Low Body Fat Does Not Influence Recovery after Muscle-Damaging Lower-Limb Plyometrics in Young Male Team Sport Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
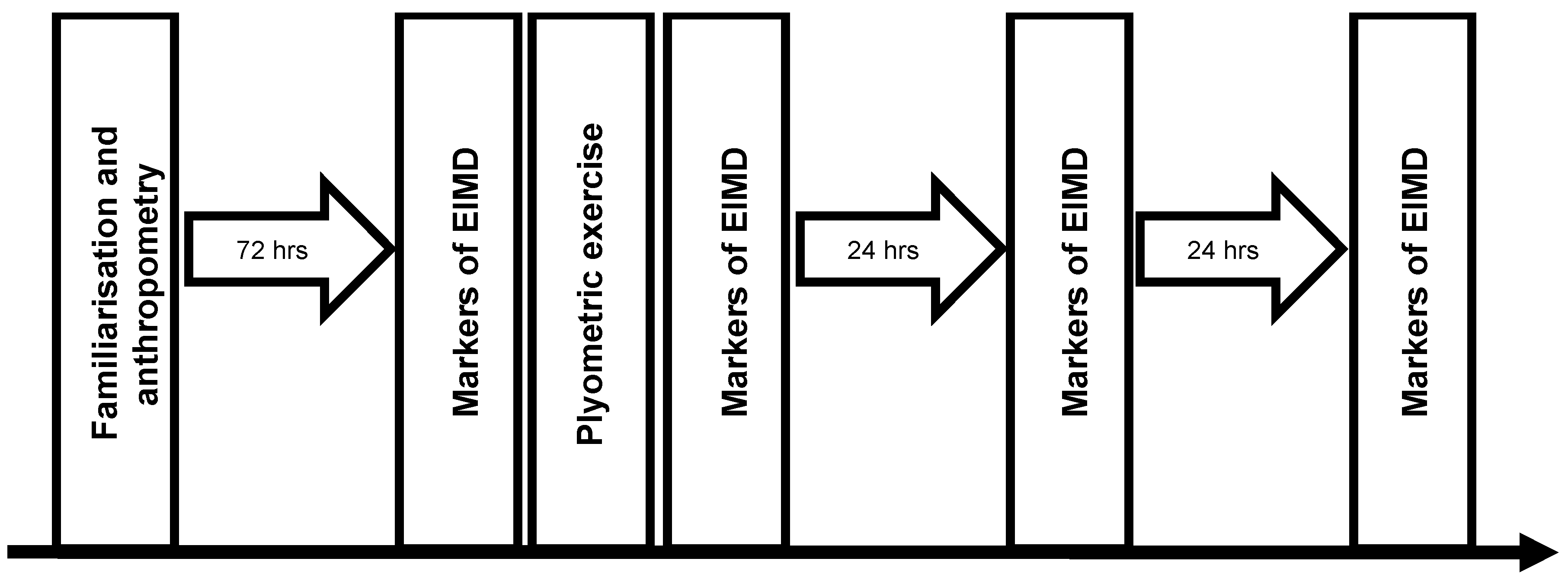
2.2. Study Design
2.3. Anthropometric Measurements
2.4. Assessment of Perceived Muscle Soreness
2.5. Assessment of Plasma Creatine Kinase Activity
2.6. Assessment of Knee Extensor Isokinetic Strength
2.7. Assessment of Maximal Vertical Jump Height
2.8. Muscle-Damaging Protocol
2.9. Statistical Analysis
3. Results
3.1. Anthropometric Characteristics
3.2. Muscle-Damaging Protocol
3.3. Plasma Creatine Kinase Activity
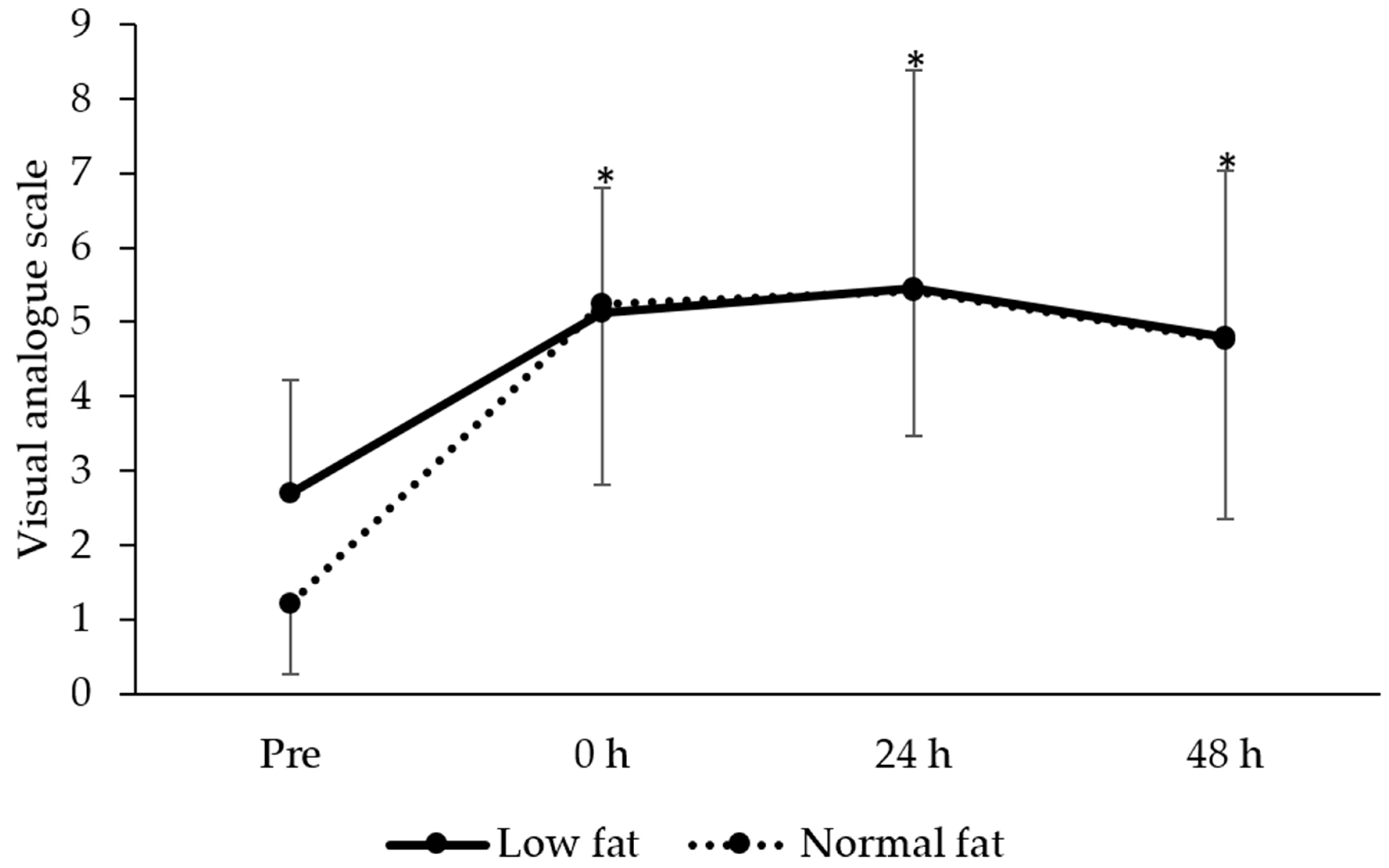
3.4. Perceived Muscle Soreness
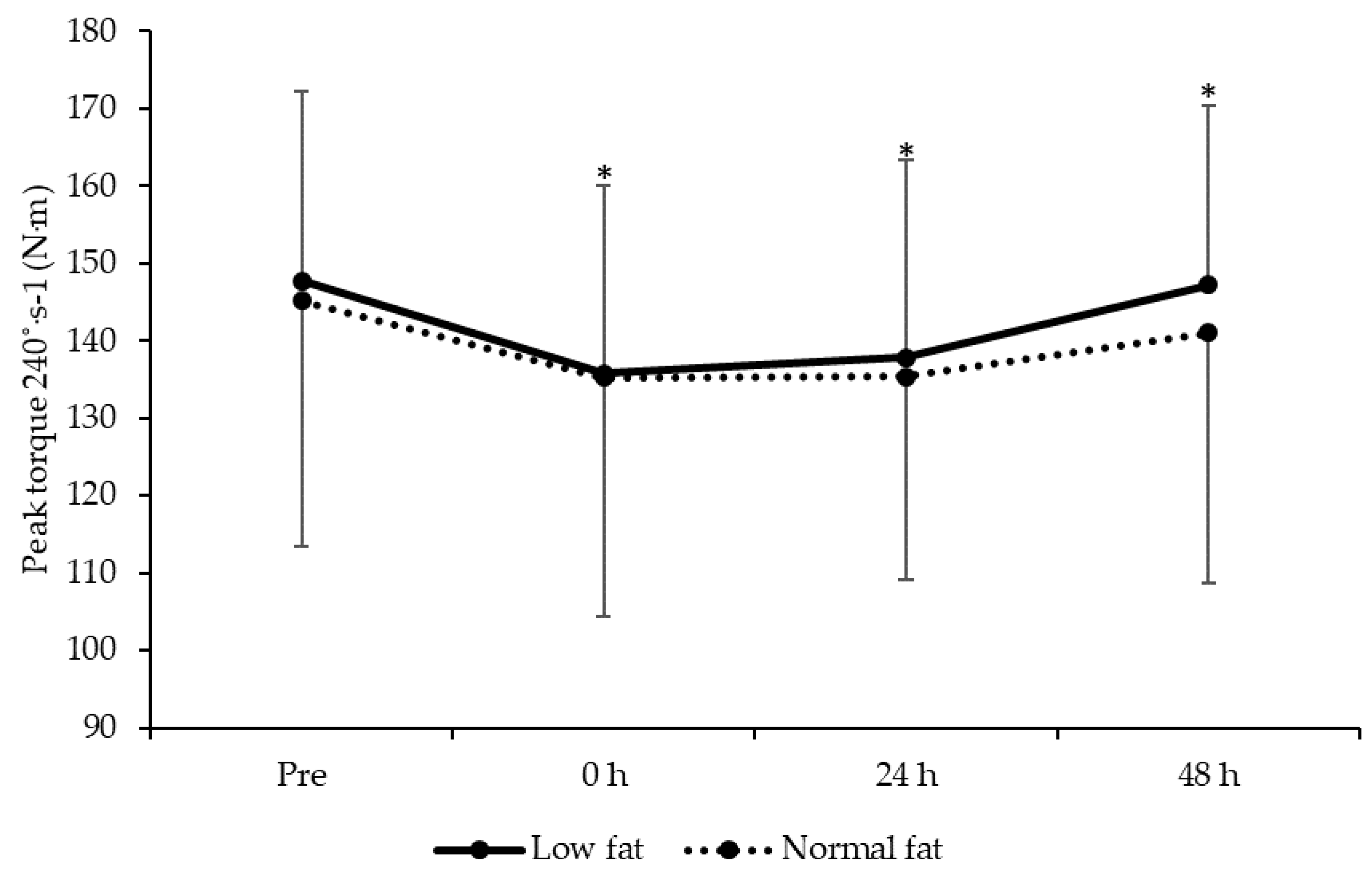
3.5. Knee Extensor Isokinetic Strength
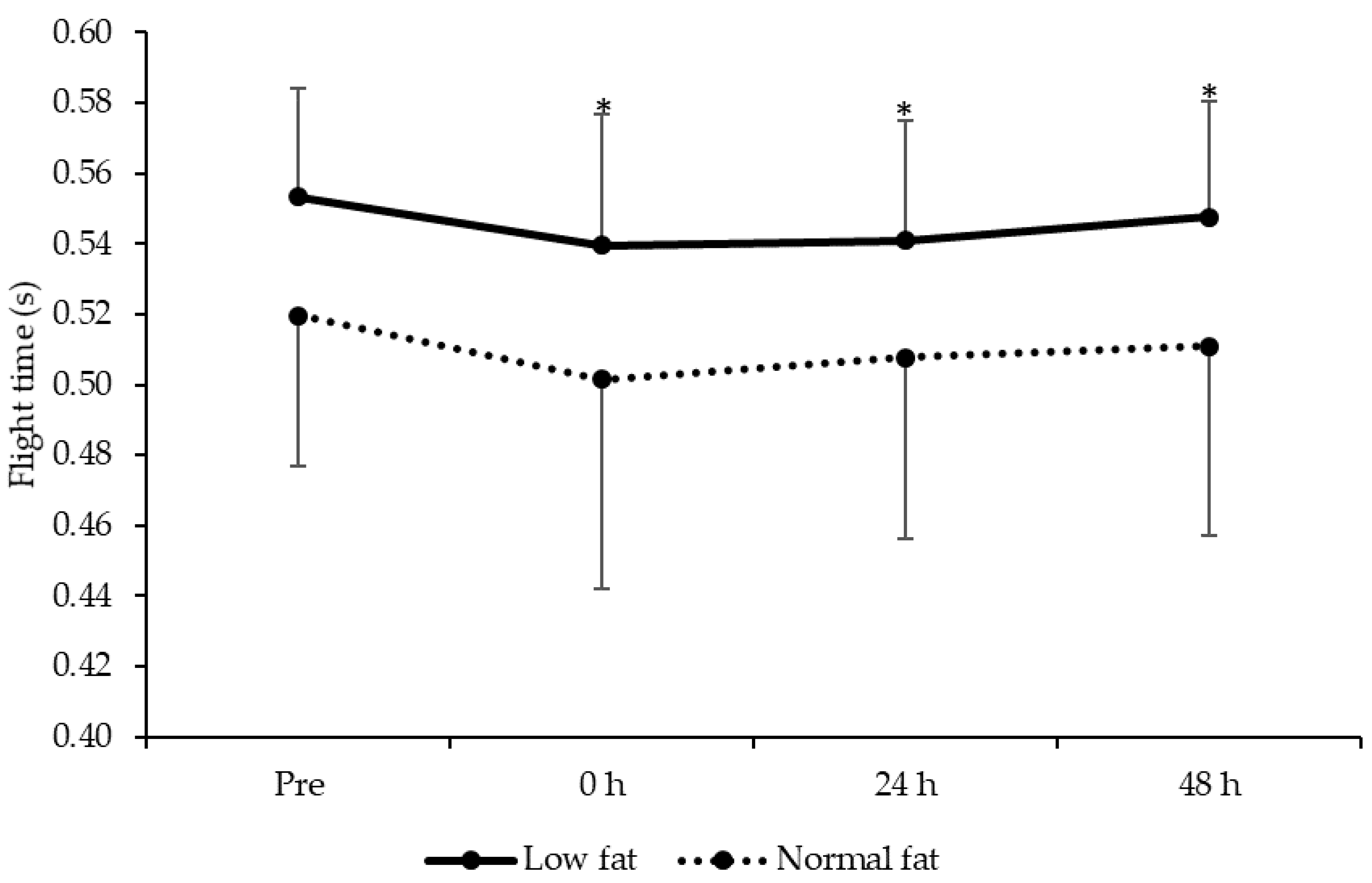
3.6. Maximal Vertical Jump Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Komi, P.V. Stretch-shortening cycle: A powerful model to study normal and fatigued muscle. J. Biomech. 2000, 33, 1197–1206. [Google Scholar] [CrossRef]
- Jones, T.W.; Smith, A.; Macnaughton, L.S.; French, D.N. Strength and Conditioning and Concurrent Training Practices in Elite Rugby Union. J. Strength Cond. Res. 2016, 30, 3354–3366. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.; Clark, C.C.T.; Ramirez-Campillo, R.; Davies, M.J.; Drury, B. A meta-analysis of plyometric training in female youth: Its efficacy and shortcomings in the literature. J. Strength Cond. Res. 2018, 33, 1996–2008. [Google Scholar] [CrossRef]
