Preliminary Evaluation of Self-Reported Training Volume as an Adjunct Measure of Female Athlete Triad Risk in Division 1 Collegiate Female Runners
Abstract
1. Introduction
2. Materials and Methods
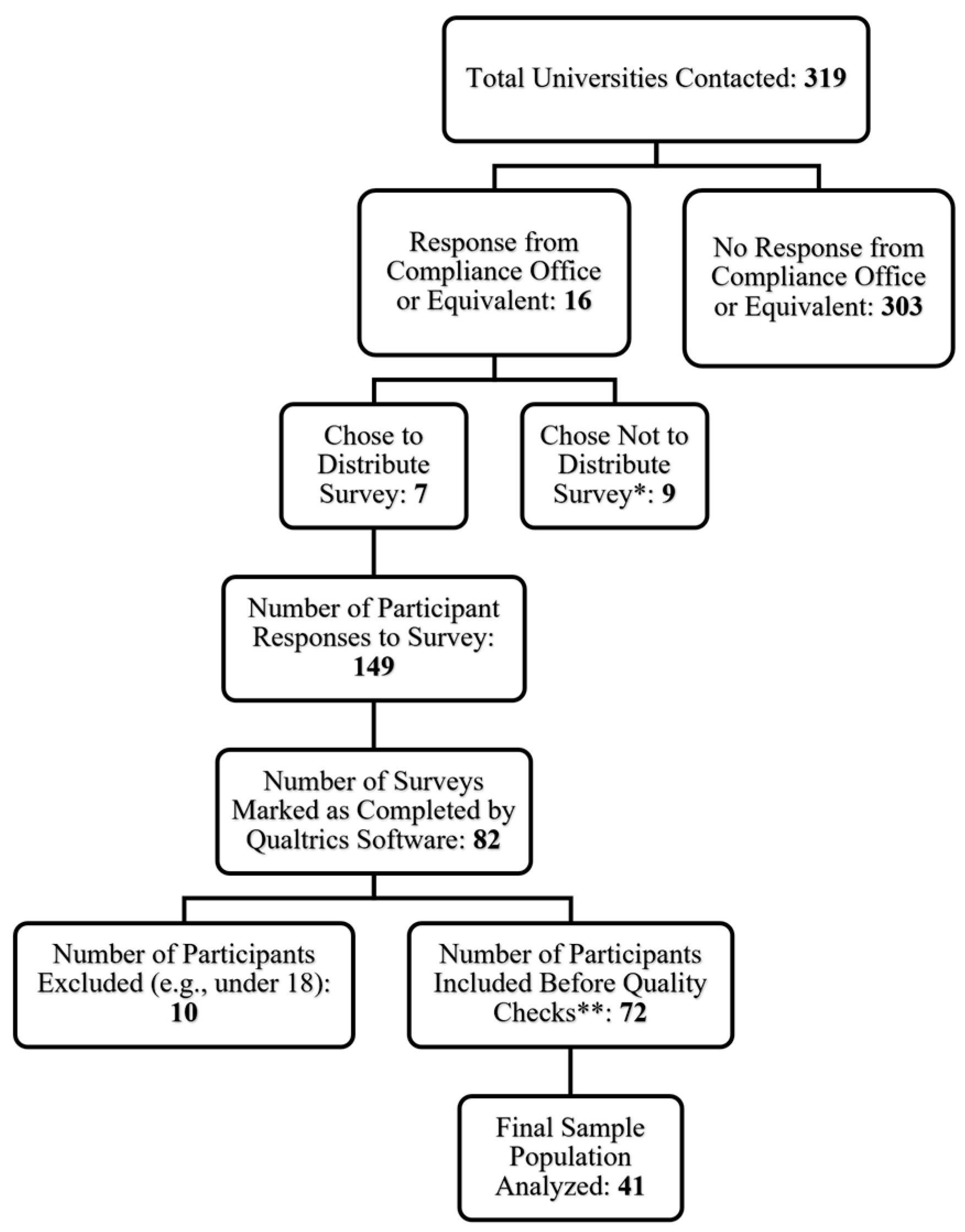
2.1. Participants and Study Design
2.2. Survey Design and Distribution
2.3. Data Computation and Statistical Approach
3. Results
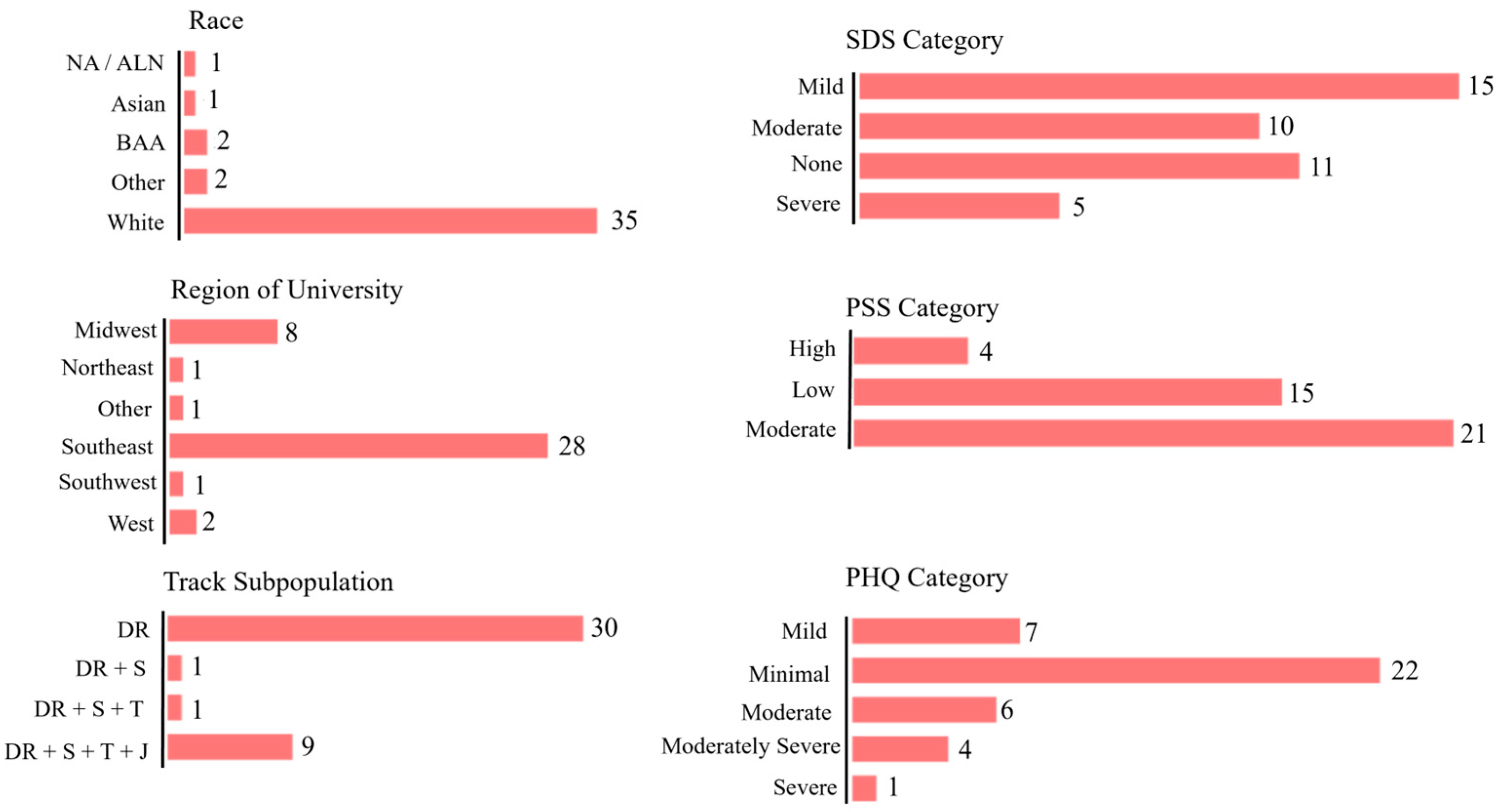
3.1. Demographics and Descriptives
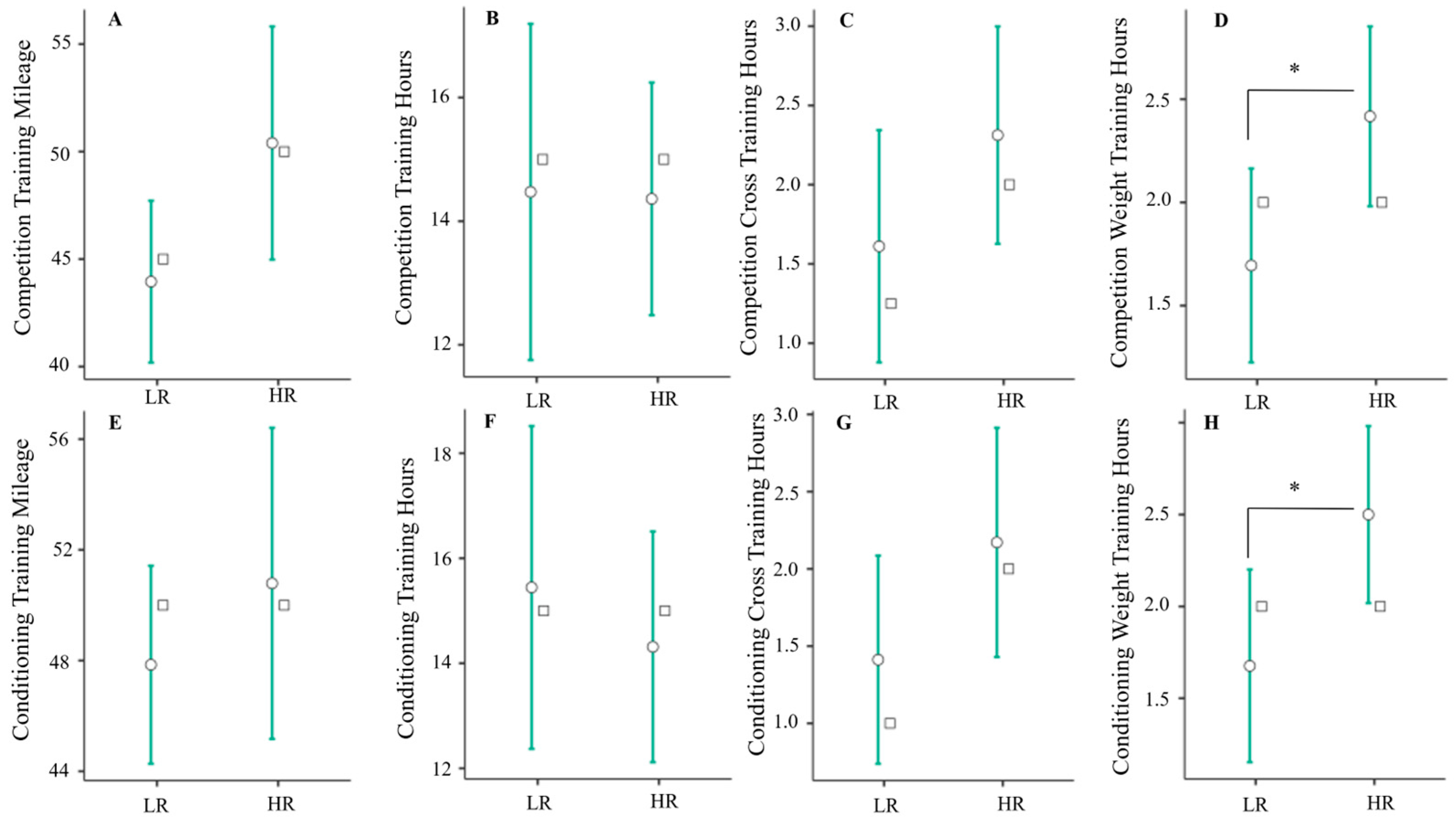
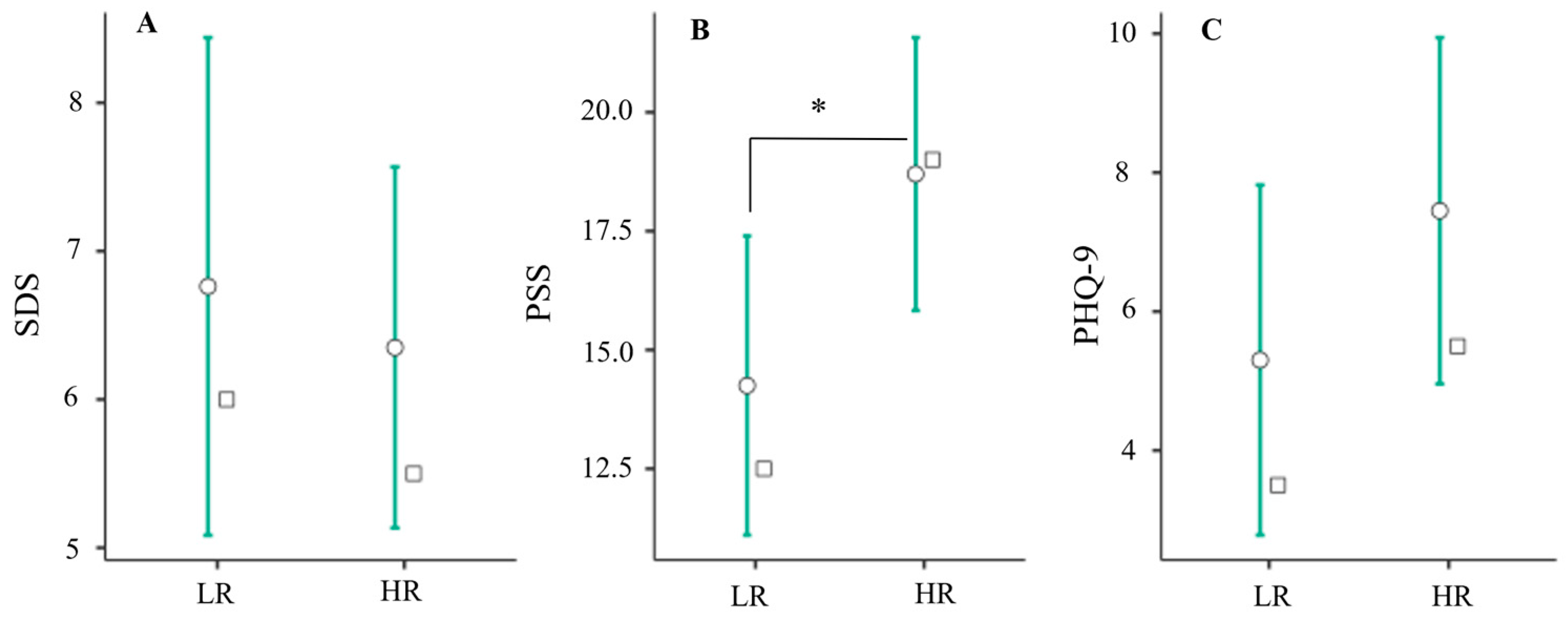
3.2. Relationship between Training Volume and Triad Risk
4. Discussion
4.1. Resistance Training as a Possible Predictor of Triad Risk
4.2. Mileage as a Predictor of Triad Risk
4.3. Implications for Survey-Based Training Volume as Predictor of Triad Risk
4.4. Limitations
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otis, C.L.; Drinkwater, B.; Johnson, M.; Loucks, A.; Wilmore, J. American College of Sports Medicine position stand. The Female Athlete Triad. Med. Sci. Sports Exerc. 1997, 29, i–ix. [Google Scholar] [CrossRef] [PubMed]
- Weiss Kelly, A.K.; Hecht, S. The Female Athlete Triad. Pediatrics 2016, 138, e20160922. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Carter, S.; Constantini, N.; Lebrun, C.; Meyer, N.; Sherman, R.; Steffen, K.; Budgett, R.; et al. The IOC consensus statement: Beyond the Female Athlete Triad—Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 2014, 48, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Nattiv, A.; Loucks, A.B.; Manore, M.M.; Sanborn, C.F.; Sundgot-Borgen, J.; Warren, M.P. American College of Sports Medicine position stand. The female athlete triad. Med. Sci. Sports Exerc. 2007, 39, 1867–1882. [Google Scholar] [CrossRef]
