Mildly Impaired Foot Control in Long-Term Treated Patients with Wilson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Clinical Examination and Scoring of Neurological Symptoms
2.3. Blood and Urine Analysis
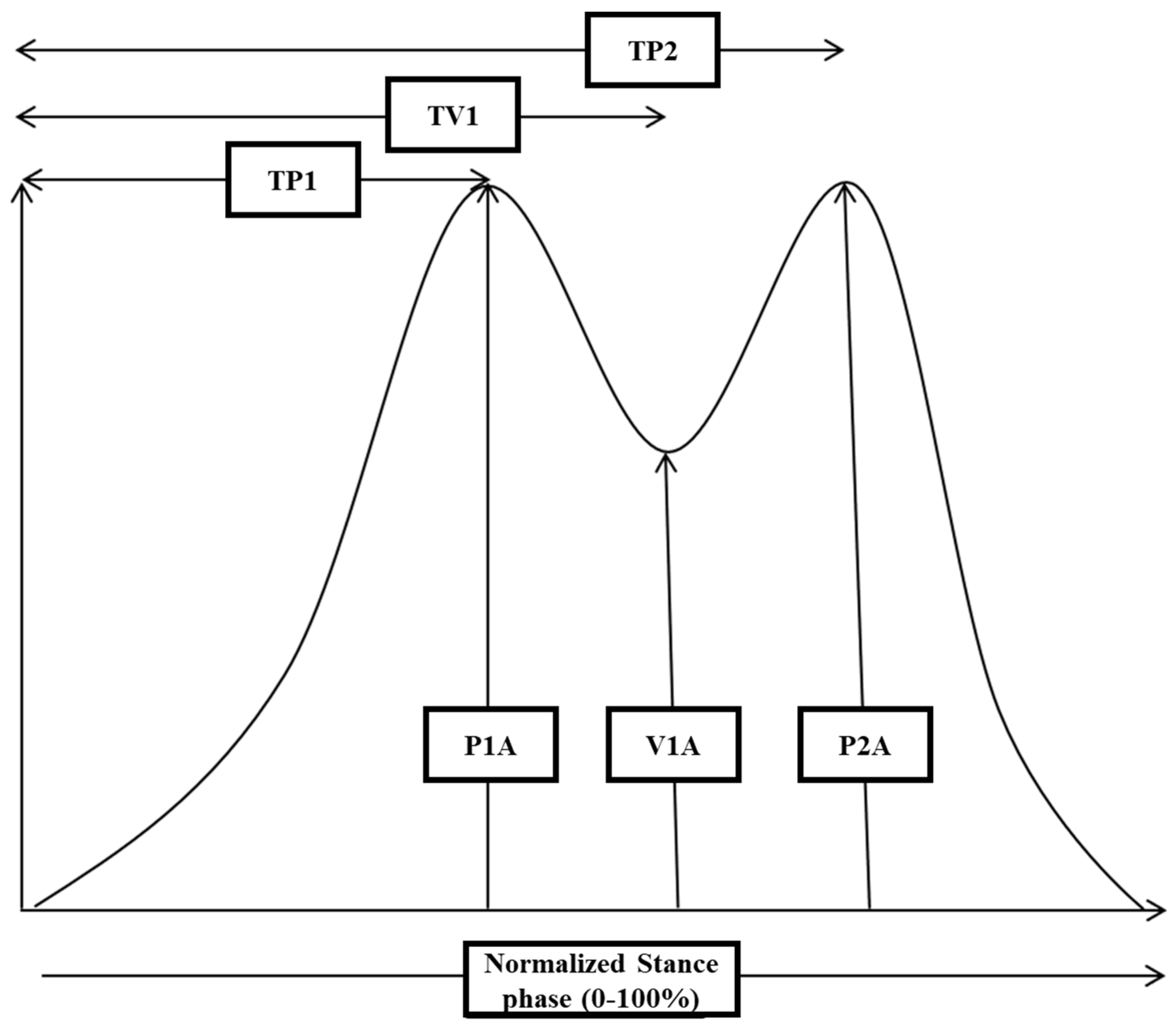
2.4. Gait Measurement
2.5. Statistics
3. Results
3.1. Comparison of Demographical Data of WD Patients and Control Subjects
3.2. Clinical Gait Abnormalities in the Patient Cohort
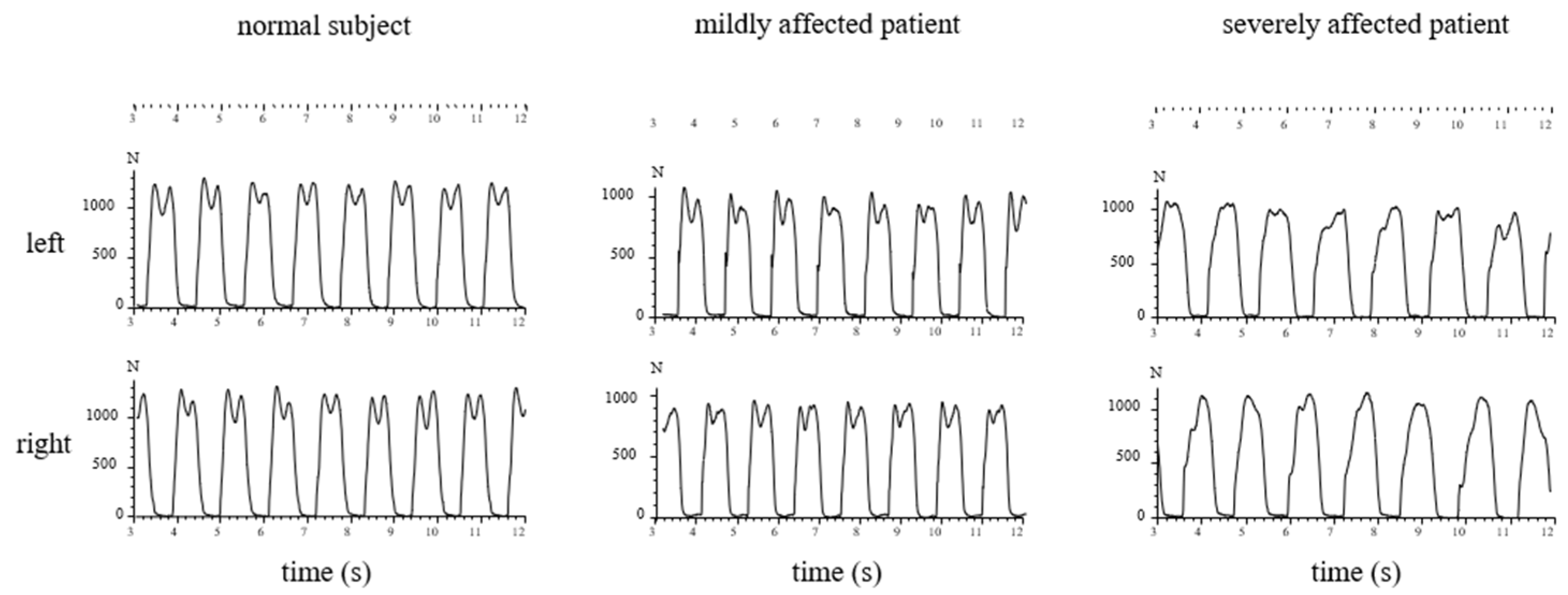
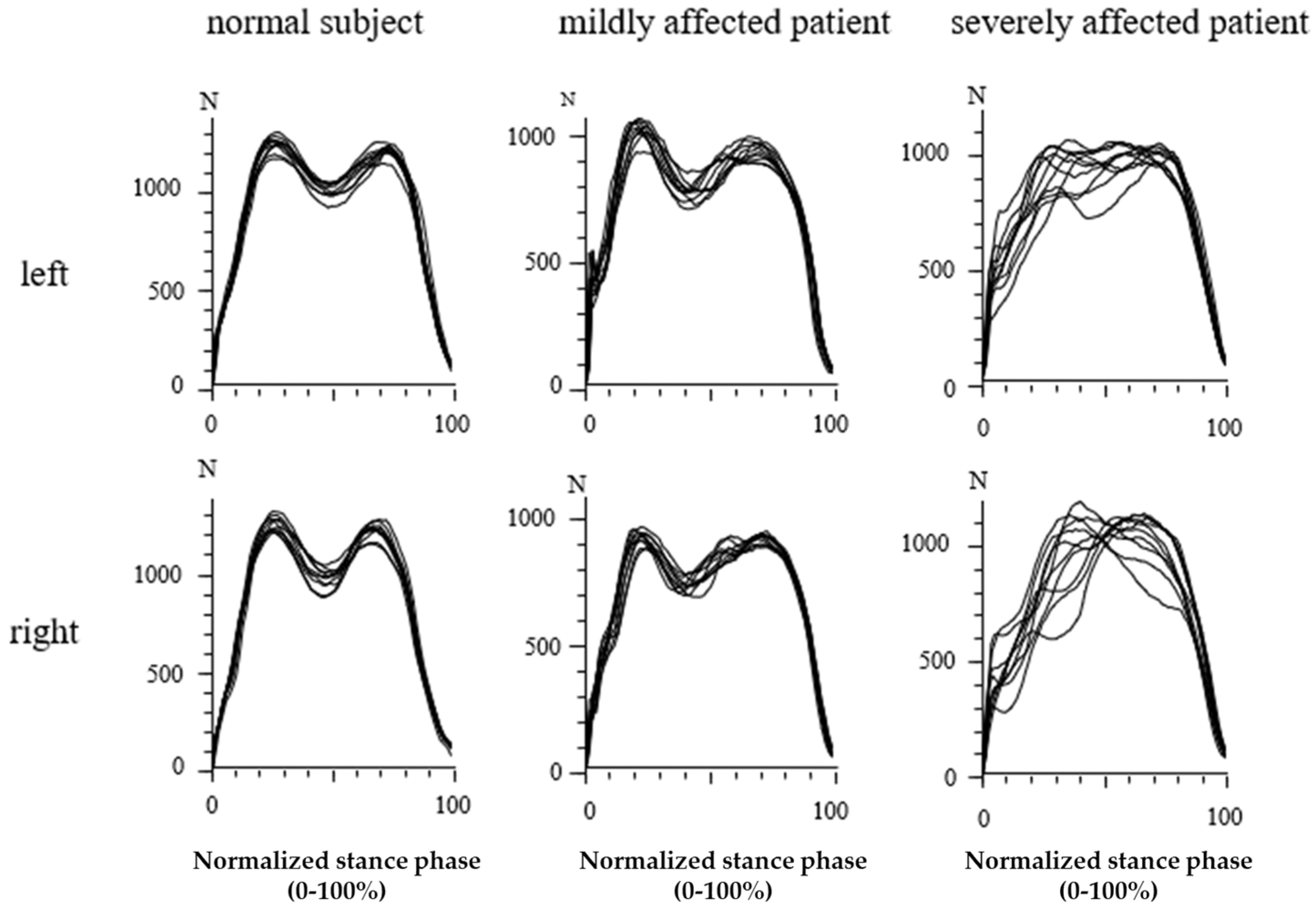
3.3. Recording of GRF-Curves in Differently Affected Patients
3.4. Percentage of WD Patients with an Abnormal Shape of GRF-Curves
3.5. Walking Speed and Cadence in WD-Patients and Control Subjects
3.6. Analysis of GRF-Curves in 24 WD Patients with a Normal Shape
3.7. Correlation of the Analysis of GRF-Curves with Clinical and Laboratory Findings
4. Discussion
4.1. Severity of Symptoms in the WD Patients
4.2. Abnormal Shape of the GRF-Curve in WD Patients
4.3. Analysis of GRF-Curves with Two Distinguishable Peaks
4.4. The Interaction between Abnormalities of the GRF-Curve and Gait Speed
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, S.A.K. Progressive lenticular degeneration: A familial nervous disease associated with cirrhosis of the liver. Brain 1912, 34, 295–507. [Google Scholar] [CrossRef]
- Cumings, J.N. The copper and iron content of brain and liver in the normal and in hepato-lenticular degeneration. Brain 1948, 71, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef]
