimi, F.; Wolf, Y.; Mehrabi, J.N. Mitigation of Postsurgical Scars Using Lasers. Plast. Reconstr. Surg. Glob. Open 2020, 8, e2746. [Google Scholar] [CrossRef]
- Seago, M.; Shumaker, P.R.; Spring, L.K.; Alam, M.; Al-Niaimi, F.; Rox Anderson, R.; Artzi, O.; Bayat, A.; Cassuto, D.; Chan, H.H.; et al. Laser Treatment of Traumatic Scars and Contractures: 2020 International Consensus Recommendations. Lasers Surg. Med. 2020, 52, 96–116. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Yang, X.; Pan, B.; Xie, H.; Bi, H. Safety and Effectiveness of Laser or Intense Pulsed Light Treatment for Early Surgical Scar: A Systematic Review and Meta-analysis. Aesthetic Plast. Surg. 2023, 48, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Friedman, O.; Gofstein, D.; Arad, E.; Gur, E.; Sprecher, E.; Artzi, O. Laser pretreatment for the attenuation of planned surgical scars: A randomized self-controlled hemi-scar pilot study. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 893–898. [Google Scholar] [CrossRef]
- González-Muñoz, A.; Perez-Montilla, J.J.; Cuevas-Cervera, M.; Aguilar-García, M.; Aguilar-Nuñez, D.; Hamed-Hamed, D.; Pruimboom, L.; Navarro-Ledesma, S. Effects of Photobiomodulation in Sports Performance: A Literature Review. Appl. Sci. 2023, 13, 3147. [Google Scholar] [CrossRef]
- Oliveira, A.F.S.S.D.; Silva, J.L.D.; Camillo, C.A.M.; Andraus, R.A.C.; Maia, L.P. Does Photobiomodulation Improve Muscle Performance and Recovery? A Systematic Review. Rev. Bras. Med. Esporte 2023, 29, e2021_0412. [Google Scholar] [CrossRef]
- Luo, W.T.; Lee, C.J.; Tam, K.W.; Huang, T.W. Effects of Low-Level Laser Therapy on Muscular Performance and Soreness Recovery in Athletes: A Meta-analysis of Randomized Controlled Trials. Sports Health Multidiscip. Approach 2022, 14, 687–693. [Google Scholar] [CrossRef]
- De Marchi, T.; Ferlito, J.V.; Ferlito, M.V.; Salvador, M.; Cesar, E.; Leal-Junior, P. Can Photobiomodulation Therapy (PBMT) Minimize Exercise-Induced Oxidative Stress? A Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 1671. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Litscher, G.; Crovella, S. Photobiomodulation and Sports: Results of a Narrative Review. Life 2021, 11, 1339. [Google Scholar] [CrossRef]
- Alves, V.M.N.; Furlan, R.M.M.M.; Motta, A.R. Immediate effects of photobiomodulation with low-level laser therapy on muscle performance: An integrative literature review. Rev. CEFAC 2019, 21, e12019. [Google Scholar] [CrossRef]
- Vanin, A.A.; Verhagen, E.; Barboza, S.D.; Costa, L.O.P.; Leal-Junior, E.C.P. Photobiomodulation therapy for the improvement of muscular performance and reduction of muscular fatigue associated with exercise in healthy people: A systematic review and meta-analysis. Lasers Med. Sci. 2018, 33, 181–214. [Google Scholar] [CrossRef]
- Ferraresi, C.; Huang, Y.Y.; Hamblin, M.R. Photobiomodulation in human muscle tissue: An advantage in sports performance? J. Biophotonics 2016, 9, 1273–1299. [Google Scholar] [CrossRef]
- Nampo, F.K.; Cavalheri, V.; dos Santos Soares, F.; de Paula Ramos, S.; Camargo, E.A. Low-level phototherapy to improve exercise capacity and muscle performance: A systematic review and meta-analysis. Lasers Med. Sci. 2016, 31, 1957–1970. [Google Scholar] [CrossRef] [PubMed]
- Borsa, P.A.; Larkin, K.A.; True, J.M. Does Phototherapy Enhance Skeletal Muscle Contractile Function and Postexercise Recovery? A Systematic Review. J. Athl. Train. 2013, 48, 57–67. [Google Scholar] [CrossRef]
- Leal-Junior, E.C.P.; Vanin, A.A.; Miranda, E.F.; de Carvalho, P.D.T.C.; Dal Corso, S.; Bjordal, J.M. Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: A systematic review with meta-analysis. Lasers Med. Sci. 2013, 30, 925–939. [Google Scholar] [CrossRef]