- Holtzman, B.; Ackerman, K.E. Recommendations and Nutritional Considerations for Female Athletes: Health and Performance. Sports Med. 2021, 51, 43–57. [Google Scholar] [CrossRef]
- Lambert, V.; Carbuhn, A.; Culp, A.; Ketterly, J.; Twombley, B.; White, D. Interassociation Consensus Statement on Sports Nutrition Models for the Provision of Nutrition Services from Registered Dietitian Nutritionists in Collegiate Athletics. J. Athl. Train 2022, 57, 717–732. [Google Scholar] [CrossRef]
- De Souza, M.J.; Nattiv, A.; Joy, E.; Misra, M.; Williams, N.I.; Mallinson, R.J.; Gibbs, J.C.; Olmsted, M.; Goolsby, M.; Matheson, G. 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play of the Female Athlete Triad: 1st International Conference held in San Francisco, California, May 2012 and 2nd International Conference held in Indianapolis, Indiana, May 2013. Br. J. Sports Med. 2014, 48, 289. [Google Scholar] [CrossRef]
- Bender, A.M.; Lawson, D.; Werthner, P.; Samuels, C.H. The Clinical Validation of the Athlete Sleep Screening Questionnaire: An Instrument to Identify Athletes that Need Further Sleep Assessment. Sports Med. Open 2018, 4, 23. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Anwer, S.; Manzar, M.D.; Alghadir, A.H.; Salahuddin, M.; Abdul Hameed, U. Psychometric Analysis of the Perceived Stress Scale Among Healthy University Students. Neuropsychiatr. Dis. Treat. 2020, 16, 2389–2396. [Google Scholar] [CrossRef]
- Mozumder, M.K. Reliability and validity of the Perceived Stress Scale in Bangladesh. PLoS ONE 2022, 17, e0276837. