The Effects of Anchor Schemes on Performance Fatigability, Neuromuscular Responses and the Perceived Sensations That Contributed to Task Termination
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethical Approval
2.3. Time Course of Procedures
2.4. Orientation Session
2.5. Test Visits
2.6. Post-Test Questionnaire
2.7. Electromyographic and Torque Acquisition
2.8. Statistical Analysis
3. Results
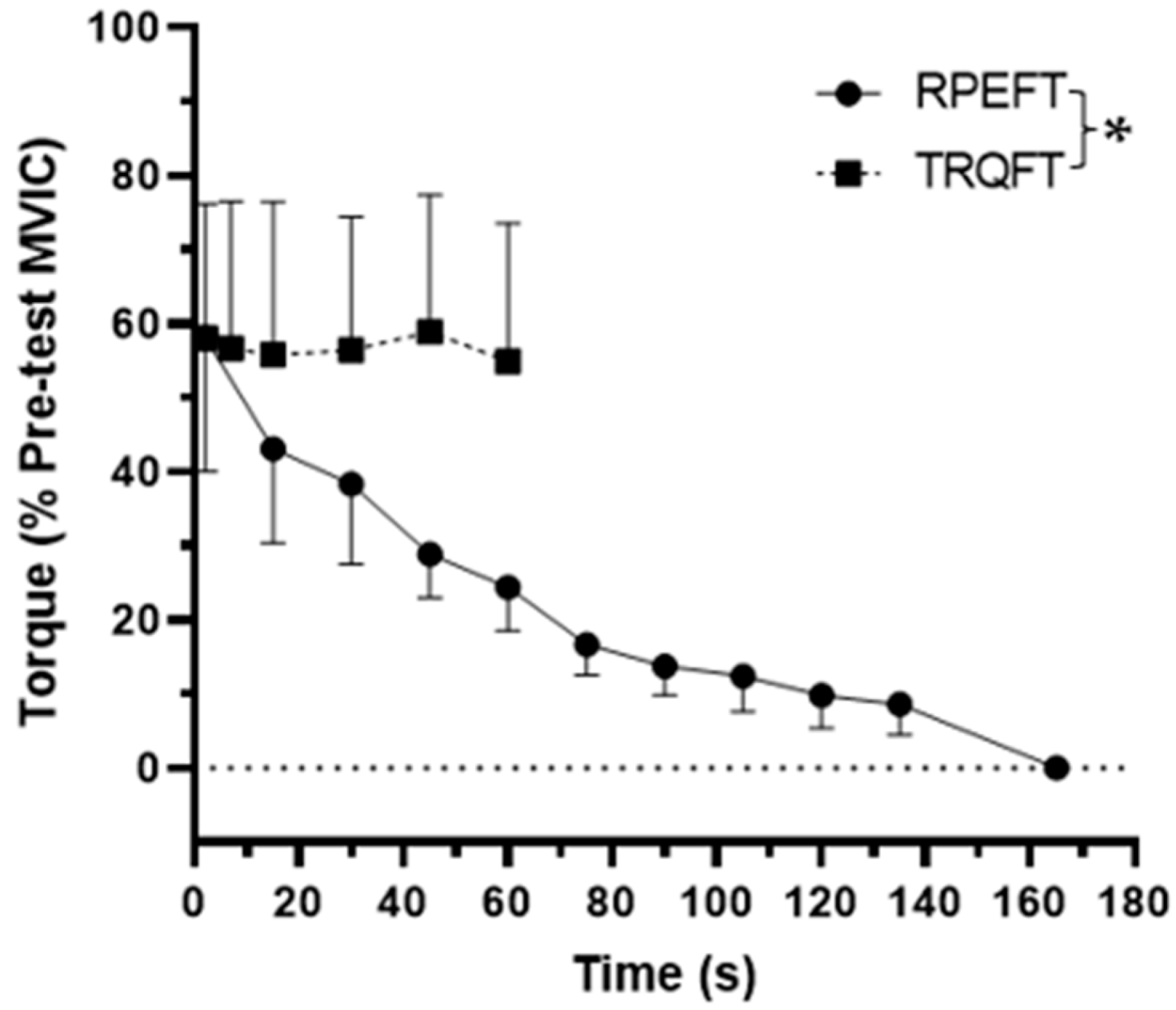
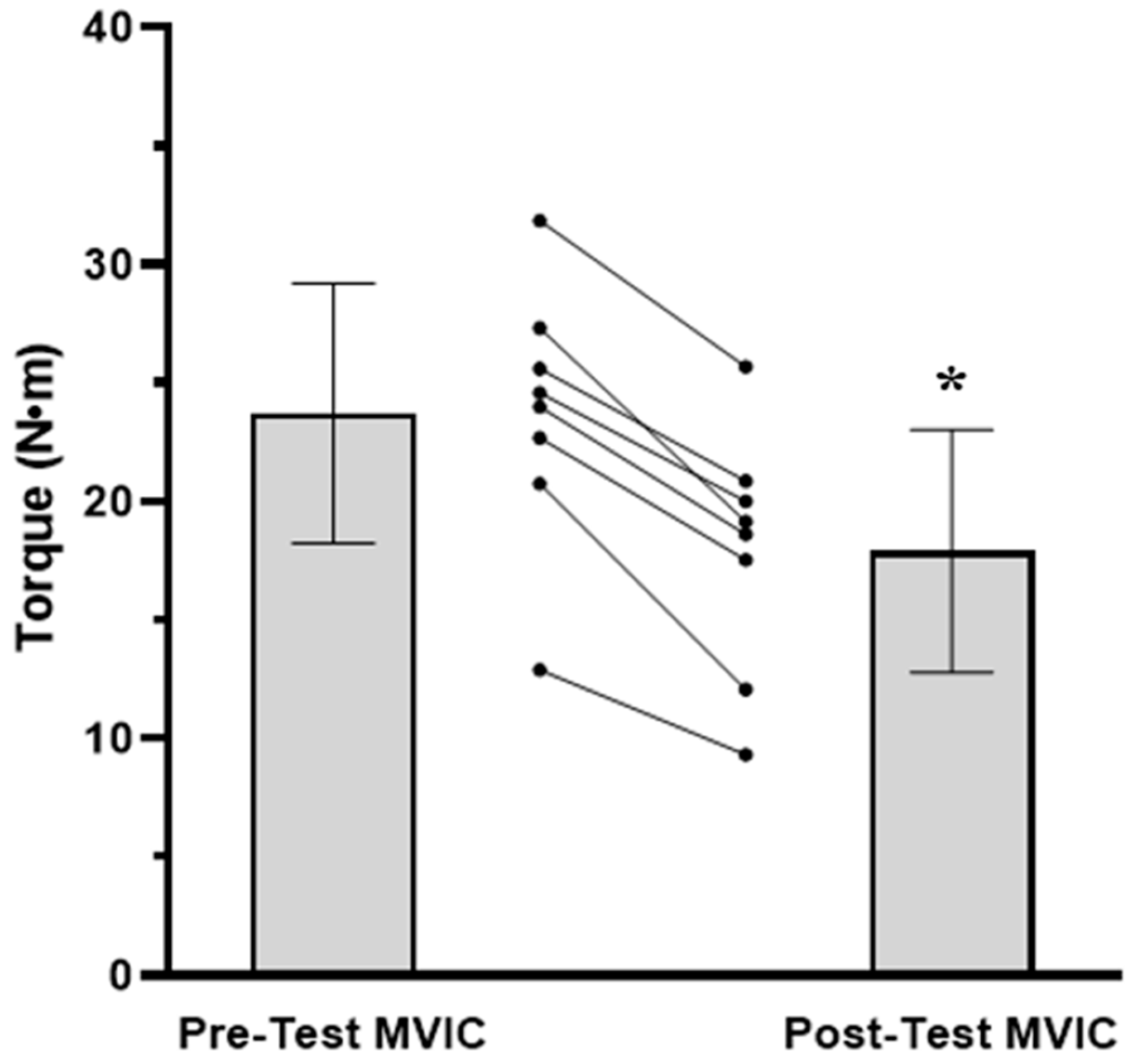
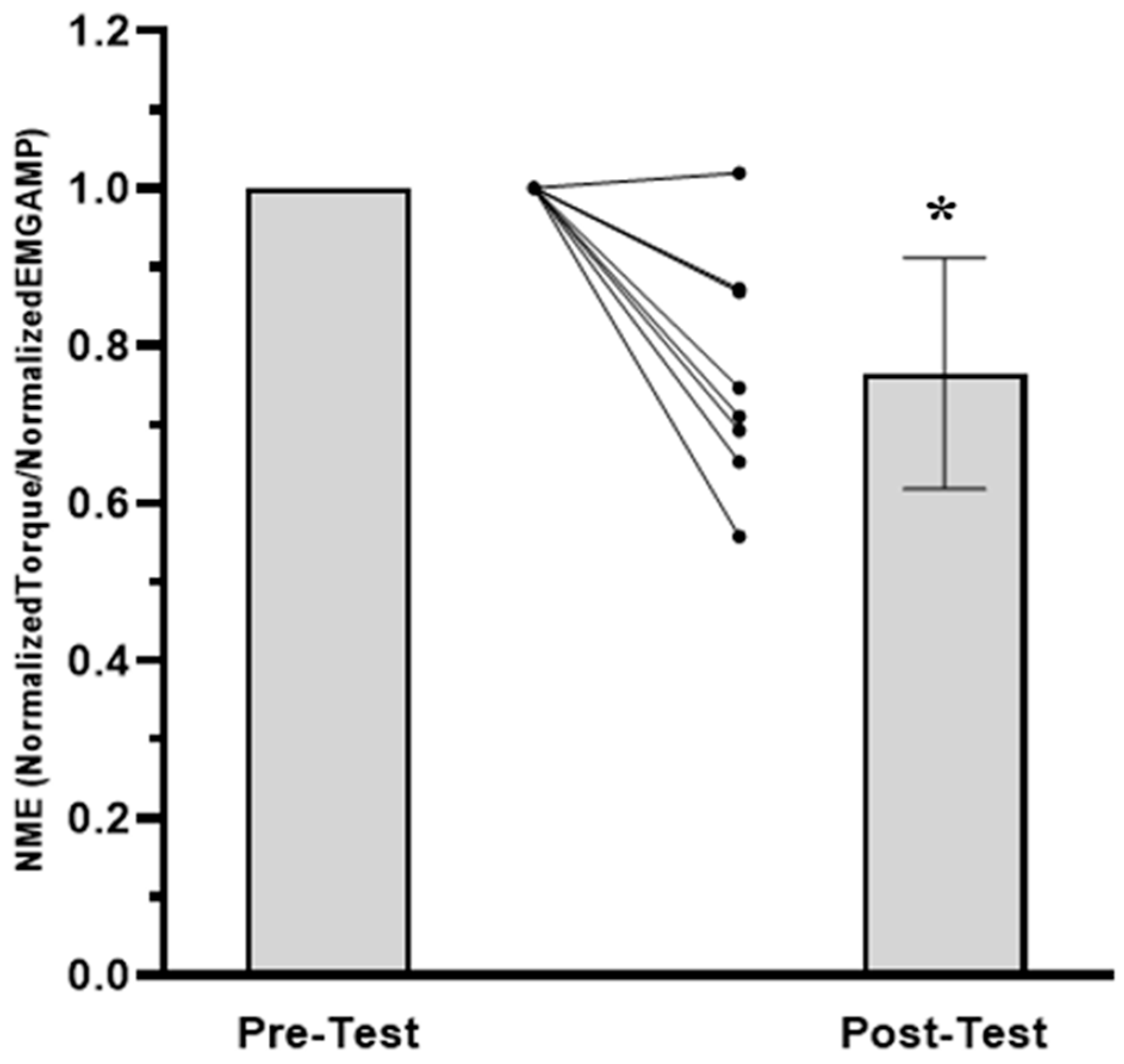
3.1. Time to Task Failure, MVIC, EMG AMP, and NME
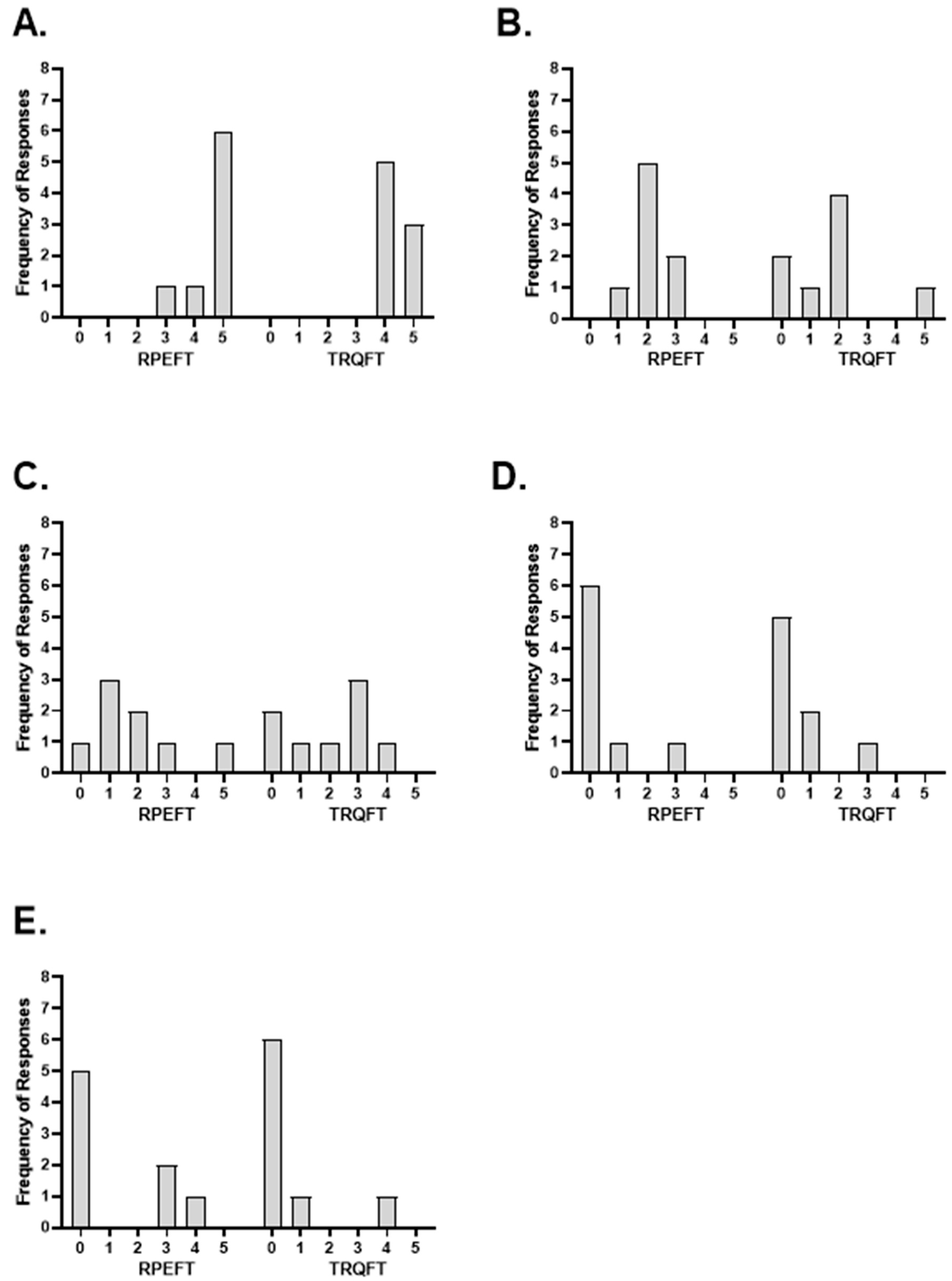
3.2. Post-Test Questionnaire
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Standardized OMNI-RES Instructions
Appendix B
| Please rate each item from 0–5 where a 0 indicates that the item had no contribution to the decision to terminate the task and a 5 indicates that the item had the greatest level of contribution to the decision to terminate the task. If you feel that the item’s intensity of contribution to terminating the task is somewhere in between these two reference points, please rate it accordingly with a 1–4 rating. Finally, if you feel that more than one item contributed equally to the decision to terminate the task, rate them the same. | ||||||
| 0 No Contribution | 1 | 2 | 3 | 4 | 5 Greatest level of Contribution | |
| Biceps Brachii | 0 | 1 | 2 | 3 | 4 | 5 |
| Forearm Muscles | 0 | 1 | 2 | 3 | 4 | 5 |
| Hand Muscles | 0 | 1 | 2 | 3 | 4 | 5 |
| Loss of Mental Focus | 0 | 1 | 2 | 3 | 4 | 5 |
| Loss of Motivation | 0 | 1 | 2 | 3 | 4 | 5 |
Instructions for the Post-Test Questionnaire
References
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and Fatigability in Neurologic Illnesses: Proposal for a Unified Taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Translating Fatigue to Human Performance. Med. Sci. Sports Exerc. 2016, 48, 2228–2238. [Google Scholar] [CrossRef]
- Behrens, M.; Gube, M.; Chaabene, H.; Prieske, O.; Zenon, A.; Broscheid, K.-C.; Schega, L.; Husmann, F.; Weippert, M. Fatigue and Human Performance: An Updated Framework. Sports Med. 2023, 53, 7–31. [Google Scholar] [CrossRef]
- Browne, S.; Renfree, A. Exercise Performance and Neuromuscular Activity at a Fixed Level of RPE following Manipulation of Peripheral Physiological Status. J. Hum. Sport Exerc. 2013, 8, 820–828. [Google Scholar] [CrossRef]
- Robertson, R.J.; Noble, B.J. Perception of Physical Exertion: Methods, Mediators, and Applications. Exerc. Sport Sci. Rev. 1997, 25, 407–452. [Google Scholar] [CrossRef]
