Histological and Immunofluorescence Study of Discal Ligaments in Human Temporomandibular Joint
Abstract
1. Introduction
2. Materials and Methods
2.1. Light Microscopy
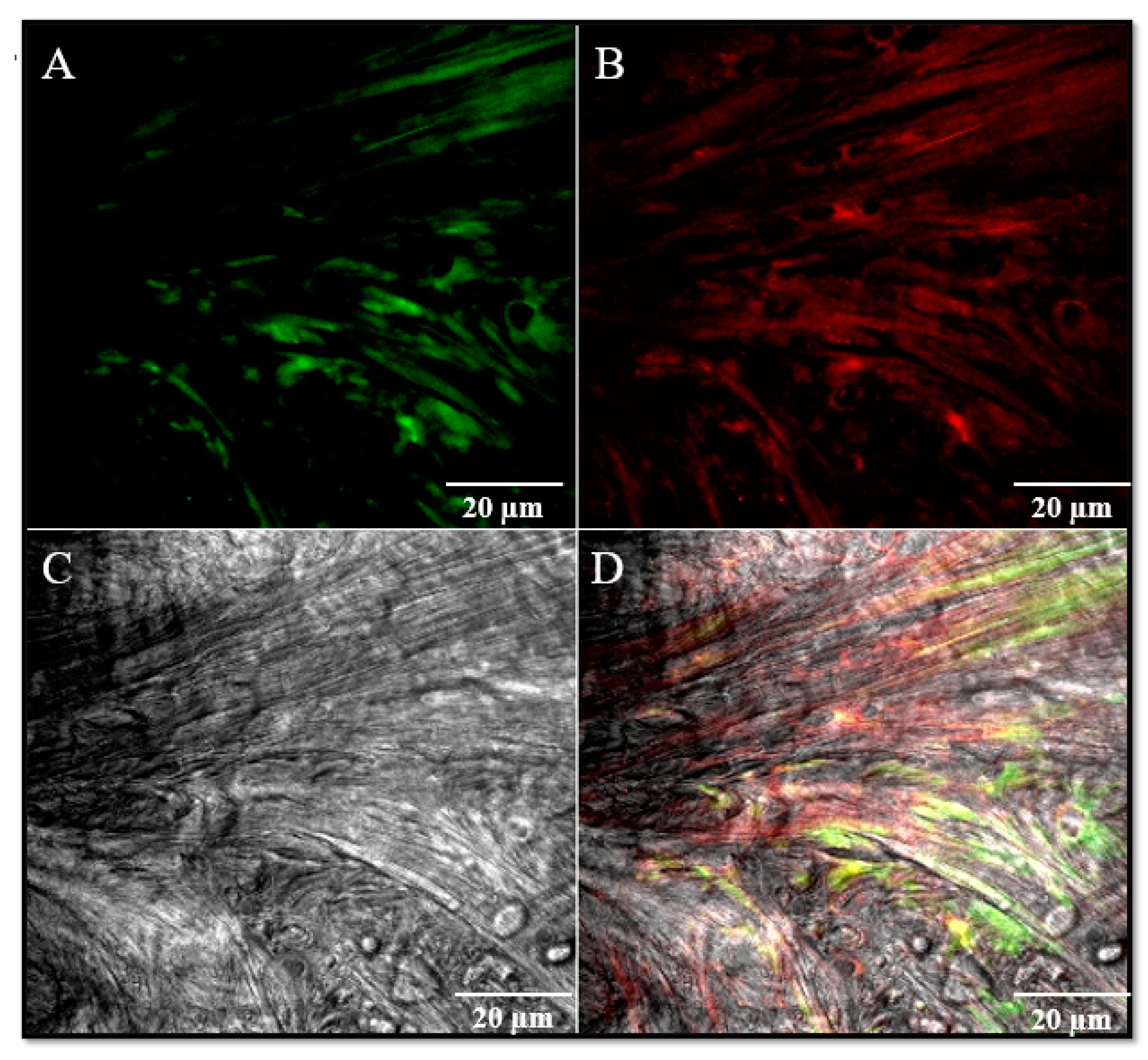
2.2. Immunofluorescence
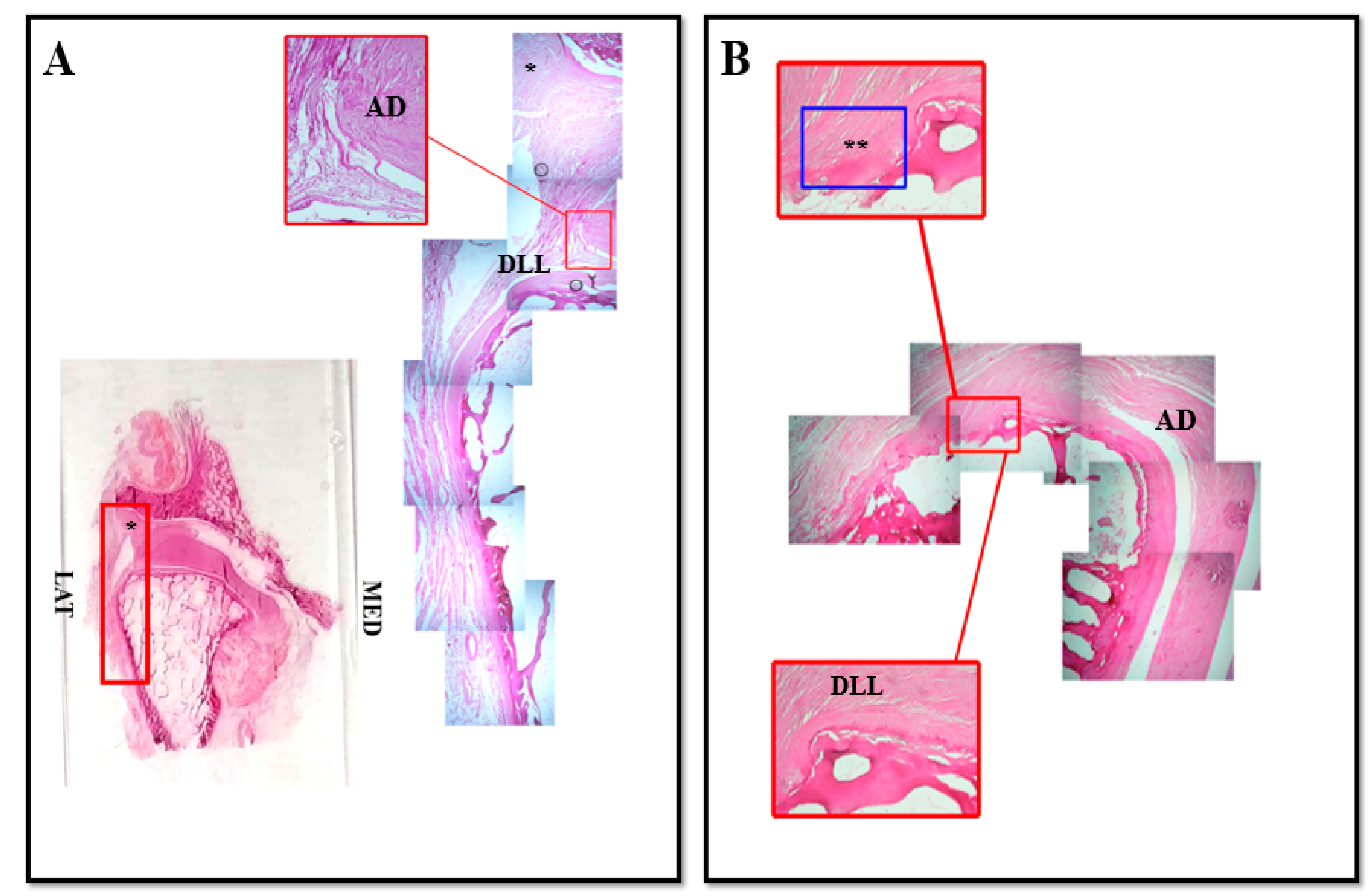
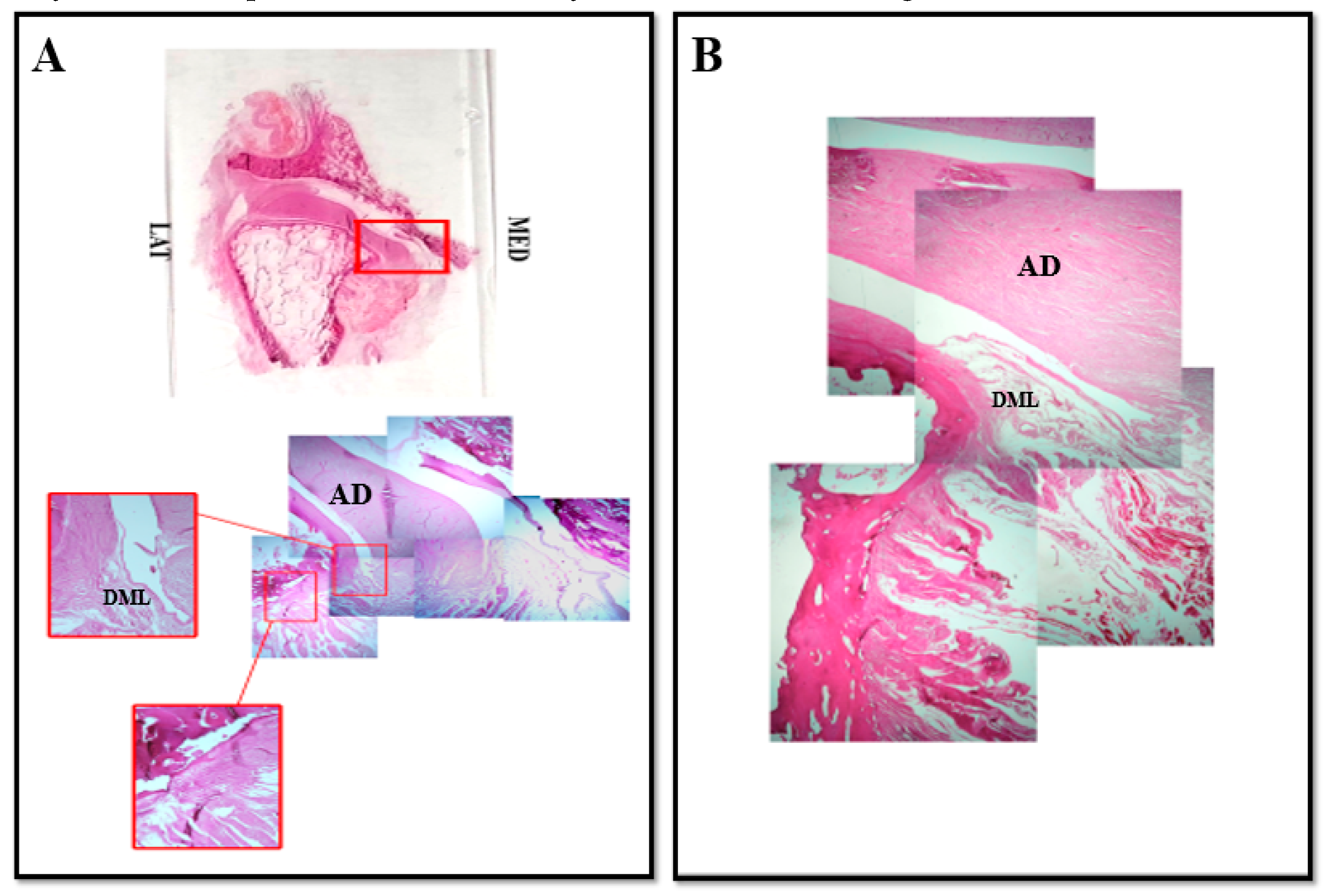
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Ponte, F.S.; Favaloro, A.; Siniscalchi, E.N.; Centofanti, A.; Runci, M.; Cutroneo, G.; Catalfamo, L. Sarcoglycans and integrins in bisphosphonate treatment: Immunohistochemical and scanning electron microscopy study. Oncol. Rep. 2013, 30, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Cascone, P.; Nicolai, G.; Vetrano, S.; Fabiani, F. TMJ biomechanical constraints: The disc and the retrodiscal tissue. Bull. Group Int. Rech. Sci. Stomatol. Odontol. 1999, 41, 26–32. [Google Scholar] [PubMed]
- Alomar, X.; Medrano, J.; Cabratosa, J.; Clavero, J.A.; Lorente, M.; Serra, I.; Monill, J.M.; Salvador, A. Anatomy of the temporomandibular joint. Semin. Ultrasound CT MRI 2007, 28, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Yuodelis, R.A. The morphogenesis of the human temporomandibular joint and its associated structures. J. Dent. Res. 1966, 45, 182–191. [Google Scholar] [CrossRef]
- Burch, J.G. Activity of the accessory ligaments of the temporomandibular joint. J. Prosthet. Dent. 1970, 24, 621–628. [Google Scholar] [CrossRef]
- Leonardi, R.; Loreto, C.; Barbato, E.; Caltabiano, R.; Lombardo, C.; Musumeci, G.; Lo Muzio, L. MMP-13 (collagenase 3) localization in human temporomandibular joint discs with internal derangement. Acta Histochem. 2008, 110, 314–318. [Google Scholar] [CrossRef]
- Leonardi, R.; Almeida, L.E.; Trevilatto, P.C.; Loreto, C. Occurrence and regional distribution of TRAIL and DR5 on temporomandibular joint discs: Comparison of disc derangement with and without reduction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 244–251. [Google Scholar] [CrossRef][Green Version]