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Maroufizadeh, S.; Omani-Samani, R.; Almasi-Hashiani, A.; Amini, P.; Sepidarkish, M. The reliability and validity of the Patient Health Questionnaire-9 (PHQ-9) and PHQ-2 in patients with infertility. Reprod. Health 2019, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fu, Z.; Bo, Q.; Mao, Z.; Ma, X.; Wang, C. The reliability and validity of PHQ-9 in patients with major depressive disorder in psychiatric hospital. BMC Psychiatry 2020, 20, 474. [Google Scholar] [CrossRef]
- Adamson, M.M.; Phillips, A.; Seenivasan, S.; Martinez, J.; Grewal, H.; Kang, X.; Coetzee, J.; Luttenbacher, I.; Jester, A.; Harris, O.A.; et al. International Prevalence and Correlates of Psychological Stress during the Global COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 9248. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Jagim, A.R.; Fields, J.; Magee, M.K.; Kerksick, C.M.; Jones, M.T. Contributing Factors to Low Energy Availability in Female Athletes: A Narrative Review of Energy Availability, Training Demands, Nutrition Barriers, Body Image, and Disordered Eating. Nutrients 2022, 14, 986. [Google Scholar] [CrossRef]
- Heikura, I.A.; Stellingwerff, T.; Burke, L.M. Self-Reported Periodization of Nutrition in Elite Female and Male Runners and Race Walkers. Front. Physiol. 2018, 9, 1732. [Google Scholar] [CrossRef]
- Graybeal, A.J.; Kreutzer, A.; Willis, J.L.; Braun-Trocchio, R.; Moss, K.; Shah, M. The impact of dieting culture is different between sexes in endurance athletes: A cross-sectional analysis. BMC Sports Sci. Med. Rehabil. 2022, 14, 157. [Google Scholar] [CrossRef]
