Age and Not the Preferred Limb Influences the Kinematic Structure of Pointing Movements
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
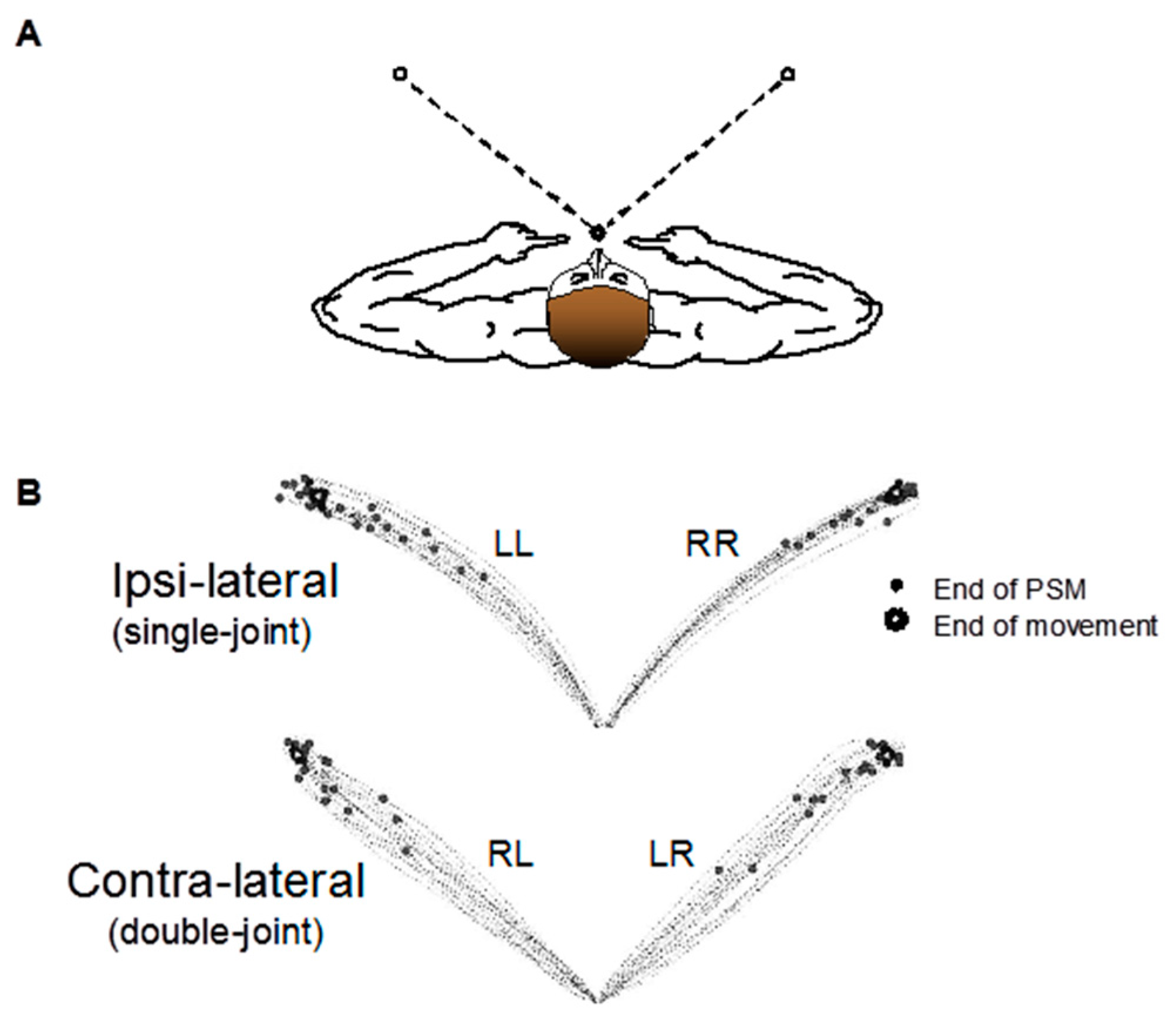
2.2. Experimental Arrangement
2.3. Experimental Procedures
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Participant’s Perferred Arm Characterization
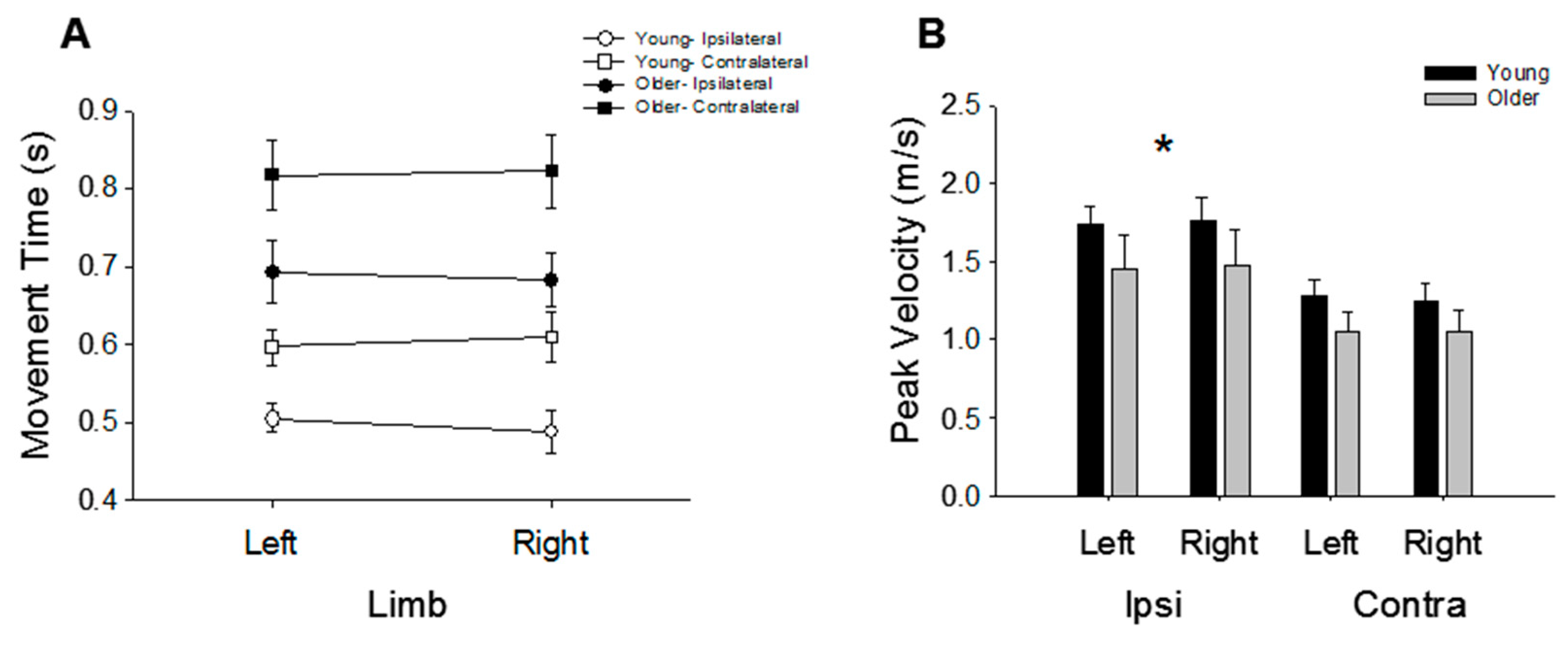
3.2. Movement Time and Speed
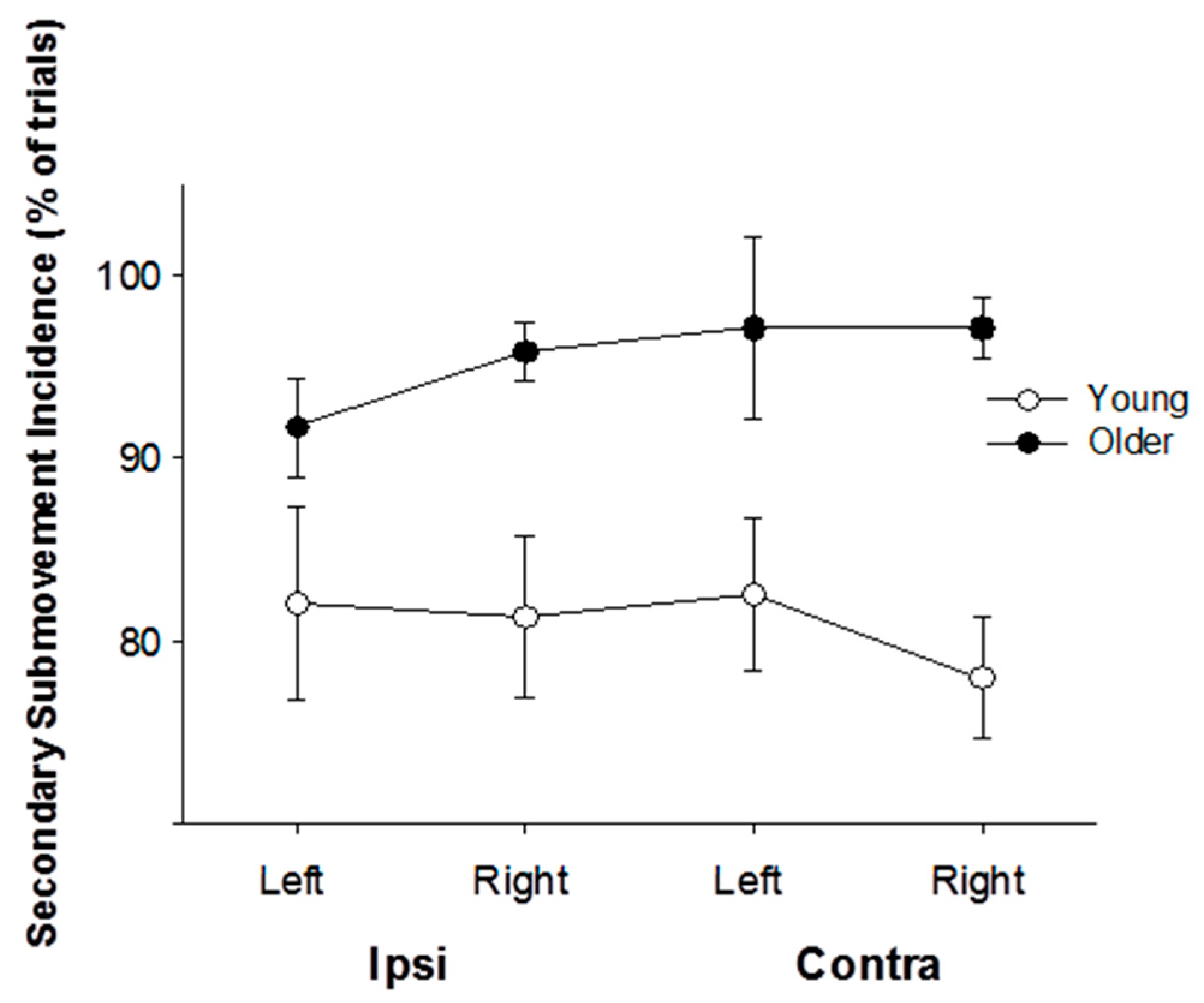
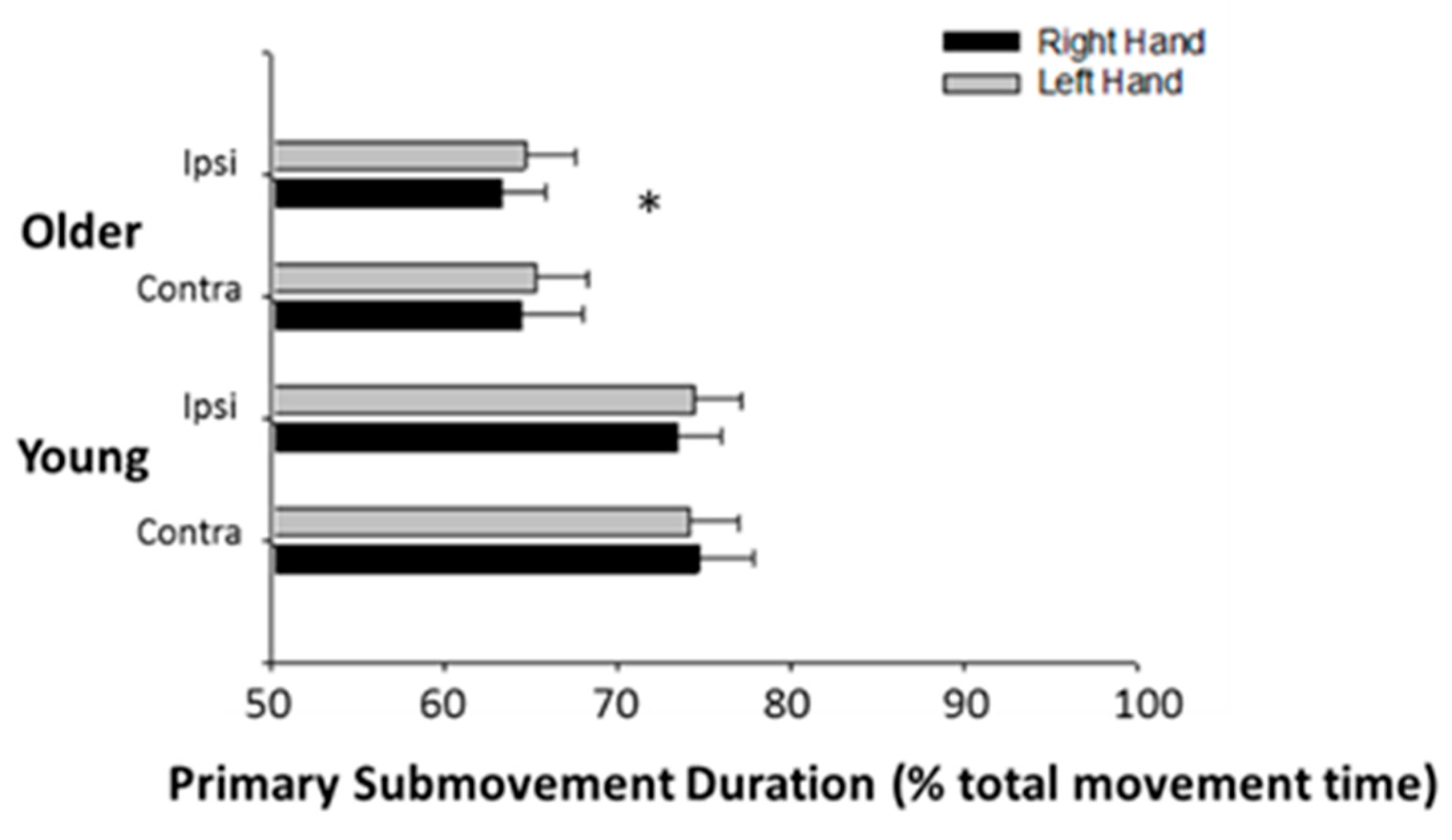
3.3. Submovement Analysis
4. Discussion
4.1. Arm Preference and Performance
4.2. The Effects of Age on Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, A.N.; Wysocki, C.J. Hand preference and age in the United States. Neuropsychologia 1992, 30, 601–608. [Google Scholar] [CrossRef]
- Todor, J.I.; Kyprie, P.M. Hand differences in the rate and variability of rapid tapping. J. Mot. Behav. 1980, 12, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Durding, B. Left-handers and right-handers compared on a motor task. J. Mot. Behav. 1979, 11, 103–111. [Google Scholar] [CrossRef]
- Woodworth, R.S. The accuracy of voluntary movement. Psychol. Rev. 1899, 3, 1–119. [Google Scholar]
- Annett, J.; Annett, M.; Hudson, P.T.; Turner, A. The control of movement in the preferred and non-preferred hands. Q. J. Exper. Psychol. 1979, 31, 641–652. [Google Scholar] [CrossRef]
- Sainburg, R.L.; Kalakanis, D. Differences in control of limb dynamics during dominant and non-dominant arm reaching. J. Neurophysiol. 2000, 83, 2661–2675. [Google Scholar] [CrossRef]
- Bagesteiro, L.B.; Sainburg, R.L. Handedness: Dominant arm advantages in control of limb dynamics. J. Neurophysiol. 2002, 88, 2408–2421. [Google Scholar] [CrossRef]
- Sainburg, R.L. Evidence for a dynamic-dominance hypothesis of handedness. Exp. Brain Res. 2002, 142, 241–258. [Google Scholar] [CrossRef]
- Sainburg, R.L. Handedness: Differential specialization for control of trajectory and position. Exerc. Sport Sci. Rev. 2005, 33, 206–213. [Google Scholar] [CrossRef]