- Dutra, Y.M.; Malta, E.S.; Elias, A.S.; Broatch, J.R.; Zagatto, A.M. Deconstructing the Ergogenic Effects of Photobiomodulation: A Systematic Review and Meta-analysis of its Efficacy in Improving Mode-Specific Exercise Performance in Humans. Sports Med. 2022, 52, 2733–2757. [Google Scholar] [CrossRef] [PubMed]
- Toma, R.L.; Vassão, P.G.; Assis, L.; Antunes, H.K.M.; Renno, A.C.M. Low level laser therapy associated with a strength training program on muscle performance in elderly women: A randomized double blind control study. Lasers Med. Sci. 2016, 31, 1219–1229. [Google Scholar] [CrossRef]
- Vassão, P.G.; Toma, R.L.; Antunes, H.K.M.; Tucci, H.T.; Renno, A.C.M. Effects of photobiomodulation on the fatigue level in elderly women: An isokinetic dynamometry evaluation. Lasers Med. Sci. 2016, 31, 275–282. [Google Scholar] [CrossRef]
- Toma, R.L.; Tucci, H.T.; Antunes, H.K.M.; Pedroni, C.R.; de Oliveira, A.S.; Buck, I.; Ferreira, P.D.; Vassão, P.G.; Renno, A.C.M. Effect of 808 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in elderly women. Lasers Med. Sci. 2013, 28, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Bublitz, C.; Renno, A.C.M.; Ramos, R.S.; Assis, L.; Sellera, C.A.C.; Trimer, R.; Borghi-Silva, A.; Arena, R.; Guizilini, S. Acute effects of low-level laser therapy irradiation on blood lactate and muscle fatigue perception in hospitalized patients with heart failure—A pilot study. Lasers Med. Sci. 2016, 31, 1203–1209. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Machado, C.D.S.M.; De Marchi, T.; Casalechi, H.L.; Bjordal, J.M.; de Carvalho, P.D.T.C.; Leal-Junior, E.C.P. Infrared Low-Level Laser Therapy (Photobiomodulation Therapy) before Intense Progressive Running Test of High-Level Soccer Players: Effects on Functional, Muscle Damage, Inflammatory, and Oxidative Stress Markers—A Randomized Controlled Trial. Oxidative Med. Cell. Longev. 2019, 2019, 6239058. [Google Scholar] [CrossRef]
- Miranda, E.F.; Tomazoni, S.S.; de Paiva, P.R.V.; Pinto, H.D.; Smith, D.; Santos, L.A.; de Tarso Camillo de Carvalho, P.; Leal-Junior, E.C.P. When is the best moment to apply photobiomodulation therapy (PBMT) when associated to a treadmill endurance-training program? A randomized, triple-blinded, placebo-controlled clinical trial. Lasers Med. Sci. 2018, 33, 719–727. [Google Scholar] [CrossRef]
- Follmer, B.; Dellagrana, R.A.; Rossato, M.; Sakugawa, R.L.; Diefenthaeler, F. Photobiomodulation therapy is beneficial in reducing muscle fatigue in Brazilian jiu-jitsu athletes and physically active men. Sport Sci. Health 2018, 14, 685–691. [Google Scholar] [CrossRef]
- De Marchi, T.; Leal-Junior, E.C.P.; Lando, K.C.; Cimadon, F.; Vanin, A.A.; da Rosa, D.P.; Salvador, M. Photobiomodulation therapy before futsal matches improves the staying time of athletes in the court and accelerates post-exercise recovery. Lasers Med. Sci. 2019, 34, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.D.S.M.; Casalechi, H.L.; Vanin, A.A.; de Azevedo, J.B.; de Carvalho, P.D.T.C.; Leal-Junior, E.C.P. Does photobiomodulation therapy combined to static magnetic field (PBMT-sMF) promote ergogenic effects even when the exercised muscle group is not irradiated? A randomized, triple-blind, placebo-controlled trial. BMC Sports Sci. Med. Rehabil. 2020, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Leal-Junior, E.C.P.; de Oliveira, M.F.D.; Joensen, J.; Stausholm, M.B.; Bjordal, J.M.; Tomazoni, S.S. What is the optimal time-response window for the use of photobiomodulation therapy combined with static magnetic field (PBMT-sMF) for the improvement of exercise performance and recovery, and for how long the effects last? A randomized, triple-blinded, placebo-controlled trial. BMC Sports Sci. Med. Rehabil. 2020, 12, 64. [Google Scholar] [CrossRef]
