Finding a Balance: A Systematic Review of the Biomechanical Effects of Vestibular Prostheses on Stability in Humans
Abstract
1. Introduction
1.1. Vestibular Dysfunction
1.2. Prosthetic Devices
1.2.1. Vestibular Implants
1.2.2. Galvanic Vestibular Stimulation (nGVS)
1.3. Research Aims
2. Methodology
2.1. Search Strategy
2.2. Selection Process
2.3. Data Extraction
3. Results
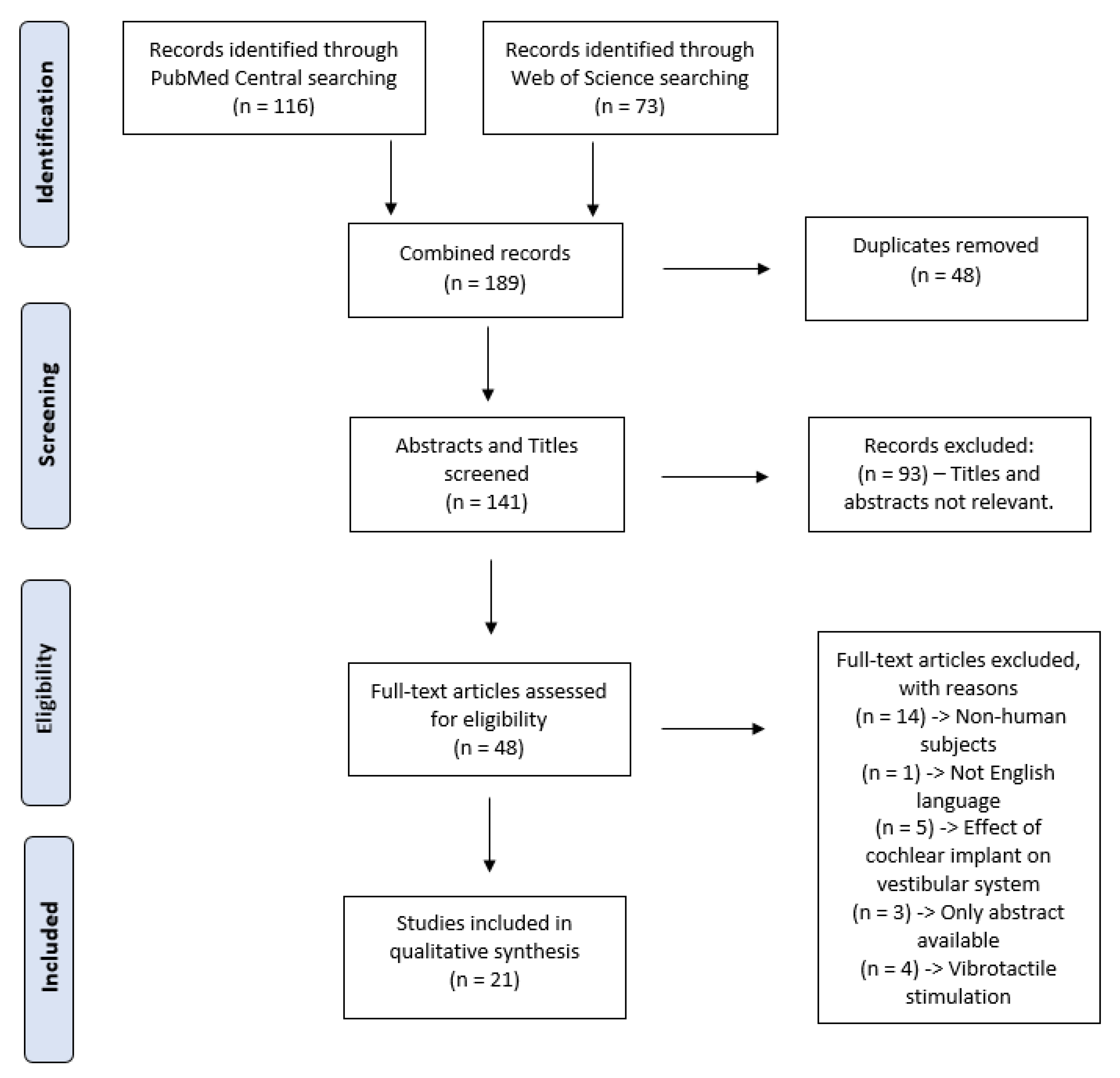
3.1. Search Results
3.2. Study Characteristics
3.3. Galvanic Vestibular Stimulation (GVS)
3.4. Vestibular Implant
4. Discussion and Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SCC | Semi-circular canal |
| BVD | Bilateral vestibular dysfunction |
| VOR | Vestibular-ocular reflex |
| aVOR | Angular vestibular-ocular reflex |
| RMS | Root mean square |
| nGVS | Noisy galvanic vestibular stimulation |
| LAN/PAN/SAN | Lateral/Posterior/Superior ampullary nerve |
| COP | Centre of pressure |
| VA | Visual acuity |
| QALY | Quality-adjusted life year |
| ML | Mediolateral |
| AP | Anteroposterior |
| ANOVA | Analysis of variance |
| Q1 | Quartile one |
| Q2 | Quartile two |
References
- Fielder, H.; Denholm, S.; Lyons, R.; Fielder, C. Measurement of health status in patients with vertigo. Clin. Otolaryngol. Allied Sci. 1996, 21, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M.; Brandt, T. Peripheral vestibular disorders. Curr. Opin. Neurol. 2013, 26, 81–89. [Google Scholar] [CrossRef]
- Cooksey, F. Rehabilitation in Vestibular Injuries; SAGE Publications: New York, NY, USA, 1946. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Peterka, R. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Dickman, J.D. The vestibular system. Fundam. Neurosci. Basic Clin. Appl. 2018, 5, 320–333. [Google Scholar]
- Baloh, R.W.; Enrietto, J.; Jacobson, K.M.; Lin, A. Age-related changes in vestibular function: A longitudinal study. Ann. New York Acad. Sci. 2001, 942, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Zingler, V.C.; Weintz, E.; Jahn, K.; Mike, A.; Huppert, D.; Rettinger, N.; Brandt, T.; Strupp, M. Follow-up of vestibular function in bilateral vestibulopathy. J. Neurol. Neurosurg. Psychiatry 2008, 79, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Herdman, S.J.; Hall, C.D.; Maloney, B.; Knight, S.; Ebert, M.; Lowe, J. Variables associated with outcome in patients with bilateral vestibular hypofunction: Preliminary study. J. Vestib. Res. 2015, 25, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kerber, K.A.; Baloh, R.W. The evaluation of a patient with dizziness. Neurol. Clin. Pract. 2011, 1, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.Q.; Ward, B.K.; Semenov, Y.R.; Carey, J.P.; Della Santina, C.C. Bilateral vestibular deficiency: Quality of life and economic implications. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 527–534. [Google Scholar] [CrossRef]