- Ferguson, L.M.; Rossi, K.A.; Ward, E.; Jadwin, E.; Miller, T.A.; Miller, W.C. Effects of Caloric Restriction and Overnight Fasting on Cycling Endurance Performance. J. Strength Cond. Res. 2009, 23, 560–570. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. Development and Validation of Prediction Equation of “Athens Authentic Marathon” Men’s Race Speed. Front. Physiol. 2021, 12, 682359. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, M.B.; Hinze, C.J.; Emborg, B.; Thomsen, F.; Hemmingsen, S.D. Compulsive exercise: Links, risks and challenges faced. Psychol. Res. Behav. Manag. 2017, 10, 85–95. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, A.P.; Oudejans, R.R.; Bakker, F.C.; Woertman, L. Contextual body image and athletes’ disordered eating: The contribution of athletic body image to disordered eating in high performance women athletes. Eur. Eat. Disord. Rev. 2011, 19, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Turton, R.; Goodwin, H.; Meyer, C. Athletic identity, compulsive exercise and eating psychopathology in long-distance runners. Eat. Behav. 2017, 26, 129–132. [Google Scholar] [CrossRef]
- Brewer, B.W.; Van Raalte, J.L.; Linder, D.E. Athletic Identity: Hercules’ Muscles or Achilles Heel? Int. J. Sport Psychol. 1993, 23, 237–254. [Google Scholar]
- Kong, P.; Harris, L.M. The sporting body: Body image and eating disorder symptomatology among female athletes from leanness focused and nonleanness focused sports. J. Psychol. 2015, 149, 141–160. [Google Scholar] [CrossRef]
- Grasdalsmoen, M.; Clarsen, B.; Sivertsen, B. Mental Health in Elite Student Athletes: Exploring the Link Between Training Volume and Mental Health Problems in Norwegian College and University Students. Front. Sports Act. Living 2022, 4, 817757. [Google Scholar] [CrossRef]
- Cobb, K.L.; Bachrach, L.K.; Greendale, G.; Marcus, R.; Neer, R.M.; Nieves, J.; Sowers, M.F.; Brown, B.W., Jr.; Gopalakrishnan, G.; Luetters, C.; et al. Disordered eating, menstrual irregularity, and bone mineral density in female runners. Med. Sci. Sports Exerc. 2003, 35, 711–719. [Google Scholar] [CrossRef]