- Petrukhin, K.; Fischer, S.G.; Pirastu, M.; Tanzi, R.E.; Chernov, I.; Devoto, M.; Brzustowicz, L.M.; Cayanis, E.; Vitale, E.; Russo, J.J.; et al. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat. Genet. 1993, 5, 338–343. [Google Scholar] [CrossRef]
- Tanzi, R.E.; Petrukhin, K.; Chernov, I.; Pellequer, J.-L.; Wasco, W.; Ross, B.; Romano, D.M.; Parano, E.; Pavone, L.; Brzustowicz, L.M.; et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 1993, 5, 344–350. [Google Scholar] [CrossRef]
- Ghika, J.; Vingerhoets, F.; Maeder, P.; Borruat, F.-X.; Bogousslavsky, J. Maladie de Wilson. EMC-Neurologie 2004, 1, 481–511. [Google Scholar] [CrossRef]
- Kuwert, T.; Hefter, H.; Scholz, D.; Milzet, M.; Weiß, P.; Arendt, G.; Herzog, H.; Loken, M.; Hennerici, M.; Feinendegen, L.E. Regional cerebral glucose consumption measured by positron emission tomography in patients with Wilson’s disease. Europ. J. Nucl. Med. 1992, 19, 96–101. [Google Scholar] [CrossRef]
- Magalhaes, A.C.A.; Caramelli, P.; Menezes, J.R.; Lo, L.S.; Bacheschi, L.A.; Barbosa, E.R.; Rosemberg, L.A.; Magalhaes, A. Wilson’s disease: MRI with clinical correlation. Neuroradiology 1994, 36, 97–100. [Google Scholar] [CrossRef]
- Machado, A.; Chien, H.F.; Deguti, M.M.; Cançado, E.; Azevedo, R.S.; Scaff, M.; Barbosa, E.R. Neurological manifestations in Wilson’s disease: Report of 119 cases. Mov. Disord. 2006, 21, 2192–2196. [Google Scholar] [CrossRef]
- Kirkham, T.H.; Kamin, D.F. Slow saccadic eye movements in Wilson’s disease. J. Neurol. Neurosurg. Psychiatry 1974, 37, 191–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ebert, D.; Georgas, E.; Rosenthal, D.; Wibowo, C.; Massing, T.; Barth, T.; Hefter, H. Phase relations between rhythmical arm movements and breathing in Wilson’s disease. Adv. Exp. Med. Biol. 2010, 669, 231–234. [Google Scholar] [PubMed]