- Leonardi, R.; Rusu, M.C.; Loreto, F.; Loreto, C.; Musumeci, G. Immunolocalization and expression of lubricin in the bilaminar zone of human temporomandibular joint disc. Acta Histochem. 2012, 114, 1–5. [Google Scholar] [CrossRef]
- Loreto, C.; Leonardi, R.; Musumeci, G.; Pannone, G.; Castorina, S. An ex vivo study on immunohistochemical localization of MMP-7 and MMP-9 in temporomandibular joint discs with internal derangement. Eur. J. Histochem. 2013, 57, e12. [Google Scholar] [CrossRef]
- Loreto, C.; Filetti, V.; Almeida, L.E.; La Rosa, G.R.M.; Leonardi, R.; Grippaudo, C.; Giudice, A.L. MMP-7 and MMP-9 are overexpressed in the synovial tissue from severe temporomandibular joint dysfunction. Eur. J. Histochem. 2020, 64, 8–12. [Google Scholar] [CrossRef]
- Okeson, J.P. Fundamentos de Oclusao e Desordens Temporomandibulares, 1th ed.; Art Med: Sao Paulo, Brazil, 2000. [Google Scholar]
- Latarjet, M.; Ruiz-Liard, A. Anatomia Humana, 4th ed.; Panamericana: Madrid, Spain, 2007. [Google Scholar]
- Kubein-Meesenburg, D.; Fanghanel, J.; Ihlow, D.; Lotozmann, U.; Hahn, W.; Theme, K.M.; Proff, P.; Gedrange, T.; Nagerl, H. Functional state of the mandible and rolling-gliding characteristics in the TMJ. Ann. Anat. 2007, 189, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Fenga, D.; Vermiglio, G.; Traina, F.; Favaloro, A.; Rosa, M.A. Scanning electron, 3D-confocal and stereotactic microscopies morpho-structural analysis of antibiotic-loaded cement in three different formulations. J. Biol. Regul. Homeost. Agents 2017, 31, 147–152. [Google Scholar] [PubMed]
- Rosa, M.A.; Gigliandolo, P.; Favaloro, A.; Vermiglio, G.; Centofanti, A.; Bruschetta, D.; Rizzo, G. Morpho-structural alterations of subchondral bone tissue in patients with osteoarthritis: A scanning electron microscopy study. Ital. J. Anat. Embryol. 2015, 120, 71–81. [Google Scholar] [PubMed]
- Rees, L.A. The structure and function of the mandibular joint. Br. Dent. J. 1954, 96, 125–133. [Google Scholar]
- Piette, E. Anatomy of the human temporomandibular joint. An update comprensive review. Acta Stomatol. Belg. 1993, 90, 103–127. [Google Scholar] [PubMed]