- De Villarreal, E.S.; Requena, B.; Cronin, J.B. The Effects of Plyometric Training on Sprint Performance: A Meta-Analysis. J. Strength Cond. Res. 2012, 26, 575–584. [Google Scholar] [CrossRef]
- Saunders, P.U.; Telford, R.D.; Pyne, D.B.; Peltola, E.M.; Cunningham, R.B.; Gore, C.J.; Hawley, J.A. Short-term plyometric training improves running economy in highly trained middle and long distance runners. J. Strength Cond. Res. 2006, 20, 947–954. [Google Scholar]
- Rubley, M.D.; Haase, A.C.; Holcomb, W.R.; Girouard, T.J.; Tandy, R.D. The Effect of Plyometric Training on Power and Kicking Distance in Female Adolescent Soccer Players. J. Strength Cond. Res. 2011, 25, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Hortobágyi, T.; Houmard, J.; Fraser, D.; Dudek, R.; Lambert, J.; Tracy, J. Normal forces and myofibrillar disruption after repeated eccentric exercise. J. Appl. Physiol. 1998, 84, 492–498. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Hubal, M.J. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 2014, 49, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2018, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Damas, F.; Nosaka, K.; Libardi, C.A.; Chen, T.C.; Ugrinowitsch, C. Susceptibility to Exercise-Induced Muscle Damage: A Cluster Analysis with a Large Sample. Int. J. Sports Med. 2016, 37, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Willardson, J.M. Short Recovery Augments Magnitude of Muscle Damage in High Responders. Med. Sci. Sports Exerc. 2010, 42, 1370–1374. [Google Scholar] [CrossRef]