- Herdman, S.J. Vestibular rehabilitation. Curr. Opin. Neurol. 2013, 26, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Cass, S.P.; Clendaniel, R.A.; Fife, T.D.; Furman, J.M.; Getchius, T.S.; Goebel, J.A.; Shepard, N.T. Vestibular rehabilitation for peripheral vestibular hypofunction: An evidence-based clinical practice guideline: From the American physical therapy association neurology section. J. Neurol. Phys. Ther. 2016, 40, 124. [Google Scholar] [CrossRef] [PubMed]
- Perez Fornos, A.; Guinand, N.; van de Berg, R.; Stokroos, R.; Micera, S.; Kingma, H.; Pelizzone, M.; Guyot, J.-P. Artificial balance: Restoration of the vestibulo-ocular reflex in humans with a prototype vestibular neuroprosthesis. Front. Neurol. 2014, 5, 66. [Google Scholar] [CrossRef]
- Phillips, C.; DeFrancisci, C.; Ling, L.; Nie, K.; Nowack, A.; Phillips, J.O.; Rubinstein, J.T. Postural responses to electrical stimulation of the vestibular end organs in human subjects. Exp. Brain Res. 2013, 229, 181–195. [Google Scholar] [CrossRef]
- Wuehr, M.; Nusser, E.; Decker, J.; Krafczyk, S.; Straube, A.; Brandt, T.; Jahn, K.; Schniepp, R. Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology 2016, 86, 2196–2202. [Google Scholar] [CrossRef]
- Miffon, M.; Guyot, J.-P. Difficulties faced by patients suffering from total bilateral vestibular loss. Orl 2015, 77, 241–247. [Google Scholar] [CrossRef]
- Chambers, J.D.; Lord, J.; Cohen, J.T.; Neumann, P.J.; Buxton, M.J. Illustrating potential efficiency gains from using cost-effectiveness evidence to reallocate Medicare expenditures. Value Health 2013, 16, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Guyot, J.-P.; Gay, A.; Izabel Kos, M.; Pelizzone, M. Ethical, anatomical and physiological issues in developing vestibular implants for human use. J. Vestib. Res. 2012, 22, 3–9. [Google Scholar] [CrossRef]
- Fridman, G.Y.; Della SAntina, C.C. Progress toward development of a multichannel vestibular prosthesis for treatment of bilateral vestibular deficiency. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 2010–2029. [Google Scholar] [CrossRef]
- Guyot, J.-P.; Sigrist, A.; Pelizzone, M.; Kos, M.I. Adaptation to steady-state electrical stimulation of the vestibular system in humans. Ann. Otol. Rhinol. Laryngol. 2011, 120, 143–149. [Google Scholar] [CrossRef]