- Tucker, R. The Anticipatory Regulation of Performance: The Physiological Basis for Pacing Strategies and the Development of a Perception-Based Model for Exercise Performance. Br. J. Sports Med. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Smith, R.W.; Housh, T.J.; Anders, J.P.V.; Neltner, T.J.; Arnett, J.E.; Ortega, D.G.; Schmidt, R.J.; Johnson, G.O. Torque and Neuromuscular Responses Are Not Joint Angle Dependent during a Sustained, Isometric Task Anchored to a High Perceptual Intensity. Am. J. Sports Sci. Med. 2022, 10, 29–39. [Google Scholar] [CrossRef]
- Smith, R.W.; Housh, T.J.; Anders, J.P.V.; Neltner, T.J.; Arnett, J.E.; Schmidt, R.J.; Johnson, G.O. Application of the Ratings of Perceived Exertion-Clamp Model to Examine the Effects of Joint Angle on the Time Course of Torque and Neuromuscular Responses during a Sustained, Isometric Forearm Flexion to Task Failure. J. Strength Cond. Res. 2022. accepted. [Google Scholar] [CrossRef]
- Smith, R.W.; Housh, T.J.; Anders, J.P.V.; Neltner, T.J.; Arnett, J.E.; Schmidt, R.J.; Johnson, G.O. Time Course of Changes in Torque and Neuromuscular Parameters during a Sustained Isometric Forearm Flexion Task to Fatigue Anchored to a Constant Rating of Perceived Exertion. J. Musculoskelet. Neuronal Interact. 2022, 22, 455–464. [Google Scholar]
- Arnett, J.E.; Smith, R.W.; Neltner, T.J.; Anders, J.P.V.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. The RPE Clamp Model and Fatigability following a Sustained, Isometric Task to Failure. J. Exerc. Physiol. Online 2022, 25, 13–26. [Google Scholar]
- Keller, J.L.; Housh, T.J.; Hill, E.C.; Smith, C.M.; Schmidt, R.J.; Johnson, G.O. Are There Sex-Specific Neuromuscular or Force Responses to Fatiguing Isometric Muscle Actions Anchored to a High Perceptual Intensity? J. Strength Cond. Res. 2022, 36, 156–161. [Google Scholar] [CrossRef]
- Smith, R.W.; Anders, J.P.V.; Neltner, T.J.; Arnett, J.E.; Keller, J.L.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. Perceptual Fatigability and Neuromuscular Responses during a Sustained, Isometric Forearm Flexion Muscle Action Anchored to a Constant Level of Perceived Exertion. NeuroSports 2021, 1, 2. [Google Scholar]
- Keller, J.L.; Housh, T.J.; Anders, J.P.V.; Neltner, T.J.; Schmidt, R.J.; Johnson, G.O. Anchor Scheme, Intensity, and Time to Task Failure Do Not Influence Performance Fatigability or Changes in Neuromuscular Responses following Bilateral Leg Extensions. J. Exerc. Physiol. Online 2020, 23, 119–134. [Google Scholar]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.S.; Vieira, T.M. Interpreting Signal Amplitudes in Surface Electromyography Studies in Sport and Rehabilitation Sciences. Front. Physiol. 2017, 8, 985. [Google Scholar] [CrossRef]