- Christo, J.E.; Bennett, S.; Wilkinson, T.M.; Townsend, G.C. Discal attachments of the human temporomandibular joint. Aust. Dent. J. 2005, 50, 152–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yung, J.P.; Pajoni, D.; Carpenitier, P. L’ATM en mouvement. Le sens de la forme. Rev. Orthop. Dentofac. 1987, 21, 531–542. [Google Scholar] [CrossRef]
- Fonzi, L. Anatomia Funzionale e Clinica Dello Splancnocranio, 5th ed.; Edi-Ermes: Milano, Italy, 2000. [Google Scholar]
- Bravetti, P.; Membre, H.; El Haddioui, A.; Gérard, H.; Fyard, J.P.; Mahler, P.; Gaudy, J.F. Histological study of the human temporo-mandibular joint and its surrounding muscles. Surg. Radiol. Anat. 2004, 26, 371–378. [Google Scholar] [CrossRef]
- Militi, A.; Cutroneo, G.; Favaloro, A.; Matarese, G.; Di Mauro, D.; Lauritano, F.; Centofanti, A.; Cervino, G.; Nicita, F.; Bramanti, A.; et al. An immunofluorescence study on VEGF and extracellular matrix proteins in human periodontal ligament during tooth movement. Heliyon 2019, 5, e02572. [Google Scholar] [CrossRef]
- Siéssere, S.; Vitti, M.; Semprini, M.; Regalo, S.C.; Iyomasa, M.M.; Dias, F.J.; Issa, J.P.; De Sousa, L.G. Macroscopic and microscopic aspects of the temporomandibular joint related to its clinical implication. Micron 2008, 39, 852–858. [Google Scholar] [CrossRef]
- Ottria, L.; Candotto, V.; Guzzo, F.; Gargari, M.; Barlattani, A. Temporomandibular joint and related structures anatomical and histological aspects. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. 1), 203–207. [Google Scholar] [PubMed]
- Chen, C.Y.; Ding, Y.; Liu, Y.J.; Zhang, Y.B. Establishment of animal model of temporomandibular joint synovitis and its histological investigation. Hua Xi Kou Qiang Yi Xue Za Zhi 2010, 28, 21–24. [Google Scholar] [PubMed]
- Orset, E.; Chaffanjon, P.; Bettega, G. Temporomandibular joint model: Anatomic and radiologic comparison between rat and human. Surg. Radiol. Anat. 2014, 36, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Scapino, R.P.; Obrez, A.; Greising, D. Organization and function of the collagen fiber system in the human temporomandibular joint disk and its attachments. Cells Tissues Organs 2006, 182, 201–225. [Google Scholar] [CrossRef] [PubMed]