- Clark, J.E.; Whitall, J. Development of Posture and Gait Across Life Span; University of South Carolina Press: Columbia, SC, USA, 1989. [Google Scholar]
- Whitall, J.; Getchell, N. Development of posture and gait across the life span. Child. Dev. 1995, 66, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Dayalu, P.; Nan, B.; Askari, F.; Brewer, G.J.; Lorincz, M.T. Prognostic significance of neurologic examination findings in Wilson disease. Park. Rel. Disord. 2011, 17, 551–556. [Google Scholar] [CrossRef]
- Giagheddu, A.; Demelia, L.; Puggioni, G.; Nurchi, A.M.; Contu, L.; Pirari, G.; Deplano, A.; Rachele, M.G. Epidemiologic study of hepatolenticular degeneration (Wilson’s disease) in Sardinia (1902–1983). Acta Neurol. Scand. 1985, 72, 43–45. [Google Scholar] [CrossRef]
- Oder, W.; Grimm, G.; Kollegger, H.; Ferenci, P.; Schneider, B.; Deecke, L. Neurological and neuropsychiatric spectrum of Wilson’s disease: A prospective study of 45 cases. J. Neurol. 1991, 238, 281–287. [Google Scholar] [PubMed]
- Hefter, H.; Arendt, G.; Stremmel, W.; Freund, H.-J. Motor impairment in Wilson’s disease I: Slowness of voluntary limb movements. Acta. Neurol. Scand. 1993, 87, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Dziezyc, K.; Litwin, T.; Chabik, G.; Czlonkowska, A. Frequencies of initial gait disturbances and falls in 100 Wilson’s disease patients. Gait. Posture 2015, 42, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Tezayak, O.; Rosenthal, D. Long-term outcome of neurological Wilson’s disease. Park. Rel. Disord. 2018, 49, 48–53. [Google Scholar] [CrossRef]