- Brenner, P.S.; DeLamater, J. Lies, Damned Lies, and Survey Self-Reports? Identity as a Cause of Measurement Bias. Soc. Psychol. Q. 2016, 79, 333–354. [Google Scholar] [CrossRef]
- Capling, L.; Beck, K.L.; Gifford, J.A.; Slater, G.; Flood, V.M.; O’Connor, H. Validity of Dietary Assessment in Athletes: A Systematic Review. Nutrients 2017, 9, 1313. [Google Scholar] [CrossRef]
| n | Whole Sample (n = 41) | Distance Runners (n = 30) | Multievent Athletes (n = 11) | p | |
|---|---|---|---|---|---|
| Baseline Demographics | |||||
| Age (yrs) | 40 | 20 ± 2 | 20 ± 2 | 20 ± 1 | 0.363 |
| Height (cm) | 41 | 164.1 ± 9.2 | 163.5 ± 9.4 | 165.5 ± 8.8 | 0.558 |
| Weight (lb) | 40 | 125.1 ± 14.4 | 122.7 ± 12.1 | 132.2 ± 18.6 | 0.068 |
| BMI (kg/m2) | 41 | 21.1 ± 2.5 | 20.9 ± 2.4 | 21.6 ± 2.8 | 0.452 |
| Competition Training Volume Measures | |||||
| Total Weekly Mileage | 41 | 47.1 ± 11.1 | 47.5 ± 10.9 | 45.9 ± 12.0 | 0.682 |
| Total Weekly Hours | 37 | 14.4 ± 5.1 | 14.2 ± 4.8 | 15.0 ± 6.1 | 0.680 |
| Total Weekly Cross Training Hours | 38 | 2.0 ± 1.6 | 1.8 ± 1.6 | 2.4 ± 1.5 | 0.351 |
| Weekly Resistance Training Hours | 36 | 2.1 ± 1.0 | 2.0 ± 1.1 | 2.1 ± 0.9 | 0.855 |
| Conditioning Training Volume Measures | |||||
| Total Weekly Mileage | 39 | 49.3 ± 10.5 | 51.1 ± 10.3 | 44.6 ± 9.9 | 0.076 |
| Total Weekly Hours | 34 | 14.9 ± 5.7 | 15.1 ± 5.7 | 14.4 ± 6.0 | 0.740 |
| Total Weekly Cross Training Hours | 36 | 1.8 ± 1.6 | 1.7 ± 1.5 | 2.1 ± 1.7 | 0.488 |
| Weekly Resistance Training Hours | 35 | 2.1 ± 1.1 | 2.1 ± 1.2 | 2.2 ± 1.1 | 0.747 |