- De Paiva, P.R.V.; Casalechi, H.L.; Tomazoni, S.S.; Machado, C.D.S.M.; Ribeiro, N.F.; Pereira, A.L.; De Oliveira, M.F.D.; Alves, M.N.D.S.; Dos Santos, M.C.; Takara, I.E.T.; et al. Does the combination of photobiomodulation therapy (PBMT) and static magnetic fields (sMF) potentiate the effects of aerobic endurance training and decrease the loss of performance during detraining? A randomised, triple-blinded, placebo-controlled trial. BMC Sports Sci. Med. Rehabil. 2020, 12, 23. [Google Scholar] [CrossRef]
- Machado, A.F.; Leal-Junior, E.C.P.; Batista, N.P.; Espinoza, R.M.C.P.P.; Hidalgo, R.B.R.; Carvalho, F.A.; Micheletti, J.K.; Vanderlei, F.M.; Pastre, C.M. Photobiomodulation therapy applied during an exercise-training program does not promote additional effects in trained individuals: A randomized placebo-controlled trial. Braz. J. Phys. Ther. 2022, 26, 100388. [Google Scholar] [CrossRef]
- Orssatto, L.B.D.R.; Detanico, D.; Kons, R.L.; Sakugawa, R.L.; Silva, J.N.D., Jr.; Diefenthaeler, F. Photobiomodulation Therapy Does Not Attenuate Fatigue and Muscle Damage in Judo Athletes: A Randomized, Triple-Blind, Placebo-Controlled Trial. Front. Physiol. 2019, 10, 811. [Google Scholar] [CrossRef]
- de Oliveira, A.R.; Vanin, A.A.; Tomazoni, S.S.; Miranda, E.F.; Albuquerque-Pontes, G.M.; De Marchi, T.; dos Santos Grandinetti, V.; de Paiva, P.R.V.; Imperatori, T.B.G.; de Carvalho, P.D.T.C.; et al. Pre-Exercise Infrared Photobiomodulation Therapy (810 nm) in Skeletal Muscle Performance and Postexercise Recovery in Humans: What Is the Optimal Power Output? Photomed. Laser Surg. 2017, 35, 595–603. [Google Scholar] [CrossRef]
- Pinto, H.D.; Vanin, A.A.; Miranda, E.F.; Tomazoni, S.S.; Johnson, D.S.; Albuquerque-Pontes, G.M.; Ivo de OAleixo, J.; Grandinetti, V.D.S.; Casalechi, H.L.; Paulo de Tarso, C.; et al. Photobiomodulation Therapy Improves Performance and Accelerates Recovery of High-Level Rugby Players in Field Test: A Randomized, Crossover, Double-Blind, Placebo-Controlled Clinical Study. J. Strength Cond. Res. 2016, 30, 3329–3338. [Google Scholar] [CrossRef]
- Larkin-Kaiser, K.A.; Borsa, P.A.; Baweja, H.S.; Moore, M.A.; Tillman, M.D.; George, S.Z.; Christou, E.A. Photobiomodulation delays the onset of skeletal muscle fatigue in a dose-dependent manner. Lasers Med. Sci. 2016, 31, 1325–1332. [Google Scholar] [CrossRef]
- Aver Vanin, A.; De Marchi, T.; Silva Tomazoni, S.; Tairova, O.; Leão Casalechi, H.; de Tarso Camillo de Carvalho, P.; Bjordal, J.M.; Leal-Junior, E.C. Pre-Exercise Infrared Low-Level Laser Therapy (810 nm) in Skeletal Muscle Performance and Postexercise Recovery in Humans, What Is the Optimal Dose? A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Photomed. Laser Surg. 2016, 34, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Dellagrana, R.A.; Rossato, M.; Sakugawa, R.L.; Lazzari, C.D.; Baroni, B.M.; Diefenthaeler, F. Dose-response effect of photobiomodulation therapy on neuromuscular economy during submaximal running. Lasers Med. Sci. 2018, 33, 329–336. [Google Scholar] [CrossRef]
- Ferraresi, C.; de Brito Oliveira, T.; de Oliveira Zafalon, L.; de Menezes Reiff, R.B.; Baldissera, V.; de Andrade Perez, S.E.; Júnior, E.M.; Parizotto, N.A. Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med. Sci. 2011, 26, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Antonialli, F.C.; De Marchi, T.; Tomazoni, S.S.; Vanin, A.A.; dos Santos Grandinetti, V.; de Paiva, P.R.V.; Pinto, H.D.; Miranda, E.F.; de Tarso Camillo de Carvalho, P.; Leal-Junior, E.C.P. Phototherapy in skeletal muscle performance and recovery after exercise: Effect of combination of super-pulsed laser and light-emitting diodes. Lasers Med. Sci. 2014, 29, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, T.; Leal-Junior, E.C.P.; Bortoli, C.; Tomazoni, S.S.; Lopes-Martins, R.