- Tezayak, O.; Rosenthal, D.; Hefter, H. Mild gait impairment in long-term treated patients with neurological Wilson’s disease. Ann. Transl. Med. 2019, 7, S57. [Google Scholar] [CrossRef]
- Grillner, S. Control of locomotion in bipeds, tetrapods, and fish. In Handbook of Physiology–The Nervous System II; Brookhart, J.M., Mountcastle, V.B., Eds.; American Physiological Society: Rockville, MD, USA, 1981; pp. 1179–1236. [Google Scholar]
- Duysens, J. Van de Crommert HWAA Neural control of locomotion, part 1: The central pattern generator from cats to humans. Gait. Posture. 1998, 7, 131–141. [Google Scholar] [CrossRef]
- Capaday, C. The special nature of human walking and its neural control. TRENDS Neurosci. 2002, 25, 370–376. [Google Scholar] [CrossRef]
- Tezayak, O. Gangstörungen bei Patienten mit Morbus Wilson. Ph.D. Thesis, University of Düsseldorf, Düsseldorf, Germany, 2021. [Google Scholar]
- Medici, V.; Mirante, V.G.; Fassati, L.R.; Pompili, M.; Forti, D.; Del Gaudio, M.; Trevisan, C.P.; Cillo, U.; Sturniolo, G.C.; Fagiuoli, S. Liver transplantation for Wilson’s disease: The burden of neurological and psychiatric disorders. Liver. Transplant. 2005, 11, 1056–1063. [Google Scholar] [CrossRef]
- Figure—Available via License: Creative Commons Attribution 2.0 Generic. Available online: https:///www.researchgate.net/figure/The-shoes-Computer-Dyno-Graphy-CDGR-system-Infotronic-Netherlands-Shown-is-the_fig1_23681105 (accessed on 15 November 2021).