- Perez Fornos, A.; Cavuscens, S.; Ranieri, M.; van de Berg, R.; Stokroos, R.; Kingma, H.; Guyot, J.-P.; Guinand, N. The vestibular implant: A probe in orbit around the human balance system. J. Vestib. Res. 2017, 27, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Fridman, G.Y.; Della Santina, C.C. Effects of vestibular prosthesis electrode implantation and stimulation on hearing in rhesus monkeys. Hear. Res. 2011, 277, 204–210. [Google Scholar] [CrossRef]
- Fitzpatrick, R.C.; Day, B.L. Probing the human vestibular system with galvanic stimulation. J. Appl. Physiol. 2004, 96, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, C.; Yamamoto, Y.; Kamogashira, T.; Kinoshita, M.; Egami, N.; Uemura, Y.; Togo, F.; Yamasoba, T.; Iwasaki, S. Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Peterka, R.J. Use of galvanic vestibular feedback for a balance prosthesis. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 6137–6140. [Google Scholar]
- Keywan, A.; Wuehr, M.; Pradhan, C.; Jahn, K. Noisy galvanic stimulation improves roll-tilt vestibular perception in healthy subjects. Front. Neurol. 2018, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Scinicariello, A.P.; Eaton, K.; Inglis, J.T.; Collins, J. Enhancing human balance control with galvanic vestibular stimulation. Biol. Cybern. 2001, 84, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Benzi, R.; Sutera, A.; Vulpiani, A. The mechanism of stochastic resonance. J. Phys. A Math. Gen. 1981, 14, L453. [Google Scholar] [CrossRef]
- Cauquil, A.S.; Martinez, P.; Ouaknine, M.; Tardy-Gervet, M.-F. Orientation of the body response to galvanic stimulation as a function of the inter-vestibular imbalance. Exp. Brain Res. 2000, 133, 501–505. [Google Scholar] [CrossRef]
- Schniepp, R.; Boerner, J.; Decker, J.; Jahn, K.; Brandt, T.; Wuehr, M. Noisy vestibular stimulation improves vestibulospinal function in patients with bilateral vestibulopathy. J. Neurol. 2018, 265, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Conder, R.; Zamani, R.; Akrami, M. The Biomechanics of Pregnancy: A Systematic Review. J. Funct. Morphol. Kinesiol. 2019, 4, 72. [Google Scholar] [CrossRef]
- Zafar, A.Q.; Zamani, R.; Akrami, M. The effectiveness of foot orthoses in the treatment of medial knee osteoarthritis: A systematic review. Gait Posture 2020, 76, 238–251. [Google Scholar] [CrossRef] [PubMed]
- S.R. Group, SJR-SCImago Journal & Country Rank (2007). Available online: http://www.scimagojr.com (accessed on 30 March 2020).