Á.B.; Salvador, M. Low-level laser therapy (LLLT) in human progressive-intensity running: Effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med. Sci. 2012, 27, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zagatto, A.M.; de Paula Ramos, S.; Nakamura, F.Y.; de Lira, F.S.; Lopes-Martins, R.Á.B.; de Paiva Carvalho, R.L. Effects of low-level laser therapy on performance, inflammatory markers, and muscle damage in young water polo athletes: A double-blind, randomized, placebo-controlled study. Lasers Med. Sci. 2016, 31, 511–521. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larkin-Kaiser, K.A.; Christou, E.; Tillman, M.; George, S.; Borsa, P.A. Near-Infrared Light Therapy to Attenuate Strength Loss After Strenuous Resistance Exercise. J. Athl. Train. 2015, 50, 45–50. [Google Scholar] [CrossRef]
- de Almeida, P.; Lopes-Martins, R.Á.B.; De Marchi, T.; Tomazoni, S.S.; Albertini, R.; Corrêa, J.C.F.; Rossi, R.P.; Machado, G.P.; da Silva, D.P.; Bjordal, J.M.; et al. Red (660 nm) and infrared (830 nm) low-level laser therapy in skeletal muscle fatigue in humans: What is better? Lasers Med. Sci. 2012, 27, 453–458. [Google Scholar] [CrossRef]
- Toma, R.L.; Oliveira, M.X.; Renno, A.C.M.; Laakso, E.L. Photobiomodulation (PBM) therapy at 904 nm mitigates effects of exercise-induced skeletal muscle fatigue in young women. Lasers Med. Sci. 2018, 33, 1197–1205. [Google Scholar] [CrossRef]
- Lanferdini, F.J.; Bini, R.R.; Baroni, B.M.; Klein, K.D.; Carpes, F.P.; Vaz, M.A. Improvement of Performance and Reduction of Fatigue With Low-Level Laser Therapy in Competitive Cyclists. Int. J. Sports Physiol. Perform. 2018, 13, 14–22. [Google Scholar] [CrossRef]
- Dellagrana, R.A.; Rossato, M.; Sakugawa, R.L.; Baroni, B.M.; Diefenthaeler, F. Photobiomodulation Therapy on Physiological and Performance Parameters During Running Tests: Dose–Response Effects. J. Strength Cond. Res. 2018, 32, 2807–2815. [Google Scholar] [CrossRef]
- Teixeira, C.L.; Mezzaroba, P.V.; Machado, F.A. Effect of Photobiomodulation on Critical Swimming Velocity: A Randomized, Crossover, Double-Blind, and Placebo-Controlled Study. Int. J. Sports Physiol. Perform. 2021, 16, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Azuma, R.H.E.; Merlo, J.K.; Jacinto, J.L.; Borim, J.M.; da Silva, R.A.; Pacagnelli, F.L.; Nunes, J.P.; Ribeiro, A.S.; Aguiar, A.F. Photobiomodulation Therapy at 808 nm Does Not Improve Biceps Brachii Performance to Exhaustion and Delayed-Onset Muscle Soreness in Young Adult Women: A Randomized, Controlled, Crossover Trial. Front. Physiol. 2021, 12, 664582. [Google Scholar] [CrossRef] [PubMed]
- Leal-Junior, E.C.P.; Lopes-Martins, R.A.B.; Baroni, B.M.; De Marchi, T.; Rossi, R.P.; Grosselli, D.; Generosi, R.A.; de Godoi, V.; Basso, M.; Mancalossi, J.L.; et al. Comparison Between Single-Diode Low-Level Laser Therapy (LLLT) and LED Multi-Diode (Cluster) Therapy (LEDT) Applications Before High-Intensity Exercise. Photomed. Laser Surg. 2009, 27, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Leal-Junior, E.C.P.; Lopes-Martins, R.Á.B.; Baroni, B.M.; De Marchi, T.; Taufer, D.; Manfro, D.S.; Rech, M.; Danna, V.; Grosselli, D.; Generosi, R.A.; et al. Effect of 830 nm low-level laser therapy applied before high-intensity exercises on skeletal muscle recovery in athletes. Lasers Med. Sci. 2009, 24, 857–863. [Google Scholar] [CrossRef]