- Jelen, P.; Wit, A.; Dudzinski, K.; Nolan, L. Expressing gait-line symmetry in able-bodied gait. Dyn. Med. 2008, 7, 17. [Google Scholar] [CrossRef]
- Sherlock, S.; Summerskill, W.H.J.; White, L.P. Portal-systemic encephalopathy. Neurological complications of liver disease. Lancet 1954, 2, 453–457. [Google Scholar] [CrossRef]
- Weissenborn, K.; Ennen, J.C.; Schomerus, H.; Rückert, N.; Heker, H. neuropsychological characterization of hepatic encephalopathy. J. Hepatol. 2001, 34, 768–773. [Google Scholar] [CrossRef]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Bleck, S.E. Gait asymmetry in community-ambulating stroke survivors. Arch. Phy. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Gage, W.H.; Brooks, D.; Bleck, S.E.; Mcllroy, W.E. Evaluation of gait symmetry after stroke: A comparison of current methods and recommendations for standardization. Gait Posture 2010, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]


| Patient | Sex | Age | Bodymass (kg) | Body Height (cm) | MotS | N-MotS | TS |
|---|---|---|---|---|---|---|---|
| patient 1 | f | 56 | 60 | 164 | 10 | 2 | 12 |
| patient 2 | f | 48 | 59 | 175 | 4 | 3 | 7 |
| patient 3 | m | 15 | 60 | 188 | 4 | 2 | 6 |
| patient 4 | m | 29 | 81 | 180 | 3 | 1 | 4 |
| patient 5 | f | 40 | 58 | 172 | 2 | 1 | 3 |
| patient 6 | f | 37 | 57 | 158 | 12 | 4 | 16 |
| mean | 37.5 | 62.5 | 172.8 | 5.8 | 2.2 | 8.0 | |
| SD | 13.1 | 8.3 | 9.9 | 3.8 | 1.1 | 4.6 |
| WD Patients with Two Peaks (n = 24) Mean (SD) | Control Subjects (n = 30) Mean (SD) | Significance p-Value | |
|---|---|---|---|
| P1A (N) | 1060 (258) | 1181 (297) | p < 0.12 (n.s.) |
| V1A (N) | 775 (219) | 801 (221) | p < 0.12 (n.s.) |
| P2A (N) | 923 (241) | 1000 (225) | p < 0.23 (n.s.) |
| PD1 (N) | 285 (117) | 380 (158) | p < 0.02 |
| PD2 (N) | 147 (75) | 200 (102) | p < 0.04 |
| TP1 (ms) | 153 (16) | 146 (16) | p < 0.13 (n.s.) |
| TP1SD (ms) | 17 (15) | 10 (4) | p < 0.02 |
| TP2 (ms) | 456 (53) | 456 (29) | p = 1.0 (n.s.) |
| TP2SD (ms) | 29 (13) | 19 (11) | p < 0.001 |
| TV1–TP1 (ms) | 159 (27) | 168 (23) | p < 0.18 (n.s.) |
| TP2–TV1 (ms) | 144 (55) | 142 (21) | p = 0.93 (n.s.) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samadzadeh, S.; Hefter, H.; Tezayak, O.; Rosenthal, D. Mildly Impaired Foot Control in Long-Term Treated Patients with Wilson’s Disease. J. Funct. Morphol. Kinesiol. 2022, 7, 5. https://doi.org/10.3390/jfmk7010005
Samadzadeh S, Hefter H, Tezayak O, Rosenthal D. Mildly Impaired Foot Control in Long-Term Treated Patients with Wilson’s Disease. Journal of Functional Morphology and Kinesiology. 2022; 7(1):5. https://doi.org/10.3390/jfmk7010005
Chicago/Turabian StyleSamadzadeh, Sara, Harald Hefter, Osman Tezayak, and Dietmar Rosenthal. 2022. "Mildly Impaired Foot Control in Long-Term Treated Patients with Wilson’s Disease" Journal of Functional Morphology and Kinesiology 7, no. 1: 5. https://doi.org/10.3390/jfmk7010005
APA StyleSamadzadeh, S., Hefter, H., Tezayak, O., & Rosenthal, D. (2022). Mildly Impaired Foot Control in Long-Term Treated Patients with Wilson’s Disease. Journal of Functional Morphology and Kinesiology, 7(1), 5. https://doi.org/10.3390/jfmk7010005