- Wang, J.; Sainburg, R.L. Limitations in interlimb transfer of visuomotor rotations. Exp. Brain Res. 2004, 149, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sainburg, R.L. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp. Brain Res. 2007, 178, 565–570. [Google Scholar] [CrossRef]
- Krings, T.; Buchbinder, B.R.; Butler, W.E.; Chiappa, K.H.; Jiang, H.J.; Cosgrove, G.R.; Rosen, B.R. Functional magnetic resonance imaging and transcranial magnetic stimulation: Complementary approaches in the evaluation of cortical motor function. Neurology 1997, 48, 1406–1416. [Google Scholar] [CrossRef]
- Dassonville, P.; Zhu, Z.H.; Uurbil, K.; Kim, S.G.; Ashe, J. Functional activation in motor cortex reflects the direction and degree of handedness. Proc. Natl. Acad. Sci. USA 1997, 94, 14015–14018. [Google Scholar] [CrossRef]
- Semmler, J.G.; Nordstrom, M.A. Hemispheric differences in motor cortex excitability during a simple index finger abduction task in humans. J. Neurophysiol. 1998, 79, 1246–1254. [Google Scholar] [CrossRef]
- Triggs, W.J.; Calvanio, R.; Macdonell, R.A.; Cros, D.; Chiappa, K.H. Physiological motor asymmetry in human handedness: Evidence from transcranial magnetic stimulation. Brain Res. 1994, 636, 270–276. [Google Scholar] [CrossRef]
- Meyer, D.E.; Abrams, R.A.; Kornblum, S.; Wright, C.E.; Smith, J.E.K. Optimality in human motor performance: Ideal control of rapid aimed movements. Psychol. Rev. 1988, 95, 340–370. [Google Scholar] [CrossRef]
- Dounskaia, N.; Wisleder, D.; Johnson, T. Influence of biomechanical factors on substructure of pointing movements. Exp. Brain Res. 2005, 164, 505–516. [Google Scholar] [CrossRef]