- Golub, J.S.; Ling, L.; Nie, K.; Nowack, A.; Shepherd, S.J.; Bierer, S.M.; Jameyson, E.; Kaneko, C.R.; Phillips, J.O.; Rubinstein, J.T. Prosthetic implantation of the human vestibular system. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2014, 35, 136. [Google Scholar] [CrossRef] [PubMed]
- Van De Berg, R.; Guinand, N.; Guyot, J.-P.; Kingma, H.; Stokroos, R. The modified ampullar approach for vestibular implant surgery: Feasibility and its first application in a human with a long-term vestibular loss. Front. Neurol. 2012, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Inukai, Y.; Masaki, M.; Otsuru, N.; Saito, K.; Miyaguchi, S.; Kojima, S.; Onishi, H. Effect of noisy galvanic vestibular stimulation in community-dwelling elderly people: A randomised controlled trial. J. Neuroeng. Rehabil. 2018, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Inukai, Y.; Otsuru, N.; Masaki, M.; Saito, K.; Miyaguchi, S.; Kojima, S.; Onishi, H. Effect of noisy galvanic vestibular stimulation on center of pressure sway of static standing posture. Brain Stimul. 2018, 11, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Yamamoto, Y.; Togo, F.; Kinoshita, M.; Yoshifuji, Y.; Fujimoto, C.; Yamasoba, T. Noisy vestibular stimulation improves body balance in bilateral vestibulopathy. Neurology 2014, 82, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pu, F.; Lv, X.; Li, S.; Li, J.; Li, D.; Li, M.; Fan, Y. Comparison of postural responses to galvanic vestibular stimulation between pilots and the general populace. BioMed Res. Int. 2015, 567690. [Google Scholar] [CrossRef]
- Fujimoto, C.; Egami, N.; Kawahara, T.; Uemura, Y.; Yamamoto, Y.; Yamasoba, T.; Iwasaki, S. Noisy galvanic vestibular stimulation sustainably improves posture in bilateral vestibulopathy. Front. Neurol. 2018, 9, 900. [Google Scholar] [CrossRef]
- Guinand, N.; van de Berg, R.; Cavuscens, S.; Stokroos, R.J.; Ranieri, M.; Pelizzone, M.; Kingma, H.; Guyot, J.-P.; Perez-Fornos, A. Vestibular implants: 8 years of experience with electrical stimulation of the vestibular nerve in 11 patients with bilateral vestibular loss. ORL 2015, 77, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Guinand, N.; Van de Berg, R.; Cavuscens, S.; Stokroos, R.; Ranieri, M.; Pelizzone, M.; Kingma, H.; Guyot, J.-P.; Pérez Fornos, A. Restoring visual acuity in dynamic conditions with a vestibular implant. Front. Neurosci. 2016, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Guinand, N.; Van de Berg, R.; Cavuscens, S.; Ranieri, M.; Schneider, E.; Lucieer, F.; Kingma, H.; Guyot, J.-P.; Perez Fornos, A. The video head impulse test to assess the efficacy of vestibular implants in humans. Front. Neurol. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.O.; Ling, L.; Nie, K.; Jameyson, E.; Phillips, C.M.; Nowack, A.L.; Golub, J.S.; Rubinstein, J.T. Vestibular implantation and longitudinal electrical stimulation of the semicircular canal afferents in human subjects. J. Neurophysiol. 2015, 113, 3866–3892. [Google Scholar] [CrossRef] [PubMed]
- Van De Berg, R.; Guinand, N.; Ranieri, M.; Cavuscens, S.; Khoa Nguyen, T.; Guyot, J.-P.; Lucieer, F.; Starkov, D.; Kingma, H.; Van Hoof, M. The vestibular implant input interacts with residual natural function. Front. Neurol. 2017, 8, 644. [Google Scholar] [CrossRef] [PubMed]
| Reference | Behaviour(s) Measured | Methods | Summary of Results |
|---|---|---|---|
| [16] | 8 gait parameters | Walking balance tested at 3 speeds, eyes closed | nGVS improved: -Stride time, stride length, base of support, phase coordination index (p < 0.05)-Benefits most pronounced during slow walking. |