- Basmajian, J.V.; De Luca, C.J. Muscles Alive: Their Functions Revealed by Electromyography; Williams & Wilkins: Baltimore, MD, USA, 1985; ISBN 0-683-00414-X. [Google Scholar]
- Keller, J.L.; Housh, T.J.; Hill, E.C.; Smith, C.M.; Schmidt, R.J.; Johnson, G.O. Neuromuscular Responses of Recreationally Active Women during a Sustained, Submaximal Isometric Leg Extension Muscle Action at a Constant Perception of Effort. Eur. J. Appl. Physiol. 2018, 118, 2499–2508. [Google Scholar] [CrossRef]
- Keller, J.L.; Housh, T.J.; Hill, E.C.; Smith, C.M.; Schmidt, R.J.; Johnson, G.O. Self-Regulated Force and Neuromuscular Responses During Fatiguing Isometric Leg Extensions Anchored to a Rating of Perceived Exertion. Appl. Psychophysiol. Biofeedback 2019, 44, 343–350. [Google Scholar] [CrossRef]
- Lagally, K.M.; Robertson, R.J. Construct Validity of the OMNI Resistance Exercise Scale. J. Strength Cond. Res. 2006, 20, 252–256. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent Validation of the OMNI Perceived Exertion Scale for Resistance Exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef]
- Robertson, R.J. Perceived Exertion for Practitioners: Rating Effort with the OMNI Picture System; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Jones, A.A.; Power, G.A.; Herzog, W. History Dependence of the Electromyogram: Implications for Isometric Steady-State EMG Parameters following a Lengthening or Shortening Contraction. J. Electromyogr. Kinesiol. 2016, 27, 30–38. [Google Scholar] [CrossRef]
- Miller, R.G.; Giannini, D.; Milner-Brown, H.S.; Layzer, R.B.; Koretsky, A.P.; Hooper, D.; Weiner, M.W. Effects of Fatiguing Exercise on High-Energy Phosphates, Force, and EMG: Evidence for Three Phases of Recovery. Muscle Nerve 1987, 10, 810–821. [Google Scholar] [CrossRef]
- Milner-Brown, H.S.; Mellenthin, M.; Miller, R.G. Quantifying Human Muscle Strength, Endurance and Fatigue. Arch. Phys. Med. Rehabil. 1986, 67, 530–535. [Google Scholar]
- Marcora, S. Psychobiology of Fatigue during Endurance Exercise. In Endurance Performance in Sport; Meijen, C., Ed.; Routledge: London, UK, 2019; pp. 15–34. ISBN 978-1-315-16731-2. [Google Scholar]
- Hureau, T.J.; Romer, L.M.; Amann, M. The “Sensory Tolerance Limit”: A Hypothetical Construct Determining Exercise Performance? Eur. J. Sport Sci. 2018, 18, 13–24. [Google Scholar] [CrossRef]