- Ketcham, C.J.; Seidler, R.D.; Van Gemmert, A.W.A.; Stelmach, G.E. Age-related kinematic differences is influenced by task difficulty, target-size, and movement amplitude. J. Gerontol. B Psychol. Sci. Soc. Sc. 2002, 57, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; Chasteen, A.L.; Abrams, R.A. Rapid aimed limb movements: Age differences and practice effects in component submovements. Psychol. Aging 1994, 9, 325–334. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, R.G.; Marteniuk, R.G.; Dugas, C.; Liske, D.; Eickmeier, B. Three-dimensional movement trajectories in Fitt’s Task; implications for control. Q. J. Exp. Psychol. 1987, 39, 629–647. [Google Scholar] [CrossRef]
- Winstein, C.J.; Pohl, P.S. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp. Brain Res. 1995, 105, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Haaland, K.Y.; Harrington, D.L. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia 1989, 27, 961–969. [Google Scholar] [CrossRef]
- Bagesteiro, L.B.; Sainburg, R.L. Nondominant arm advantages in load compensation during rapid elbow joint movements. J. Neurophysiol. 2003, 90, 1503–1513. [Google Scholar] [CrossRef]
- Carson, R.G.; Goodman, D.; Chua, R.; Elliott, D. Asymmetries in the regulation of visually guided aiming. Mot. Behav. 1993, 25, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Haaland, K.Y.; Prestopnik, J.L.; Knight, R.T.; Lee, R.R. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain 2004, 127, 1145–1158. [Google Scholar] [CrossRef]
- Grabiner, M.D.; Enoka, R.M. Change in movement capabilities with aging. Exerc. Sport Sci. Rev. 1995, 23, 65–104. [Google Scholar] [CrossRef]
- Masakado, Y.; Noda, Y.; Nagata, M.; Kimura, A.; Chino, N.; Akaboshi, K. Macro-EMG and motor unit recruitment threshold: Differences between the young and the aged. Neurosci. Lett. 1994, 179, 1–4. [Google Scholar] [CrossRef]
- Campbell, M.J.; McComas, A.J.; Petito, F. Physiological changes in ageing muscles. J. Neurol. Neurosurg. Psychiatry 1973, 36, 174–182. [Google Scholar] [CrossRef]
- McComas, A.J.; Fawcett, P.R.W.; Campbell, M.J.; Sica, R.E. Electrophysiological estimation of the number of motor units within a human muscle. J. Neurol. Neurosurg. Psychol. 1971, 34, 121–131. [Google Scholar] [CrossRef]