- Hubal, M.J.; Rubinstein, S.R.; Clarkson, P.M. Mechanisms of Variability in Strength Loss after Muscle-Lengthening Actions. Med. Sci. Sports Exerc. 2007, 39, 461–468. [Google Scholar] [CrossRef]
- Messier, S.P.; Ettinger, W.H.; Doyle, T.E.; Morgan, T.; James, M.K.; O’Toole, M.L.; Burns, R. Obesity: Effects on Gait in an Osteoarthritic Population. J. Appl. Biomech. 1996, 12, 161–172. [Google Scholar] [CrossRef]
- Hickner, R.C.; Mehta, P.M.; Dyck, D.; DeVita, P.; Houmard, J.A.; Koves, T.; Byrd, P. Relationship between fat-to-fat-free mass ratio and decrements in leg strength after downhill running. J. Appl. Physiol. 2001, 90, 1334–1341. [Google Scholar] [CrossRef]
- Paschalis, V.; Nikolaidis, M.G.; Theodorou, A.A.; Deli, C.K.; Raso, V.; Jamurtas, A.Z.; Giakas, G.; Koutedakis, Y. The Effects of Eccentric Exercise on Muscle Function and Proprioception of Individuals Being Overweight and Underweight. J. Strength Cond. Res. 2013, 27, 2542–2551. [Google Scholar] [CrossRef]
- Twist, C.; Sykes, D. Evidence of exercise-induced muscle damage following a simulated rugby league match. Eur. J. Sport Sci. 2011, 11, 401–409. [Google Scholar] [CrossRef]
- Nosaka, K.; Sakamoto, K.E.I.; Newton, M.; Sacco, P. How long does the protective effect on eccentric exercise-induced muscle damage last? Med. Sci. Sports Exerc. 2001, 33, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Demerath, E.W.; Guo, S.S.; Chumlea, W.C.; Towne, B.; Roche, A.F.; Siervogel, R.M. Comparison of percent body fat estimates using air displacement plethysmography and hydrodensitomery in adults and children. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 389–397. [Google Scholar] [CrossRef]
