A Competitive Sprinter’s Resting Blood Lactate Levels Fluctuate with a One-Year Training Cycle: Case Reports
Abstract
:1. Introduction
2. Materials and Methods
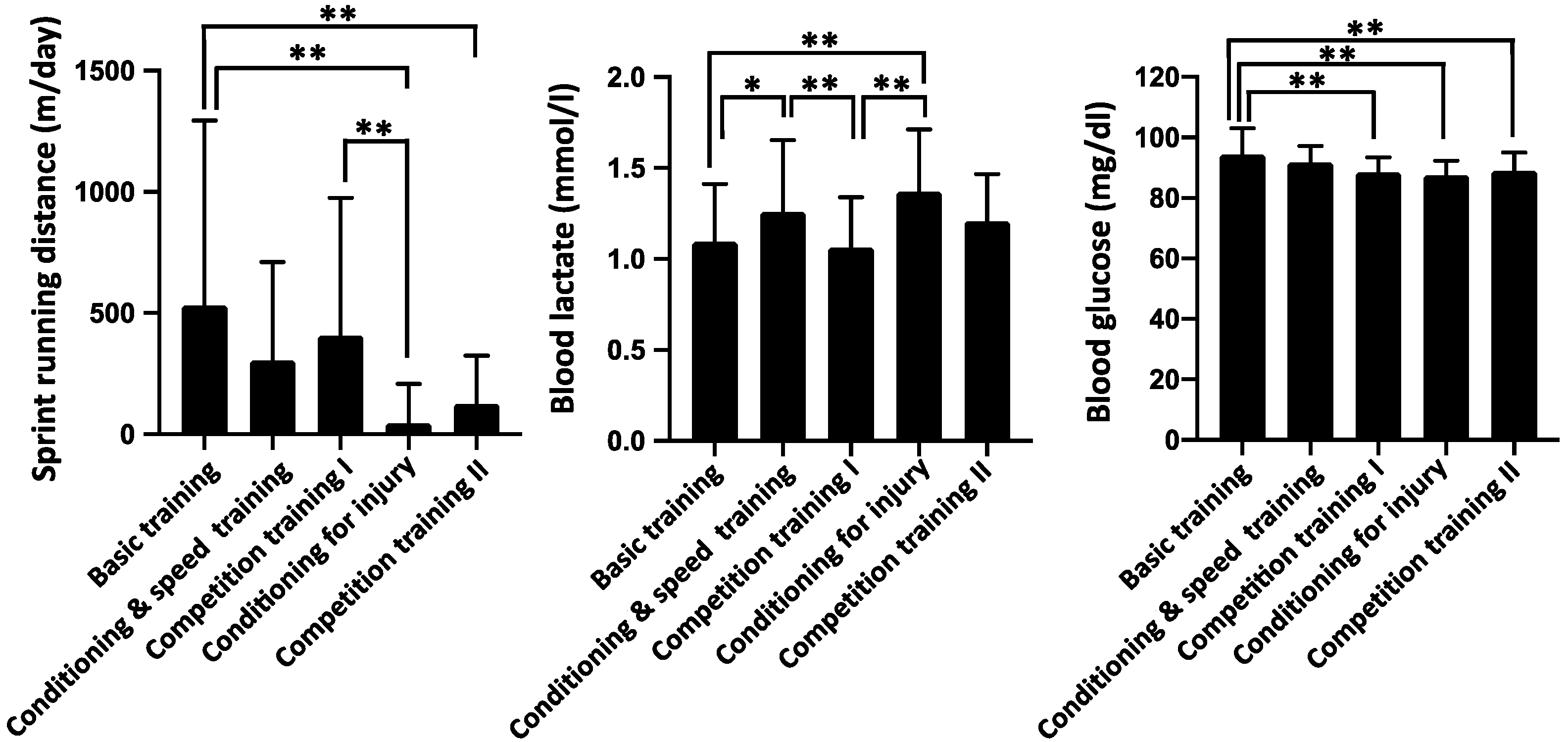
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Broskey, N.T.; Zou, K.; Dohm, G.L.; Houmard, J.A. Plasma Lactate as a Marker for Metabolic Health. Exerc. Sport Sci. Rev. 2020, 48, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Selvin, E.; Miller, E.R.; Brancati, F.L.; Young, J.H. Plasma lactate and diabetes risk in 8045 participants of the atherosclerosis risk in communities study. Ann. Epidemiol. 2013, 23, 791–796.e794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avogaro, A.; Toffolo, G.; Miola, M.; Valerio, A.; Tiengo, A.; Cobelli, C.; Del Prato, S. Intracellular lactate- and pyruvate-interconversion rates are increased in muscle tissue of non-insulin-dependent diabetic individuals. J. Clin. Investig. 1996, 98, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, T.E.; Pories, W.J.; Houmard, J.A.; Tanner, C.J.; Zheng, D.; Zou, K.; Coen, P.M.; Goodpaster, B.H.; Kraus, W.E.; Dohm, G.L. Plasma lactate as a marker of metabolic health: Implications of elevated lactate for impairment of aerobic metabolism in the metabolic syndrome. Surgery 2019, 166, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, G.; Manning, P.; Newton, J.L. Understanding Muscle Dysfunction in Chronic Fatigue Syndrome. J. Aging Res. 2016, 2016, 2497348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghali, A.; Lacout, C.; Ghali, M.; Gury, A.; Beucher, A.B.; Lozac’h, P.; Lavigne, C.; Urbanski, G. Elevated blood lactate in resting conditions correlate with post-exertional malaise severity in patients with Myalgic encephalomyelitis/Chronic fatigue syndrome. Sci. Rep. 2019, 9, 18817. [Google Scholar] [CrossRef] [PubMed]
- Puskarich, M.A.; Trzeciak, S.; Shapiro, N.I.; Albers, A.B.; Heffner, A.C.; Kline, J.A.; Jones, A.E. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest 2013, 143, 1548–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rishu, A.H.; Khan, R.; Al-Dorzi, H.M.; Tamim, H.M.; Al-Qahtani, S.; Al-Ghamdi, G.; Arabi, Y.M. Even mild hyperlactatemia is associated with increased mortality in critically ill patients. Crit. Care 2013, 17, R197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardigo, L.P.; Palermi, S.; Padulo, J.; Dhahbi, W.; Russo, L.; Linetti, S.; Cular, D.; Tomljanovic, M. External Responsiveness of the SuperOp(TM) Device to Assess Recovery After Exercise: A Pilot Study. Front. Sports Act. Living 2020, 2, 67. [Google Scholar] [CrossRef] [PubMed]