- De Ponte, F.S.; Falzea, R.; Runci, M.; Nastro Siniscalchi, E.; Lauritano, F.; Bramanti, E.; Cervino, G.; Cicciu, M. Histomorphological and clinical evaluation of maxillary alveolar ridge reconstruction after craniofacial trauma by applying combination of allogeneic and autogenous bone graft. Chin. J. Traumatol. 2017, 20, 14–17. [Google Scholar] [CrossRef] [PubMed]
- De Ponte, F.S.; Catalfamo, L.; Micali, G.; Runci, M.; Cutroneo, G.; Vermiglio, G.; Centofanti, A.; Rizzo, G. Effect of bisphosphonates on the mandibular bone and gingival epithelium of rats without tooth extraction. Exp. Ther. Med. 2016, 11, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- De Ponte, F.S.; Cutroneo, G.; Falzea, R.; Rizzo, G.; Catalfamo, L.; Favaloro, A.; Vermiglio, G.; Runci, M.; Centofanti, A.; Anastasi, G. Histochemical and morphological aspects of fresh frozen bone: A preliminary study. Eur. J. Histochem. 2016, 60, 2642. [Google Scholar] [CrossRef]
- Cutroneo, G.; Vermiglio, G.; Centofanti, A.; Rizzo, G.; Runci, M.; Favaloro, A.; Piancino, M.G.; Bracco, P.; Ramieri, G.; Bianchi, F.; et al. Morphofunctional compensation of masseter muscles in unilateral posterior crossbite patients. Eur. J. Histochem. 2016, 60, 2605. [Google Scholar] [CrossRef]
- Cutroneo, G.; Centofanti, A.; Speciale, F.; Rizzo, G.; Favaloro, A.; Santoro, G.; Bruschetta, D.; Milardi, D.; Micali, A.; Di Mauro, D.; et al. Sarcoglycan complex in masseter and sternocleidomastoid muscles of baboons: An immunohistochemical study. Eur. J. Histochem. 2015, 59, 2509. [Google Scholar] [CrossRef][Green Version]
- Ventura Spagnolo, E.; Mondello, C.; Di Mauro, D.; Vermiglio, G.; Asmundo, A.; Filippini, E.; Alibrandi, A.; Rizzo, G. Analysis on sarcoglycans expression as markers of septic cardiomyopathy in sepsis-related death. Int. J. Legal Med. 2018, 132, 1685–1692. [Google Scholar] [CrossRef]