| Linear Regression | Binomial Logistic Regression | |||||
|---|---|---|---|---|---|---|
| R2 | β | p | OR | 95% CI | p | |
| Dependent Variable | Triad Risk Factor Count | Triad Risk Factor Group | ||||
| Competition Training Measures | ||||||
| Total Weekly Mileage | 0.077 | 0.099 | 0.568 | 1.05 | 0.98/1.13 | 0.156 |
| Total Weekly Hours | 0.068 | −0.040 | 0.818 | 0.988 | 0.87/1.13 | 0.860 |
| Total Weekly Cross Training Hours | 0.135 | 0.271 | 0.117 | 1.373 | 0.86/2.21 | 0.190 |
| Weekly Resistance Training Hours | 0.216 | 0.390 * | 0.022 | 2.86 * | 1.06/7.57 | 0.037 |
| Conditioning Training Measures | ||||||
| Total Weekly Mileage | 0.069 | 0.058 | 0.738 | 1.01 | 0.95/1.09 | 0.703 |
| Total Weekly Hours | 0.0641 | −0.017 | 0.923 | 0.963 | 0.85/1.09 | 0.552 |
| Total Weekly Cross Training Hours | 0.115 | 0.237 | 0.176 | 1.37 | 0.84/2.23 | 0.204 |
| Weekly Resistance Training Hours | 0.180 | 0.355 * | 0.044 | 2.38 * | 1.03/5.50 | 0.042 |
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|---|
| Dependent Variable | SDS | PSS | PHQ-9 | ||||||
| Competition Training Measures | |||||||||
| Total Weekly Mileage | 1.39 | 0.76/2.61 | 0.263 | 0.98 | 0.91/1.05 | 0.597 | 0.98 | 0.91/1.05 | 0.550 |
| Total Weekly Hours | 1.07 | 0.95/1.21 | 0.272 | 1.18 * | 1.03/1.38 | 0.031 | 0.98 | 0.87/1.11 | 0.788 |
| Total Weekly Cross Training Hours | 1.25 | 0.82/1.98 | 0.312 | 1.26 | 0.77/2.16 | 0.374 | 1.20 | 0.74/1.92 | 0.455 |
| Weekly Resistance Training Hours | 1.35 | 0.72/2.58 | 0.939 | 1.277 | 0.65/2.68 | 0.489 | 0.408 * | 0.19/0.83 | 0.017 |
| Conditioning Training Measures | |||||||||
| Total Weekly Mileage | 1.09 * | 1.03/1.18 | 0.009 | 1.00 | 0.93/1.07 | 0.975 | 1.04 | 0.96/1.12 | 0.338 |