- Wibom, R.; Hultman, E.; Johansson, M.; Matherei, K.; Constantin-Teodosiu, D.; Schantz, P.G. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J. Appl. Physiol. 1992, 73, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M. Molecular responses to high-intensity interval exercise. Appl. Physiol. Nutr. Metab. 2009, 34, 428–432. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgomaster, K.A.; Cermak, N.M.; Phillips, S.M.; Benton, C.R.; Bonen, A.; Gibala, M.J. Divergent response of metabolite transport proteins in human skeletal muscle after sprint interval training and detraining. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1970–R1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilegaard, H.; Domino, K.; Noland, T.; Juel, C.; Hellsten, Y.; Halestrap, A.P.; Bangsbo, J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am. J. Physiol. 1999, 276, E255–E261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, L.; Barrett-Connor, E. Seasonal variation in fasting plasma glucose levels in man. Diabetologia 1982, 22, 250–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarrett, R.J.; Murrells, T.J.; Shipley, M.J.; Hall, T. Screening blood glucose values: Effects of season and time of day. Diabetologia 1984, 27, 574–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyono-Enguelle, S.; Marbach, J.; Heitz, A.; Ott, C.; Gartner, M.; Pape, A.; Vollmer, J.C.; Freund, H. Lactate removal ability and graded exercise in humans. J. Appl. Physiol. 1990, 68, 905–911. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kano, R.; Sato, K. A Competitive Sprinter’s Resting Blood Lactate Levels Fluctuate with a One-Year Training Cycle: Case Reports. J. Funct. Morphol. Kinesiol. 2021, 6, 95. https://doi.org/10.3390/jfmk6040095
Kano R, Sato K. A Competitive Sprinter’s Resting Blood Lactate Levels Fluctuate with a One-Year Training Cycle: Case Reports. Journal of Functional Morphology and Kinesiology. 2021; 6(4):95. https://doi.org/10.3390/jfmk6040095
Chicago/Turabian StyleKano, Ryotaro, and Kohei Sato. 2021. "A Competitive Sprinter’s Resting Blood Lactate Levels Fluctuate with a One-Year Training Cycle: Case Reports" Journal of Functional Morphology and Kinesiology 6, no. 4: 95. https://doi.org/10.3390/jfmk6040095
APA StyleKano, R., & Sato, K. (2021). A Competitive Sprinter’s Resting Blood Lactate Levels Fluctuate with a One-Year Training Cycle: Case Reports. Journal of Functional Morphology and Kinesiology, 6(4), 95. https://doi.org/10.3390/jfmk6040095