- Katzman, R. Human Nervous System; Masoro, E.J., Ed.; Oxford University Press: New York, NY, USA, 1995; Volume 11, pp. 325–343. [Google Scholar]
- Jan, J.H.; Thomas, J.R.; Stelmach, G.E. Aging and rapid aiming arm movement control. Exp. Aging Res. 2000, 24, 155–168. [Google Scholar]
- Fradet, L.; Lee, G.; Dounskaia, N. Origins of submovements in movements of elderly adults. J. Neuro. Eng. Rehab. 2008, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Seidler, R.D.; Alberts, J.L.; Stelmach, G.E. Changes in multi-joint performance with age. Mot. Control 2002, 6, 19–31. [Google Scholar] [CrossRef]
- Ketcham, C.J.; Dounskaia, N.V.; Stelmach, G.E. Age-related differences in the control of multijoint movements. Mot. Control 2004, 8, 422–436. [Google Scholar] [CrossRef]
- Ketcham, C.J.; Dounskaia, N.V.; Stelmach, G.E. Multijoint movement control: The importance of interactive torque. Prog. Brain Res. 2004, 143, 207–218. [Google Scholar] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Wisleder, D.; Dounskaia, N. The role of different submovement types during pointing to a target. Exp. Brain Res. 2007, 176, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Novak, K.E.; Miller, L.E.; Houk, J.C. Kinematic properties of rapid hand movements in a knob turning task. Exp. Brain Res. 2000, 132, 419–433. [Google Scholar] [CrossRef]
- Novak, K.E.; Miller, L.E.; Houk, J.C. The use of overlapping submovements in the control of rapid hand movements. Exp. Brain Res. 2002, 144, 351–364. [Google Scholar] [CrossRef]
- Gazzaniga, M.S. Cerebral specialization and interhemispheric communication. Brain 2000, 123, 1293–1326. [Google Scholar] [CrossRef] [PubMed]
- Poston, B.; Van Gemmert, A.W.A.; Barduson, B.; Stelmach, G.E. Movement structure in young and elderly adults during goal-directed movements of the left and right arm. Brain Cogn. 2009, 69, 30–38. [Google Scholar] [CrossRef][Green Version]