| Total Weekly Hours | 1.08 | 0.97/1.22 | 0.172 | 1.15 * | 1.02/1.35 | 0.035 | 0.99 | 0.88/1.11 | 0.858 |
| Total Weekly Cross Training Hours | 0.927 | 0.62/1.39 | 0.710 | 1.24 | 0.74/2.17 | 0.423 | 0.93 | 0.56/1.49 | 0.753 |
| Weekly Resistance Training Hours | 1.85 * | 1.05/3.39 | 0.036 | 1.28 | 0.68/2.51 | 0.454 | 0.452 * | 0.22/0.87 | 0.022 |
| Total Risk Factor Count | 0.86 | 0.67/1.09 | 0.217 | 1.11 | 0.86/1.45 | 0.442 | 1.17 | 0.92/1.49 | 0.211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parnell, S.; Graybeal, A.J.; Renna, M.E.; Stavres, J. Preliminary Evaluation of Self-Reported Training Volume as an Adjunct Measure of Female Athlete Triad Risk in Division 1 Collegiate Female Runners. J. Funct. Morphol. Kinesiol. 2024, 9, 179. https://doi.org/10.3390/jfmk9040179
Parnell S, Graybeal AJ, Renna ME, Stavres J. Preliminary Evaluation of Self-Reported Training Volume as an Adjunct Measure of Female Athlete Triad Risk in Division 1 Collegiate Female Runners. Journal of Functional Morphology and Kinesiology. 2024; 9(4):179. https://doi.org/10.3390/jfmk9040179
Chicago/Turabian StyleParnell, Sarah, Austin J. Graybeal, Megan E. Renna, and Jon Stavres. 2024. "Preliminary Evaluation of Self-Reported Training Volume as an Adjunct Measure of Female Athlete Triad Risk in Division 1 Collegiate Female Runners" Journal of Functional Morphology and Kinesiology 9, no. 4: 179. https://doi.org/10.3390/jfmk9040179
APA StyleParnell, S., Graybeal, A. J., Renna, M. E., & Stavres, J. (2024). Preliminary Evaluation of Self-Reported Training Volume as an Adjunct Measure of Female Athlete Triad Risk in Division 1 Collegiate Female Runners. Journal of Functional Morphology and Kinesiology, 9(4), 179. https://doi.org/10.3390/jfmk9040179