- Utter, A.C.; Goss, F.L.; Swan, P.D.; Harris, G.S.; Robertson, R.J.; Trone, G.A. Evaluation of Air Displacement for Assessing Body Composition of Collegiate Wrestlers. Med. Sci. Sports Exerc. 2003, 35, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Purcell, S.A.; Prado, C.M.; Gonzalez, M.C. Fat mass to fat-free mass ratio reference values from NHANES III using bioelectrical impedance analysis. Clin. Nutr. 2018, 37, 2284–2287. [Google Scholar] [CrossRef]
- Burt, D.G.; Lamb, K.; Nicholas, C.; Twist, C. Effects of exercise-induced muscle damage on resting metabolic rate, sub-maximal running and post-exercise oxygen consumption. Eur. J. Sport Sci. 2014, 14, 337–344. [Google Scholar] [CrossRef]
- Wilhite, M.R.; Cohen, E.R.; Wilhite, S.C. Reliability of Concentric and Eccentric Measurements of Quadriceps Performance Using the KIN-COM Dynamometer: The Effect of Testing Order for Three Different Speeds. J. Orthop. Sports Phys. Ther. 1992, 15, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Highton, J.M.; Twist, C.; Eston, R.G. The Effects of Exercise-Induced Muscle Damage on Agility and Sprint Running Performance. J. Exerc. Sci. Fit. 2009, 7, 24–30. [Google Scholar] [CrossRef]
- Byrne, C.; Eston, R. The effect of exercise-induced muscle damage on isometric and dynamic knee extensor strength and vertical jump performance. J. Sports Sci. 2002, 20, 417–425. [Google Scholar] [CrossRef]