- Noakes, T.D.; St Clair Gibson, A.; Lambert, E.V. From Catastrophe to Complexity: A Novel Model of Integrative Central Neural Regulation of Effort and Fatigue during Exercise in Humans. Br. J. Sports Med. 2004, 38, 511–514. [Google Scholar] [CrossRef]
- St Clair Gibson, A.; Swart, J.; Tucker, R. The Interaction of Psychological and Physiological Homeostatic Drives and Role of General Control Principles in the Regulation of Physiological Systems, Exercise and the Fatigue Process—The Integrative Governor Theory. Eur. J. Sport Sci. 2018, 18, 25–36. [Google Scholar] [CrossRef]
- Venhorst, A.; Micklewright, D.P.; Noakes, T.D. The Psychophysiological Determinants of Pacing Behaviour and Performance During Prolonged Endurance Exercise: A Performance Level and Competition Outcome Comparison. Sports Med. 2018, 48, 2387–2400. [Google Scholar] [CrossRef]
- Greenhouse-Tucknott, A.; Butterworth, J.B.; Wrightson, J.G.; Harrison, N.A.; Dekerle, J. Effect of the Subjective Intensity of Fatigue and Interoception on Perceptual Regulation and Performance during Sustained Physical Activity. PLoS ONE 2022, 17, e0262303. [Google Scholar] [CrossRef]
- De Paula Caraça Smirmaul, B. Sense of Effort and Other Unpleasant Sensations during Exercise: Clarifying Concepts and Mechanisms. Br. J. Sports Med. 2012, 46, 308–311. [Google Scholar] [CrossRef]
- Mauger, A.R. Fatigue Is a Pain-the Use of Novel Neurophysiological Techniques to Understand the Fatigue-Pain Relationship. Front. Physiol. 2013, 4, 104. [Google Scholar] [CrossRef]
- Venhorst, A.; Micklewright, D.; Noakes, T.D. Towards a Three-Dimensional Framework of Centrally Regulated and Goal-Directed Exercise Behaviour: A Narrative Review. Br. J. Sports Med. 2018, 52, 957–966. [Google Scholar] [CrossRef]
- Hall, E.E.; Ekkekakis, P.; Petruzzello, S.J. Is the Relationship of RPE to Psychological Factors Intensity-Dependent? Med. Sci. Sports Exerc. 2005, 37, 1365–1373. [Google Scholar] [CrossRef]
- Drouin, P.J.; Tschakovsky, M.E. The Oxygen-Conformer Response and Its Contribution to Task Failure in Exhaustive Exercise. J. Appl. Physiol. 2019, 126, 796. [Google Scholar] [CrossRef]
- Mauger, A.R. Factors Affecting the Regulation of Pacing: Current Perspectives. Open Access J. Sports Med. 2014, 5, 209–214. [Google Scholar] [CrossRef]
- Smits, B.L.M.; Pepping, G.-J.; Hettinga, F.J. Pacing and Decision Making in Sport and Exercise: The Roles of Perception and Action in the Regulation of Exercise Intensity. Sports Med. 2014, 44, 763–775. [Google Scholar] [CrossRef]