- Gordon, J.; Ghilardi, M.F.; Cooper, S.E.; Ghez, C. Accuracy of planar reaching movemetns. II. Systematic extent errors resulting from inertial anisotrophy. Exp. Brain Res. 1994, 99, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.; Chua, R. Manual asymmetries in goal-directed movement. In Manual Asymmetries in Motor Performance; Elliott, D., Roy, E.A., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 143–172. [Google Scholar]
- Flowers, K. Handedness and controlled movement. Br. J. Psychol. 1975, 66, 39–52. [Google Scholar] [CrossRef]
- Fradet, L.; Lee, G.; Dounskaia, N. Origins of submovements during pointing movements. Acta Psychol. 2008, 129, 91–100. [Google Scholar] [CrossRef]
- Tretriluxana, J.; Gordon, J.; Winstein, C.J. Manual asymmetries in grasp pre-shaping and transport-grasp coordination. Exp. Brain Res. 2008, 188, 305–315. [Google Scholar] [CrossRef]
- Poston, B.; Van Gemmert, A.W.A.; Sharma, S.; Chakrabarti, S.; Zavaremi, S.H.; Stelmach, G. Movement trajectory smoothness is not associated with the endpoint accuracy of rapid multi-joint arm movements in young and older adults. Acta Psychol. 2013, 143, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Christou, E.A.; Carlton, L.G. Age and contraction type influence motor output variability in rapid discrete tasks. J. Appl. Physiol. 2002, 93, 489–498. [Google Scholar] [CrossRef][Green Version]
- Haaland, K.Y.; Harrington, D.L.; Grice, J.W. Effects of aging on planning and implementing arm movements. Psychol. Aging 1993, 8, 617–632. [Google Scholar] [CrossRef]
- Pohl, P.S.; Winstein, C.J.; Fisher, B.E. The locus of age-related movement slowing: Sensory processing in continuous goal-directed aiming. J. Gerontol. 1996, 51B, 94–102. [Google Scholar] [CrossRef]
- Goggin, N.L.; Meeuwsen, H.J. Age-related differences in the control of spatial aiming movements. Res. Q. Exerc. Sport 1992, 63, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Fitts, P.M. The information capacity of the human motor system in controlling the amplitude of movement. J. Exper. Psych. 1954, 47, 381–391. [Google Scholar] [CrossRef]