- Glatthorn, J.F.; Gouge, S.; Nussbaumer, S.; Stauffacher, S.; IMpellizzeri, F.M.; Maffiuletti, N.A. Validity and Reliability of Optojump Photoelectic Cells for estimating Vertical Jump Height. J. Strength Cond. Res. 2011, 25, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Twist, C.; Eston, R. The effects of exercise-induced muscle damage on maximal intensity intermittent exercise performance. Eur. J. Appl. Physiol. 2005, 94, 652–658. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef]
- Warren, G.; Lowe, D.; Armstrong, R. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999, 27, 43–59. [Google Scholar] [CrossRef]
- Burt, D.; Twist, C. The Effects of Exercise-Induced Muscle Damage on Cycling Time-Trial Performance. J. Strength Cond. Res. 2011, 25, 2185–2192. [Google Scholar] [CrossRef]
- Twist, C.; Eston, R.G. The effect of muscle-damaging exercise on maximal intensity cycling and drop jump performance. J. Exerc. Sci. Fit. 2007, 5, 79–87. [Google Scholar]
- Eston, R.G.; Finney, S.; Baker, S.; Baltzopoulos, V. Muscle tenderness and peak torque changes after downhill running following a prior bout of isokinetic eccentric exercise. J. Sports Sci. 1996, 14, 291–299. [Google Scholar] [CrossRef]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
- Marginson, V.; Rowlands, A.V.; Gleeson, N.P.; Eston, R.G. Comparison of the symptoms of exercise-induced muscle damage after an initial and repeated bout of plyometric exercise in men and boys. J. Appl. Physiol. 2005, 99, 1174–1181. [Google Scholar] [CrossRef]
- Fernandes, J.F.T.; Lamb, K.L.; Twist, C. Internal Loads, but Not External Loads and Fatigue, Are Similar in Young and Middle-Aged Resistance-Trained Males during High Volume Squatting Exercise. J. Funct. Morphol. Kinesiol. 2018, 3, 45. [Google Scholar] [CrossRef]