- Amann, M.; Venturelli, M.; Ives, S.J.; McDaniel, J.; Layec, G.; Rossman, M.J.; Richardson, R.S. Peripheral Fatigue Limits Endurance Exercise via a Sensory Feedback-Mediated Reduction in Spinal Motoneuronal Output. J. Appl. Physiol. 2013, 115, 355–364. [Google Scholar] [CrossRef]
- Rejeski, W.J. The Perception of Exertion: A Social Psychophysiological Integration. J. Sport Exerc. Psychol. 1981, 3, 305–320. [Google Scholar] [CrossRef]
- Hutchinson, J.C.; Tenenbaum, G. Perceived Effort and Exertion. In APA Handbook of Sport and Exercise Psychology, Volume 2: Exercise Psychology; Anshel, M.H., Ed.; APA Handbooks in Psychology Series; American Psychological Association: Washington, DC, USA, 2019; pp. 159–182. ISBN 1433830418. [Google Scholar]
- Arnett, J.E.; Smith, R.W.; Neltner, T.J.; Anders, J.P.V.; Ortega, D.G.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. The Effects of Joint Angle and Anchoring Scheme on Performance Fatigability and Neuromuscular Responses following Isometric Forearm Flexion Tasks to Failure. NeuroSports 2023, 1, 7. [Google Scholar]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Kulig, K.; Andrews, J.G.; Hay, J.G. Human Strength Curves. Exerc. Sport Sci. Rev. 1984, 12, 417–466. [Google Scholar] [CrossRef]
- Gearhart, R.E.; Goss, F.L.; Lagally, K.M.; Jakicic, J.M.; Gallagher, J.; Robertson, R.J. Standardized Scaling Procedures for Rating Perceived Exertion during Resistance Exercise. J. Strength Cond. Res. 2001, 15, 320–325. [Google Scholar]
- Albertus, Y.; Tucker, R.; St Clair Gibson, A.; Lambert, E.V.; Hampson, D.B.; Noakes, T.D. Effect of Distance Feedback on Pacing Strategy and Perceived Exertion during Cycling. Med. Sci. Sports Exerc. 2005, 37, 461–468. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Scheff, S.W. Chapter 8—Nonparametric Statistics. In Fundamental Statistical Principles for the Neurobiologist; Scheff, S.W., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 157–182. ISBN 978-0-12-804753-8. [Google Scholar]
- Carr, J.C.; Beck, T.W.; Ye, X.; Wages, N.P. Intensity-Dependent EMG Response for the Biceps Brachii during Sustained Maximal and Submaximal Isometric Contractions. Eur. J. Appl. Physiol. 2016, 116, 1747–1755. [Google Scholar] [CrossRef]
- Farina, D.; Merletti, R.; Enoka, R.M. The Extraction of Neural Strategies from the Surface EMG. J. Appl. Physiol. 2004, 96, 1486–1495. [Google Scholar] [CrossRef]