| [25] | Postural stability | Two-legged stance task, eyes closed | nGVS improved: -Postural stability (p < 0.01) (measurements were taken 2 h after stimulation) |
| [38] | Postural stability | Two-legged stance task, eyes open | nGVS improved: -ML mean velocity(p < 0.01)-AP mean velocity (p < 0.01)-Sway path length (p < 0.01) |
| [39] | Postural Sway | Two-legged stance task, eyes open | nGVS improved: -Postural sway (p < 0.01) |
| [40] | Postural stability | Two-legged stance task, eyes open | nGVS improved postural stability in:-76% of healthy subjects (p < 0.01)-91% of patients with BVD (p < 0.01) |
| [27] | Vestibular motion perception during roll rotations | Direction-recognition tasks on a motion platform, eyes closed | nGVS Improved:-Direction-recognition thresholds at 0.5 μA and 1.0 μA (p < 0.05)-No significance at 0.2 μA (p > 0.05) |
| [26] | ML balance control | Balance tested on the sway-referenced surface, eyes closed | nGVS improved: ML balance control (p < 0.05) |
| [41] | ML body sway | Two-legged stance task, eyes open | nGVS caused:-ML body sway in both groups(p < 0.01)-The response was lower in pilots compared to the general population (p < 0.001) |
| [31] | Vestibulospinal function | Body motion response to stimulation, eyes open | nGVS improved: -vestibulospinal function in 90% of patients (p < 0.05)-No response to nGVS in patients with complete BVD |
| [28] | ML body sway | Two-legged stance task on motorised platform, eyes open | GVS improved:-ML sway latency (p < 0.01)-ML sway amplitude (p < 0.01) |
| [42] | Postural stability | Two-legged stance, eyes closed | nGVS improved: -AP latency and sway, even 6-h post-stimulation (p < 0.01) |
| Reference | Journal (SJR Quartile) | Participant Details Provided | Description of Vestibulopathy |
|---|---|---|---|
| [16] | Neurology (Q1) | 13 Adults (5 female), mean age = 50.1 ± 5.5 years | BVD |
| [25] | Scientific Reports (Q1) | 30 Adults (13 Female), mean age = 67.0 ± 0.03 years | Healthy |
| [36] | Otology and Neurotology (Q1) | 1 Adult (Male), age = 56 years | Ménière’s disease |
| [43] | ORL (Q1) | 11 Adults (3 Female), mean age = 60.2 years | BVD and unilateral or bilateral hearing loss |
| [44] | Frontiers in Neuroscience (Q1) | 6 Adults (2 Female), mean age = 58 years | BVD and unilateral or bilateral hearing loss |
| [45] | Journal of Frontiers in Neurology (Q1) | 3 Adults (1 Female), mean age = 53.3 years | BVD |
| [21] | Annals of Otology, Rhinology and Laryngology (Q2) | 1 Adult (Male), mean age = 69 years | BVD and bilateral hearing loss |
| [38] | Journal of Neuroengineering and Rehabilitation (Q1) | 32 Adults (25 Female), mean age = 75.8 ± 0.8 years | Healthy |
| [39] | Brain Stimulation (Q1) | 18 Adults (12 Female), mean age = 21.8 ± 1.1 years | Healthy |
| 24 Adults (11 Female), mean age = 22.2 ± 1.8 years | Healthy | ||
| 16 Adults (10 Female), mean age = 21.9 ± 1.2 years | Healthy | ||
| [40] | Neurology (Q1) | 21 Adults (10 Female), mean age = 38.7 ± 2.6 years | Healthy |
| 11 Adults (5 Female), mean age = 46.4 ± 5.2 years | BVD | ||
| [27] | Frontiers in Neurology (Q1) | 15 Adults (7 Female), mean age = 25.1 ± 1.7 years | Healthy |
| [14] | Frontiers in Neurology (Q1) | 3 Adults (2 Female), mean age = 63.7 years | BVD, unilateral or bilateral hearing loss |
| [46] | Neurophysiology (Q1) | 4 Adults (2 Female), mean age = 67 ± 9 years | Unilateral Ménière’s disease |
| [26] | Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS (Q1) | 2 Adults (Sex and age unspecified) | 1 Healthy, 1 BVD |
| [15] | Experimental Brain Research (Q2) | 4 Adults (2 female), mean ag e = 67 ± 9 years | Unilateral Ménière’s disease |
| [41] | BioMed Research International (Q1) | 12 Students, mean age =23.8 years | Healthy |