| Characteristic | Low Fat (n = 10) | Normal Fat (n = 10) |
|---|---|---|
| Body mass (kg) | 76.9 ± 6.1 | 77.2 ± 15.5 |
| Fat mass (kg) | 8.0 ± 1.8 | 16.7 ± 8.2 * |
| Fat-free mass (kg) | 68.6 ± 6.6 | 60.4 ± 7.7 * |
| Body fat (%) | 10.6 ± 2.5 | 20.8 ± 5.1 * |
| FM:FFM ratio | 0.12 ± 0.03 | 0.27 ± 0.09 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, J.F.T.; Lamb, K.L.; Twist, C. Low Body Fat Does Not Influence Recovery after Muscle-Damaging Lower-Limb Plyometrics in Young Male Team Sport Athletes. J. Funct. Morphol. Kinesiol. 2020, 5, 79. https://doi.org/10.3390/jfmk5040079
Fernandes JFT, Lamb KL, Twist C. Low Body Fat Does Not Influence Recovery after Muscle-Damaging Lower-Limb Plyometrics in Young Male Team Sport Athletes. Journal of Functional Morphology and Kinesiology. 2020; 5(4):79. https://doi.org/10.3390/jfmk5040079
Chicago/Turabian StyleFernandes, John F. T., Kevin L. Lamb, and Craig Twist. 2020. "Low Body Fat Does Not Influence Recovery after Muscle-Damaging Lower-Limb Plyometrics in Young Male Team Sport Athletes" Journal of Functional Morphology and Kinesiology 5, no. 4: 79. https://doi.org/10.3390/jfmk5040079
APA StyleFernandes, J. F. T., Lamb, K. L., & Twist, C. (2020). Low Body Fat Does Not Influence Recovery after Muscle-Damaging Lower-Limb Plyometrics in Young Male Team Sport Athletes. Journal of Functional Morphology and Kinesiology, 5(4), 79. https://doi.org/10.3390/jfmk5040079