- Moritani, T.; Nagata, A.; Muro, M. Electromyographic Manifestations of Muscular Fatigue. Med. Sci. Sports Exerc. 1982, 14, 198–202. [Google Scholar] [CrossRef]
- Thomas, K.; Goodall, S.; Howatson, G. Performance Fatigability Is Not Regulated to A Peripheral Critical Threshold. Exerc. Sport Sci. Rev. 2018, 46, 240–246. [Google Scholar] [CrossRef]
- Carlson, B.M. Chapter 5—The Muscular System. In The Human Body; Carlson, B.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 111–136. ISBN 978-0-12-804254-0. [Google Scholar]
- Duchateau, J.; Enoka, R.M. Distribution of Motor Unit Properties across Human Muscles. J. Appl. Physiol. 2022, 132, 1–13. [Google Scholar] [CrossRef]
- Laett, C.; Gavilão, U.; do Rio, J.; Cossich, V.; de Oliveira, C.G. Relationship between Upper and Lower Limbs Muscle Explosive Strength with the Vastus Lateralis and Biceps Brachii Architecture. Rev. Bras. Ciênc. Esporte 2021, 43, e012820. [Google Scholar] [CrossRef]
- Ohtaka, C.; Fujiwara, M. Force Control Characteristics for Generation and Relaxation Compared between the Upper and Lower Limbs. J. Hum. Sport Exerc. 2022, 17, 181–196. [Google Scholar] [CrossRef]
- Wan, J.; Qin, Z.; Wang, P.; Sun, Y.; Liu, X. Muscle Fatigue: General Understanding and Treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal Muscle Energy Metabolism during Exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Anders, J.P.V.; Keller, J.L.; Neltner, T.J.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. Task-Specific Performance Fatigability and the Bilateral Deficit during Isokinetic Leg Extensions. J. Musculoskelet. Neuronal Interact. 2021, 21, 4–12. [Google Scholar] [PubMed]
- Hunter, S.K. Sex Differences in Human Fatigability: Mechanisms and Insight to Physiological Responses. Acta Physiol. 2014, 210, 768–789. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Taylor, J.L.; Gandevia, S.C. A Comparison of Central Aspects of Fatigue in Submaximal and Maximal Voluntary Contractions. J. Appl. Physiol. 2008, 104, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, D.; Kayser, B.; Place, N. Are There Critical Fatigue Thresholds? Aggregated vs. Individual Data. Front. Physiol. 2016, 7, 376. [Google Scholar] [CrossRef]
- Krogh-Lund, C. Myo-Electric Fatigue and Force Failure from Submaximal Static Elbow Flexion Sustained to Exhaustion. Eur. J. Appl. Physiol. 1993, 67, 389–401. [Google Scholar] [CrossRef]