- Galganski, M.E.; Fuglevand, A.J.; Enoka, R.M. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J. Neurophysiol. 1993, 69, 2108–2115. [Google Scholar] [CrossRef]
- Seidler-Dobrin, R.D.; He, J.; Stelmach, G.E. Coactivation to reduce variability in the elderly. Mot. Control 1998, 2, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Christou, E.A.; Hunter, S.K.; Kornatz, K.W.; Semmler, J.G.; Taylor, A.M.; Tracy, B.L. Mechanisms that contribute to differences in motor performance between young and old adults. J. Electromyogr. Kines. 1993, 13, 1–12. [Google Scholar] [CrossRef]
- Laidlaw, D.H.; Bilodeau, M.; Enoka, R.M. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve 2000, 23, 600–612. [Google Scholar] [CrossRef]
- Semmler, J.G.; Kornatz, K.W.; Meyer, F.G.; Enoka, R.M. Diminished task-related adjustments of common inputs to hand muscle motor neurons in older adults. Exp. Brain Res. 2006, 172, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Christou, E.A.; Enoka, R.M. Multiple features of motor-unit activity influence force fluctuations during isometric contractions. J. Neurophysiol. 2003, 90, 1350–1361. [Google Scholar] [CrossRef]
- Harris, C.M.; Wolpert, D.M. Signal-dependent noise determines motor planning. Nature 1998, 394, 780–784. [Google Scholar] [CrossRef]
- Christou, E.A.; Shinohara, M.; Enoka, R.M. Fluctuations in acceleration during voluntary contractions lead to greater impairment of movement accuracy in old adults. J. Appl. Physiol. 2003, 95, 373–384. [Google Scholar] [CrossRef][Green Version]
- Graves, A.G.; Kornatz, K.W.; Enoka, R.M. Older adults use a unique strategy to lift inertial loads with the elbow flexor muscles. J. Neurophysiol. 2000, 83, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, T.; DeVita, P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc. Sport Sci. Rev. 2006, 34, 29–35. [Google Scholar] [CrossRef]
- Darling, W.G.; Cooke, J.D.; Brown, S.H. Control of simple arm movements in elderly humans. Neurobiol. Aging 1989, 10, 149–157. [Google Scholar] [CrossRef]