- Arco, A.; Favaloro, A.; Gioffrè, M.; Santoro, G.; Speciale, F.; Vermiglio, G.; Cutroneo, G. Sarcoglycans in the normal and pathological breast tissue of humans: An immunohistochemical and molecular study. Cell Tissues Organs 2012, 195, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, D.; Sengar, M.; Lauriano, E.R.; Pergolizzi, S.; Macri’, F.; Salpietro, L.; Favaloro, A.; Satora, L.; Dabrowski, K.; Zaccone, G. Morphology and innervation of the teleost physostome swim bladders and their functional evolution in non-teleostean lineages. Acta Histochem. 2012, 114, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, G.; Cutroneo, G.; Sidoti, A.; Santoro, G.; D’Angelo, R.; Rizzo, G.; Rinaldi, C.; Giacobbe, O.; Bramanti, P.; Navarra, G.; et al. Sarcoglycan subcomplex in normal human smooth muscle: An immunohistochemical and molecular study. Int. J. Mol. Med. 2005, 16, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, G.; Cutroneo, G.; Rizzo, G.; Favaloro, A. Sarcoglycan subcomplex in normal and pathological human muscle fibers. Eur. J. Histochem. 2007, 51 (Suppl. 1), 29–33. [Google Scholar] [PubMed]
- Anastasi, G.; Cutroneo, G.; Sidoti, A.; Rinaldi, C.; Bruschetta, D.; Rizzo, G.; D’Angelo, R.; Tarone, G.; Amato, A.; Favaloro, A. Sarcoglycan subcomplex expression in normal human smooth muscle. J. Histochem. Cytochem. 2007, 55, 831–843. [Google Scholar] [CrossRef]
- Di Mauro, D.; Gaeta, R.; Arco, A.; Milardi, D.; Lentini, S.; Runci, M.; Rizzo, G.; Magaudda, L. Distribution of costameric proteins in normal human ventricular and atrial cardiac muscle. Folia Histochem. Cytobiol. 2009, 47, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, A.; Milardi, D.; Anastasi, G.; Basile, G.; Ciolli, P.; Irrera, M.; Cutroneo, G.; Bruschetta, D.; Rizzo, G.; Mondello, S.; et al. A direct cortico-nigral pathway as revealed by constrained spherical deconvolution tractography in humans. Front. Hum. Neurosci. 2016, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.J.; Hawthorn, R.; Harris, R. Anatomy and Histology of temporalmandibular joint. Monogr. Oral Sci. 1975, 4, 1–26. [Google Scholar] [PubMed]