- Leal, E.C.P.; Lopes-Martins, R.Á.B.; Frigo, L.; De Marchi, T.; Rossi, R.P.; De Godoi, V.; Tomazoni, S.S.; Silva, D.P.; Basso, M.; Filho, P.L.; et al. Effects of Low-Level Laser Therapy (LLLT) in the Development of Exercise-Induced Skeletal Muscle Fatigue and Changes in Biochemical Markers Related to Postexercise Recovery. J. Orthop. Sports Phys. Ther. 2010, 40, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Leal-Junior, E.C.P.; Lopes-Martins, R.Á.B.; Vanin, A.A.; Baroni, B.M.; Grosselli, D.; De Marchi, T.; Iversen, V.V.; Bjordal, J.M. Effect of 830 nm low-level laser therapy in exercise-induced skeletal muscle fatigue in humans. Lasers Med. Sci. 2009, 24, 425–431. [Google Scholar] [CrossRef]
- Rossato, M.; Dellagrana, R.A.; Sakugawa, R.L.; Baroni, B.M.; Diefenthaeler, F. Dose–Response Effect of Photobiomodulation Therapy on Muscle Performance and Fatigue During a Multiple-Set Knee Extension Exercise: A Randomized, Crossover, Double-Blind Placebo-Controlled Trial. Photobiomodul. Photomed. Laser Surg. 2020, 38, 758–765. [Google Scholar] [CrossRef]
- Rossato, M.; Dellagrana, R.A.; Sakugawa, R.L.; Lazzari, C.D.; Baroni, B.M.; Diefenthaeler, F. Time Response of Photobiomodulation Therapy on Muscular Fatigue in Humans. J. Strength Cond. Res. 2018, 32, 3285–3293. [Google Scholar] [CrossRef]
- Miranda, E.F.; Vanin, A.A.; Tomazoni, S.S.; Grandinetti, V.D.S.; de Paiva, P.R.V.; Machado, C.D.S.M.; Monteiro, K.K.D.S.; Casalechi, H.L.; de Tarso, P.; de Carvalho, C.; et al. Using Pre-Exercise Photobiomodulation Therapy Combining Super-Pulsed Lasers and Light-Emitting Diodes to Improve Performance in Progressive Cardiopulmonary Exercise Tests. J. Athl. Train. 2016, 51, 129–135. [Google Scholar] [CrossRef]
- Orssatto, L.B.R.; Rossato, M.; Vargas, M.; Diefenthaeler, F.; de la Rocha Freitas, C. Photobiomodulation Therapy Effects on Resistance Training Volume and Discomfort in Well-Trained Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Photobiomodul. Photomed. Laser Surg. 2020, 38, 720–726. [Google Scholar] [CrossRef]
- Dellagrana, R.A.; Rossato, M.; Orssatto, L.B.R.; Sakugawa, R.L.; Baroni, B.M.; Diefenthaeler, F. Effect of Photobiomodulation Therapy in the 1500 m Run: An Analysis of Performance and Individual Responsiveness. Photobiomodul. Photomed. Laser Surg. 2020, 38, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Higashi, R.H.; Toma, R.L.; Tucci, H.T.; Pedroni, C.R.; Ferreira, P.D.; Baldini, G.S.; Aveiro, M.C.; Borghi-Silva, A.; de Oliveira, A.S.; Renno, A.C.M. Effects of Low-Level Laser Therapy on Biceps Braquialis Muscle Fatigue in Young Women. Photomed. Laser Surg. 2013, 31, 586–594. [Google Scholar] [CrossRef] [PubMed]
- da Silva Alves, M.A.; Pinfildi, C.E.; Neto, L.N.; Lourenço, R.P.; de Azevedo, P.H.S.M.; Dourado, V.Z. Acute effects of low-level laser therapy on physiologic and electromyographic responses to the cardiopulmonary exercise testing in healthy untrained adults. Lasers Med. Sci. 2014, 29, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Maciel, T.; Muñoz, I.S.S.; Nicolau, R.A.; Nogueira, D.V.; Hauck, L.A.; Osório, R.A.L.; de Paula Júnior, A.R. Phototherapy effect on the muscular activity of regular physical activity practitioners. Lasers Med. Sci. 2014, 29, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, R.A.; Pinfildi, C.E.; de Castro Pochini, A.; Cohen, M. Photobiomodulation therapy and NMES improve muscle strength and jumping performance in young volleyball athletes: A randomized controlled trial study in Brazil. Lasers Med. Sci. 2020, 35, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, F.A.; da Silva, B.A.K.; Laraia, E.M.S.; de Melo, R.M.; Silva, P.H.; Leal-Junior, E.C.P.; de Carvalho, P.D.T.C. Effects of Pre- or Post-Exercise Low-Level Laser Therapy (830 nm) on Skeletal Muscle Fatigue and Biochemical Markers of Recovery in Humans: Double-Blind Placebo-Controlled Trial. Photomed. Laser Surg. 2014, 32, 106–112. [Google Scholar] [CrossRef]