- Proac, C.; Coren, S.; Duncan, P. Life-span age trends in laterality. J. Gerontol. 1980, 35, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Proac, C.; Friesen, I.C. Hand preference side and its relation to hand preference switch history among old and oldest-old adults. Dev. Neuropsychol. 2000, 17, 225–239. [Google Scholar] [CrossRef]
- Brown, J.W.; Jaffe, J. Hypothesis on cerebral dominance. Neuropsychologia 1975, 13, 107–110. [Google Scholar] [CrossRef]
- Goldstein, G.; Shelly, C. Does the right hemisphere age more rapidly than the left? J. Clin. Neuropsychol. 1981, 3, 65–78. [Google Scholar] [CrossRef] [PubMed]
| Purdue Pegboard Test (Pegs/30 s) | ||||
|---|---|---|---|---|
| Age (Years) | Laterality Quotient | Right Hand | Left Hand | |
| Young (n = 12) | 22 ± 2 | 0.81 ± 0.21 | 14.9 ± 1.5 * † | 13.8 ± 2.0 † |
| Older (n = 12) | 72 ± 8 | 0.83 ± 0.18 | 13.0 ± 2.2 * | 11.7 ± 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornatz, K.W.; Poston, B.; Stelmach, G.E. Age and Not the Preferred Limb Influences the Kinematic Structure of Pointing Movements. J. Funct. Morphol. Kinesiol. 2021, 6, 100. https://doi.org/10.3390/jfmk6040100
Kornatz KW, Poston B, Stelmach GE. Age and Not the Preferred Limb Influences the Kinematic Structure of Pointing Movements. Journal of Functional Morphology and Kinesiology. 2021; 6(4):100. https://doi.org/10.3390/jfmk6040100
Chicago/Turabian StyleKornatz, Kurt W., Brach Poston, and George E. Stelmach. 2021. "Age and Not the Preferred Limb Influences the Kinematic Structure of Pointing Movements" Journal of Functional Morphology and Kinesiology 6, no. 4: 100. https://doi.org/10.3390/jfmk6040100
APA StyleKornatz, K. W., Poston, B., & Stelmach, G. E. (2021). Age and Not the Preferred Limb Influences the Kinematic Structure of Pointing Movements. Journal of Functional Morphology and Kinesiology, 6(4), 100. https://doi.org/10.3390/jfmk6040100