- Bonde-Petersen, F.; Mork, A.L.; Nielsen, E. Local Muscle Blood Flow and Sustained Contractions of Human Arm and Back Muscles. Eur. J. Appl. Physiol. 1975, 34, 43–50. [Google Scholar] [CrossRef]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and Peripheral Fatigue in Physical Exercise Explained: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal Muscle Fatigue: Cellular Mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef]
- Allen, D.G.; Trajanovska, S. The Multiple Roles of Phosphate in Muscle Fatigue. Front. Physiol. 2012, 3, 463. [Google Scholar] [CrossRef]
- Maclaren, D.P.; Gibson, H.; Parry-Billings, M.; Edwards, R.H. A Review of Metabolic and Physiological Factors in Fatigue. Exerc. Sport Sci. Rev. 1989, 17, 29–66. [Google Scholar] [CrossRef]
- Hureau, T.J.; Broxterman, R.M.; Weavil, J.C.; Lewis, M.T.; Layec, G.; Amann, M. On the Role of Skeletal Muscle Acidosis and Inorganic Phosphates as Determinants of Central and Peripheral Fatigue: A 31 P-MRS Study. J. Physiol. 2022, 600, 3069–3081. [Google Scholar] [CrossRef]
- Orizio, C.; Gobbo, M.; Diemont, B.; Esposito, F.; Veicsteinas, A. The Surface Mechanomyogram as a Tool to Describe the Influence of Fatigue on Biceps Brachii Motor Unit Activation Strategy. Historical Basis and Novel Evidence. Eur. J. Appl. Physiol. 2003, 90, 326–336. [Google Scholar] [CrossRef]
- Zénon, A.; Sidibé, M.; Olivier, E. Disrupting the Supplementary Motor Area Makes Physical Effort Appear Less Effortful. J. Neurosci. 2015, 35, 8737–8744. [Google Scholar] [CrossRef]
- Kim, S.-I. Neuroscientific Model of Motivational Process. Front. Psychol. 2013, 4, 98. [Google Scholar] [CrossRef]
- Benedek, M.; Jauk, E.; Beaty, R.E.; Fink, A.; Koschutnig, K.; Neubauer, A.C. Brain Mechanisms Associated with Internally Directed Attention and Self-Generated Thought. Sci. Rep. 2016, 6, 22959. [Google Scholar] [CrossRef]
- Seidler, R.D.; Noll, D.C.; Thiers, G. Feedforward and Feedback Processes in Motor Control. NeuroImage 2004, 22, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Halperin, I.; Emanuel, A. Rating of Perceived Effort: Methodological Concerns and Future Directions. Sports Med. 2020, 50, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Arabadzhiev, T.I.; Dimitrov, V.G.; Dimitrova, N.A.; Dimitrov, G.V. Interpretation of EMG Integral or RMS and Estimates of “Neuromuscular Efficiency” Can Be Misleading in Fatiguing Contraction. J. Electromyogr. Kinesiol. 2010, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.E.C.; Bryan, A.D.; Eaton, M. Menstrual Cycle Effects on Perceived Exertion and Pain during Exercise among Sedentary Women. J. Women’s Health 2011, 20, 439–446. [Google Scholar] [CrossRef]