- Lanferdini, F.J.; Krüger, R.L.; Baroni, B.M.; Lazzari, C.; Figueiredo, P.; Reischak-Oliveira, A.; Vaz, M.A. Low-level laser therapy improves the VO2 kinetics in competitive cyclists. Lasers Med. Sci. 2018, 33, 453–460. [Google Scholar] [CrossRef]
- Leal-Junior, E.C.P.; Lopes-Martins, R.Á.B.; Dalan, F.; Ferrari, M.; Sbabo, F.M.; Generosi, R.A.; Baroni, B.M.; Penna, S.C.; Iversen, V.V.; Bjordal, J.M. Effect of 655-nm Low-Level Laser Therapy on Exercise-Induced Skeletal Muscle Fatigue in Humans. Photomed. Laser Surg. 2008, 26, 419–424. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H. Effect of Low-Level Laser Therapy on Exercise-Induced Skeletal Muscle Fatigue and Exercise Performance. Acta Medica Mediterr. 2020, 36, 2019–2025. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Aimi, M.; Vaz, M.A.; Baroni, B.M. Effects of low-level laser therapy on hamstring strain injury rehabilitation: A randomized controlled trial. Phys. Ther. Sport 2020, 42, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Pinto, H.D.; Casalechi, H.L.; de Marchi, T.; dos Santos Monteiro Machado, C.; Dias, L.B.; Lino, M.M.A.; de Azevedo, J.B.; Tomazoni, S.S.; Leal-Junior, E.C.P. Photobiomodulation Therapy Combined with a Static Magnetic Field Applied in Different Moments Enhances Performance and Accelerates Muscle Recovery in CrossFit® Athletes: A Randomized, Triple-Blind, Placebo-Controlled Crossover Trial. Oxidative Med. Cell. Longev. 2022, 2022, 9968428. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, P.R.V.; Tomazoni, S.S.; Johnson, D.S.; Vanin, A.A.; Albuquerque-Pontes, G.M.; Machado, C.D.S.M.; Casalechi, H.L.; de Carvalho, P.D.T.C.; Leal-Junior, E.C.P. Photobiomodulation therapy (PBMT) and/or cryotherapy in skeletal muscle restitution, what is better? A randomized, double-blinded, placebo-controlled clinical trial. Lasers Med. Sci. 2016, 31, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.A.; Miranda, E.F.; Machado, C.S.M.; de Paiva, P.R.V.; Albuquerque-Pontes, G.M.; Casalechi, H.L.; de Tarso Camillo de Carvalho, P.; Leal-Junior, E.C.P. What is the best moment to apply phototherapy when associated to a strength training program? A randomized, double-blinded, placebo-controlled trial. Lasers Med. Sci. 2016, 31, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- de Brito Vieira, W.H.; Bezerra, R.M.; Queiroz, R.A.S.; Maciel, N.F.B.; Parizotto, N.A.; Ferraresi, C. Use of Low-Level Laser Therapy (808 nm) to Muscle Fatigue Resistance: A Randomized Double-Blind Crossover Trial. Photomed. Laser Surg. 2014, 32, 678–685. [Google Scholar] [CrossRef]
- Florianovicz, V.C.; Ferraresi, C.; Kuriki, H.U.; Marcolino, A.M.; Barbosa, R.I. Effects of Photobiomodulation Therapy and Restriction of Wrist Extensor Blood Flow on Grip: Randomized Clinical Trial. Photobiomodul. Photomed. Laser Surg. 2020, 38, 743–749. [Google Scholar] [CrossRef]
- Baroni, B.M.; Leal-Junior, E.C.P.; De Marchi, T.; Lopes, A.L.; Salvador, M.; Vaz, M.A. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur. J. Appl. Physiol. 2010, 110, 789–796. [Google Scholar] [CrossRef]
- de Brito Vieira, W.H.; Ferraresi, C.; de Andrade Perez, S.E.; Baldissera, V.; Parizotto, N.A. Effects of low-level laser therapy (808 nm) on isokinetic muscle performance of young women submitted to endurance training: A randomized controlled clinical trial. Lasers Med. Sci. 2012, 27, 497–504. [Google Scholar] [CrossRef]
- Baroni, B.M.; Rodrigues, R.; Freire, B.B.; Franke, R.D.A.; Geremia, J.M.; Vaz, M.A. Effect of low-level laser therapy on muscle adaptation to knee extensor eccentric training. Eur. J. Appl. Physiol. 2015, 115, 639–647. [Google Scholar] [CrossRef]
- Kakihata, C.M.M.; Malanotte, J.A.; Higa, J.Y.; Errero, T.K.; Balbo, S.L.; Bertolini, G.R.F. Influence of low-level laser therapy on vertical jump in sedentary individuals. Einstein 2015, 13, 41–46. [Google Scholar] [CrossRef]