- Heylings, D.J.; Nielsen, I.L.; McNeil, C. Lateral Pterygoid muscle and temporomandibular disc. J. Orofac. Pain 1995, 9, 9–16. [Google Scholar] [PubMed]
- Anastasi, G.; Cutroneo, G.; Santoro, G.; Arco, A.; Rizzo, G.; Trommino, C.; Bramanti, P.; Soscia, L.; Favaloro, A. Integrins, muscle agrin and sarcoglycans during muscular inactivity conditions: An immunohistochemical study. Eur. J. Histochem. 2006, 50, 327–336. [Google Scholar]
- Smolke, C. The relationship between the temporomandibular joint capsule, articular disc and jaw muscles. J. Anat. 1994, 184, 335–345. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Runci Anastasi, M.; Centofanti, A.; Arco, A.; Vermiglio, G.; Nicita, F.; Santoro, G.; Cascone, P.; Anastasi, G.P.; Rizzo, G.; Cutroneo, G. Histological and Immunofluorescence Study of Discal Ligaments in Human Temporomandibular Joint. J. Funct. Morphol. Kinesiol. 2020, 5, 90. https://doi.org/10.3390/jfmk5040090
Runci Anastasi M, Centofanti A, Arco A, Vermiglio G, Nicita F, Santoro G, Cascone P, Anastasi GP, Rizzo G, Cutroneo G. Histological and Immunofluorescence Study of Discal Ligaments in Human Temporomandibular Joint. Journal of Functional Morphology and Kinesiology. 2020; 5(4):90. https://doi.org/10.3390/jfmk5040090
Chicago/Turabian StyleRunci Anastasi, Michele, Antonio Centofanti, Alba Arco, Giovanna Vermiglio, Fabiana Nicita, Giuseppe Santoro, Piero Cascone, Giuseppe Pio Anastasi, Giuseppina Rizzo, and Giuseppina Cutroneo. 2020. "Histological and Immunofluorescence Study of Discal Ligaments in Human Temporomandibular Joint" Journal of Functional Morphology and Kinesiology 5, no. 4: 90. https://doi.org/10.3390/jfmk5040090
APA StyleRunci Anastasi, M., Centofanti, A., Arco, A., Vermiglio, G., Nicita, F., Santoro, G., Cascone, P., Anastasi, G. P., Rizzo, G., & Cutroneo, G. (2020). Histological and Immunofluorescence Study of Discal Ligaments in Human Temporomandibular Joint. Journal of Functional Morphology and Kinesiology, 5(4), 90. https://doi.org/10.3390/jfmk5040090