- Mattu, A.T.; Iannetta, D.; MacInnis, M.J.; Doyle-Baker, P.K.; Murias, J.M. Menstrual and Oral Contraceptive Cycle Phases Do Not Affect Submaximal and Maximal Exercise Responses. Scand. J. Med. Sci. Sports 2020, 30, 472–484. [Google Scholar] [CrossRef]

Orientation Session
| Test Visit 1
| Test Visit 2
|
| Subjects | RPEFT | TRQFT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biceps Brachii | Forearm Muscles | Hand Muscles | Loss of Motivation | Loss of Focus | Biceps Brachii | Forearm Muscles | Hand Muscles | Loss of Motivation | Loss of Focus | |
| 1 | 5 | 2 | 2 | 0 | 0 | 5 | 1 | 1 | 0 | 0 |
| 2 | 5 | 2 | 1 | 0 | 0 | 5 | 2 | 0 | 0 | 0 |
| 3 | 5 | 2 | 3 | 0 | 0 | 4 | 2 | 3 | 0 | 0 |
| 4 | 5 | 3 | 2 | 0 | 0 | 4 | 2 | 2 | 0 | 0 |
| 5 | 5 | 3 | 1 | 1 | 4 | 4 | 5 | 3 | 1 | 1 |
| 6 | 4 | 2 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| 7 | 3 | 1 | 5 | 3 | 3 | 4 | 2 | 4 | 3 | 4 |
| 8 | 5 | 2 | 1 | 0 | 3 | 5 | 0 | 3 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, R.W.; Housh, T.J.; Arnett, J.E.; Anders, J.P.V.; Neltner, T.J.; Ortega, D.G.; Schmidt, R.J.; Johnson, G.O. The Effects of Anchor Schemes on Performance Fatigability, Neuromuscular Responses and the Perceived Sensations That Contributed to Task Termination. J. Funct. Morphol. Kinesiol. 2023, 8, 49. https://doi.org/10.3390/jfmk8020049
Smith RW, Housh TJ, Arnett JE, Anders JPV, Neltner TJ, Ortega DG, Schmidt RJ, Johnson GO. The Effects of Anchor Schemes on Performance Fatigability, Neuromuscular Responses and the Perceived Sensations That Contributed to Task Termination. Journal of Functional Morphology and Kinesiology. 2023; 8(2):49. https://doi.org/10.3390/jfmk8020049
Chicago/Turabian StyleSmith, Robert W., Terry J. Housh, Jocelyn E. Arnett, John Paul V. Anders, Tyler J. Neltner, Dolores G. Ortega, Richard J. Schmidt, and Glen O. Johnson. 2023. "The Effects of Anchor Schemes on Performance Fatigability, Neuromuscular Responses and the Perceived Sensations That Contributed to Task Termination" Journal of Functional Morphology and Kinesiology 8, no. 2: 49. https://doi.org/10.3390/jfmk8020049
APA StyleSmith, R. W., Housh, T. J., Arnett, J. E., Anders, J. P. V., Neltner, T. J., Ortega, D. G., Schmidt, R. J., & Johnson, G. O. (2023). The Effects of Anchor Schemes on Performance Fatigability, Neuromuscular Responses and the Perceived Sensations That Contributed to Task Termination. Journal of Functional Morphology and Kinesiology, 8(2), 49. https://doi.org/10.3390/jfmk8020049