- de Souza, C.G.; Borges, D.T.; de Brito Macedo, L.; Brasileiro, J.S. Low-level laser therapy reduces the fatigue index in the ankle plantar flexors of healthy subjects. Lasers Med. Sci. 2016, 31, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Felismino, A.S.; Costa, E.C.; Aoki, M.S.; Ferraresi, C.; de Araújo Moura Lemos, T.M.; de Brito Vieira, W.H. Effect of low-level laser therapy (808 nm) on markers of muscle damage: A randomized double-blind placebo-controlled trial. Lasers Med. Sci. 2014, 29, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, C.G.; Dornelles, M.P.; Severo-Silveira, L.; Marques, V.B.; Rosso, I.D.A.; Baroni, B.M. Effects of low-level laser therapy applied before or after plyometric exercise on muscle damage markers: Randomized, double-blind, placebo-controlled trial. Lasers Med. Sci. 2016, 31, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Wadee, A.N.; Sobhi, N.N. The Effect of Low-Level Laser Therapy on Electrically Induced Muscle Fatigue: A Pilot Study. Photomed. Laser Surg. 2008, 26, 501–506. [Google Scholar] [CrossRef]
- Rossato, M.; Dellagrana, R.A.; Lanferdini, F.J.; Sakugawa, R.L.; Lazzari, C.D.; Baroni, B.M.; Diefenthaeler, F. Effect of pre-exercise phototherapy applied with different cluster probe sizes on elbow flexor muscle fatigue. Lasers Med. Sci. 2016, 31, 1237–1244. [Google Scholar] [CrossRef]
- Craig, J.A.; Barlas, P.; Baxter, G.D.; Walsh, D.M.; Allen, J.M. Delayed-Onset Muscle Soreness: Lack of Effect of Combined Phototherapy/Low-Intensity Laser Therapy at Low Pulse Repetition Rates. J. Clin. Laser Med. Surg. 1996, 14, 375–380. [Google Scholar] [CrossRef]
- Craig, J.A.; Barron, J.; Walsh, D.M.; Baxter, G.D. Lack of effect of combined low intensity laser therapy/phototherapy (CLILT) on delayed onset muscle soreness in humans. Lasers Surg. Med. 1999, 24, 223–230. [Google Scholar] [CrossRef]
- Hainline, B.; Derman, W.; Vernec, A.; Budgett, R.; Deie, M.; Dvořák, J.; Harle, C.; Herring, S.A.; McNamee, M.; Meeuwisse, W.; et al. International Olympic Committee consensus statement on pain management in elite athletes. Br. J. Sports Med. 2017, 51, 1245–1258. [Google Scholar] [CrossRef]
- Tripodi, N.; Feehan, J.; Husaric, M.; Sidiroglou, F.; Apostolopoulos, V. The effect of low-level red and near-infrared photobiomodulation on pain and function in tendinopathy: A systematic review and meta-analysis of randomized control trials. BMC Sports Sci. Med. Rehabil. 2021, 13, 91. [Google Scholar] [CrossRef]
- Taylor, D.N.; Winfield, T.; Wynd, S. Low-Level Laser Light Therapy Dosage Variables vs Treatment Efficacy of Neuromusculoskeletal Conditions: A Scoping Review. J. Chiropr. Med. 2020, 19, 119–127. [Google Scholar] [CrossRef]
- Awotidebe, A.W.; Inglis-Jassiem, G.; Young, T. Does Low-level Laser Therapy Provide Additional Benefits to Exercise in Patients with Shoulder Musculoskeletal Disorders? A Meta-analysis of Randomised Controlled Trials. Ortop. Traumatol. Rehabil. 2019, 21, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Clijsen, R.; Brunner, A.; Barbero, M.; Clarys, P.; Taeymans, J. Effects of low-level laser therapy on pain in patients with musculoskeletal disorders: A systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2017, 53, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Haslerud, S.; Magnussen, L.H.; Joensen, J.; Lopes-Martins, R.A.B.; Bjordal, J.M. The Efficacy of Low-Level Laser Therapy for Shoulder Tendinopathy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Physiother. Res. Int. 2015, 20, 108–125. [Google Scholar] [CrossRef]
- Emanet, S.K.; Altan, L.İ.; Yurtkuran, M. Investigation of the Effect of GaAs Laser Therapy on Lateral Epicondylitis. Photomed. Laser Surg. 2010, 28, 397–403. [Google Scholar] [CrossRef]