| 12 pilots, mean age =25.3 years | Healthy | ||
| [31] | Journal of neurology (Q1) | 12 Adults (6 female), age unspecified | 2 complete BVD 10 partial BVD |
| [28] | Biological Cybernetics (Q1) | 9 Adults (6 female), mean age =22 years | Healthy |
| [42] | Frontiers in neurology (Q1) | 13 Adults (5 female), mean age =63.1 ± 4.0 years | BVD |
| [37] | Frontiers in neurology (Q1) | 1 Adult (female), age =21 years | Complete BVD and bilateral hearing loss |
| [47] | Frontiers in neurology (Q1) | 4 Adults (2 female), mean age =58.8 years | BVD |
| Reference | Details of Prosthesis | Behaviour(s) Measured | Summary of Results | Adverse Effects Reported |
|---|---|---|---|---|
| [14] | Modified cochlear implant, intralabyrinthine ampullar approach | VOR response | Activation of LAN electrode elicited VOR response (p < 0.01) | N/A |
| [36] | Modified cochlear implant, intralabyrinthine ampullar approach | Electrically evoked eye movements | Stimulation of canal-specific eye movements successful in 2/3 electrodes | Severe loss of horizontal canal and auditory function |
| [45] | Modified cochlear implant, both surgical approaches used | High-frequency aVOR response | aVOR restored in a broad frequency range | N/A |
| [44] | Modified cochlear implant, both surgical approaches used | Visual acuity in dynamic conditions | VA improved when the implant was turned on (p < 0.001) | N/A |
| [43] | Modified cochlear implant, both surgical approaches used | VOR response | Stimulation of canal-specific eye movements successful in 17/24 available electrodes. | N/A |
| [21] | Modified cochlear implant, extralabyrinthine approach | Electrically evoked eye movements | Stimulation of PAN caused smooth canal-specific eye movement | Initial strong nystagmus abated after vestibular adaptation |
| [15] | Modified cochlear implant, intralabyrinthine ampullar approach | Postural responses | 2 s of electrical stimulation of each SCC all elicited sway response | Reduced vestibular function in implanted ear |
| [46] | Modified cochlear implant, intralabyrinthine ampullar approach | VOR response | Stimulation of ampullary nerves caused a proportional VOR response | Hearing and vestibular loss in implanted ear |
| [37] | Modified cochlear implant, intralabyrinthine ampullar approach | Electrically evoked eye movements | Ampullar stimulation evoked eye movements in a patient after 20 years of no vestibular function | N/A |
| [47] | Modified cochlear implant, intralabyrinthine ampullar approach | VOR | Artificial stimulation of the vestibular nerve branches interacts with residual vestibular function | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haxby, F.; Akrami, M.; Zamani, R. Finding a Balance: A Systematic Review of the Biomechanical Effects of Vestibular Prostheses on Stability in Humans. J. Funct. Morphol. Kinesiol. 2020, 5, 23. https://doi.org/10.3390/jfmk5020023
Haxby F, Akrami M, Zamani R. Finding a Balance: A Systematic Review of the Biomechanical Effects of Vestibular Prostheses on Stability in Humans. Journal of Functional Morphology and Kinesiology. 2020; 5(2):23. https://doi.org/10.3390/jfmk5020023
Chicago/Turabian StyleHaxby, Felix, Mohammad Akrami, and Reza Zamani. 2020. "Finding a Balance: A Systematic Review of the Biomechanical Effects of Vestibular Prostheses on Stability in Humans" Journal of Functional Morphology and Kinesiology 5, no. 2: 23. https://doi.org/10.3390/jfmk5020023
APA StyleHaxby, F., Akrami, M., & Zamani, R. (2020). Finding a Balance: A Systematic Review of the Biomechanical Effects of Vestibular Prostheses on Stability in Humans. Journal of Functional Morphology and Kinesiology, 5(2), 23. https://doi.org/10.3390/jfmk5020023