- Kaydok, E. Short-Term Efficacy Comparison of High-Intensity and Low-Intensity Laser Therapy in the Treatment of Lateral Epicondylitis: A Randomized Double-Blind Clinical Study. Arch. Rheumatol. 2020, 35, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.K.Y.; Cheing, G.L.Y. Effects of 904-nm Low-Level Laser Therapy in the Management of Lateral Epicondylitis: A Randomized Controlled Trial. Photomed. Laser Surg. 2007, 25, 65–71. [Google Scholar] [CrossRef]
- Eslamian, F.; Shakouri, S.K.; Ghojazadeh, M.; Nobari, O.E.; Eftekharsadat, B. Effects of low-level laser therapy in combination with physiotherapy in the management of rotator cuff tendinitis. Lasers Med. Sci. 2012, 27, 951–958. [Google Scholar] [CrossRef]
- Bal, A.; Eksioglu, E.; Gurcay, E.; Gulec, B.; Karaahmet, O.; Cakci, A. Low-Level Laser Therapy in Subacromial Impingement Syndrome. Photomed. Laser Surg. 2009, 27, 31–36. [Google Scholar] [CrossRef]
- World Association for Photobiomodulation Therapy. WALT Recommendations—Dosage Recommendations 2010. 2010. Available online: https://waltpbm.org/documentation-links/recommendations/ (accessed on 31 July 2024).
- Bingöl, Ü.; Altan, L.; Yurtkuran, M. Low-Power Laser Treatment for Shoulder Pain. Photomed. Laser Surg. 2005, 23, 459–464. [Google Scholar] [CrossRef]
- Yeldan, I.; Cetin, E.; Razak Ozdincler, A. The effectiveness of low-level laser therapy on shoulder function in subacromial impingement syndrome. Disabil. Rehabil. 2009, 31, 935–940. [Google Scholar] [CrossRef]
- Tascioglu, F.; Degirmenci, N.A.; Ozkan, S.; Mehmetoglu, O. Low-level laser in the treatment of carpal tunnel syndrome: Clinical, electrophysiological, and ultrasonographical evaluation. Rheumatol. Int. 2012, 32, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.P. Mitochondrial cytochrome c oxidase is not the primary acceptor for near infrared light—It is mitochondrial bound water: The principles of low-level light therapy. Ann. Transl. Med. 2019, 7 (Suppl. 1), S13. [Google Scholar] [CrossRef] [PubMed]
- RHamblin, M. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.G.; Ribeiro, R.S.; Mencalha, A.L.; de Souza Fonseca, A. Photobiomodulation at molecular, cellular, and systemic levels. Lasers Med. Sci. 2023, 38, 136. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Esnouf, A.; Wright, P.A.; Moore, J.C.; Ahmed, S. Depth of Penetration of an 850nm Wavelength Low Level Laser in Human Skin. Acupunct. Electro-Ther. Res. 2007, 32, 81–86. [Google Scholar] [CrossRef]
- Souza-Barros, L.; Dhaidan, G.; Maunula, M.; Solomon, V.; Gabison, S.; Lilge, L.; Nussbaum, E.L. Skin color and tissue thickness effects on transmittance, reflectance, and skin temperature when using 635 and 808 nm lasers in low intensity therapeutics. Lasers Surg. Med. 2018, 50, 291–301. [Google Scholar] [CrossRef]
- De Marchi, T.; Schmitt, V.M.; Machado, G.P.; de Sene, J.S.; de Col, C.D.; Tairova, O.; Salvador, M.; Leal-Junior, E.C.P. Does photobiomodulation therapy is better than cryotherapy in muscle recovery after a high-intensity exercise? A randomized, double-blind, placebo-controlled clinical trial. Lasers Med. Sci. 2017, 32, 429–437. [Google Scholar] [CrossRef]
- Bettleyon, J.; Kaminski, T.W. Does Low-Level Laser Therapy Decrease Muscle-Damaging Mediators After Performance in Soccer Athletes Versus Sham Laser Treatment? A Critically Appraised Topic. J. Sport Rehabil. 2020, 29, 1210–1213. [Google Scholar] [CrossRef]
- Berni, M.; Brancato, A.M.; Torriani, C.; Bina, V.; Annunziata, S.; Cornella, E.; Trucchi, M.; Jannelli, E.; Mosconi, M.; Gastaldi, G.; et al. The Role of Low-Level Laser Therapy in Bone Healing: Systematic Review. Int. J. Mol. Sci. 2023, 24, 7094. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).