Reliability of a New Test of Balance Function in Healthy and Concussion Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Post-Hoc Analysis: Group Comparison
2.2. Instrumentation
3. Results
3.1. Descriptive Analysis
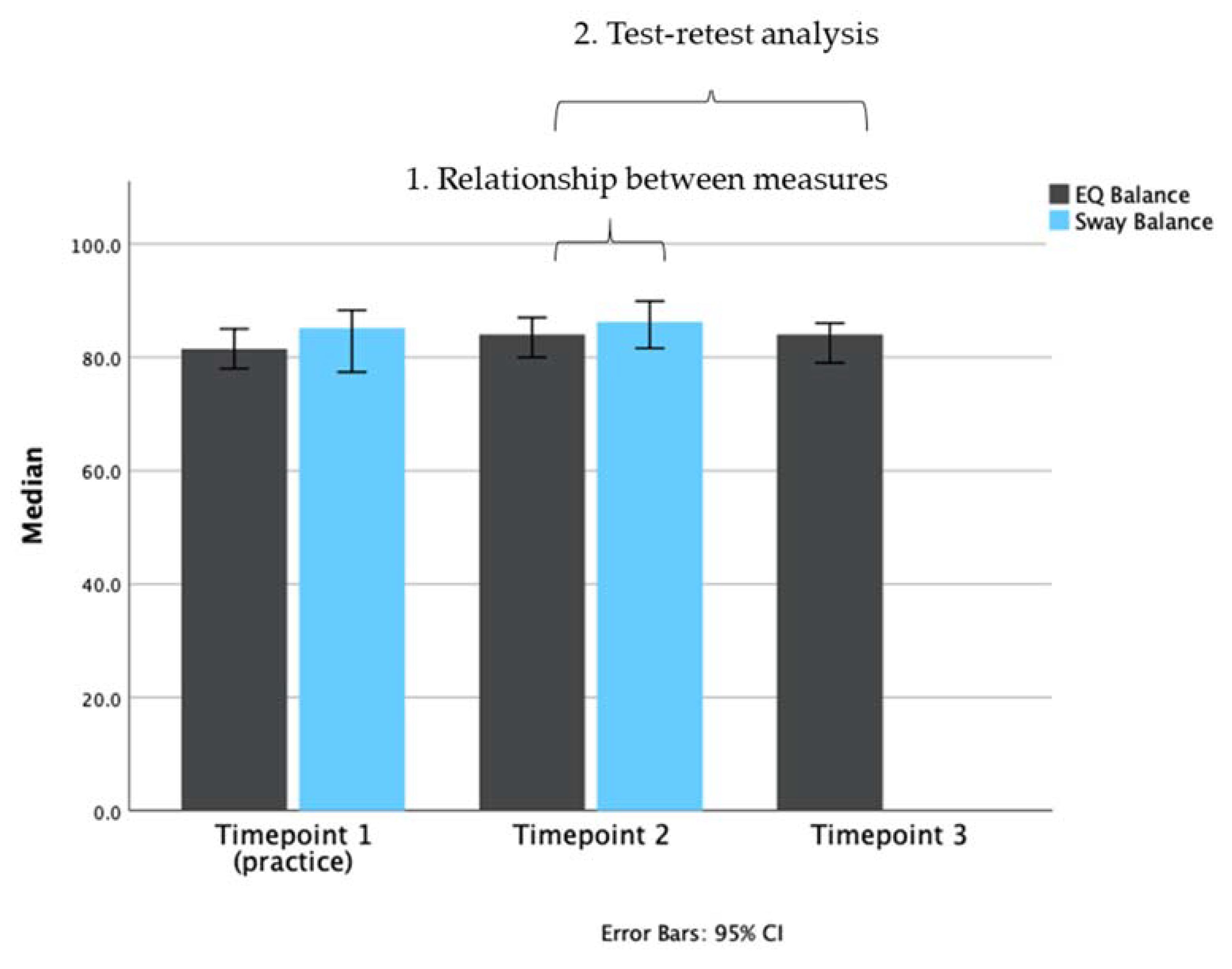
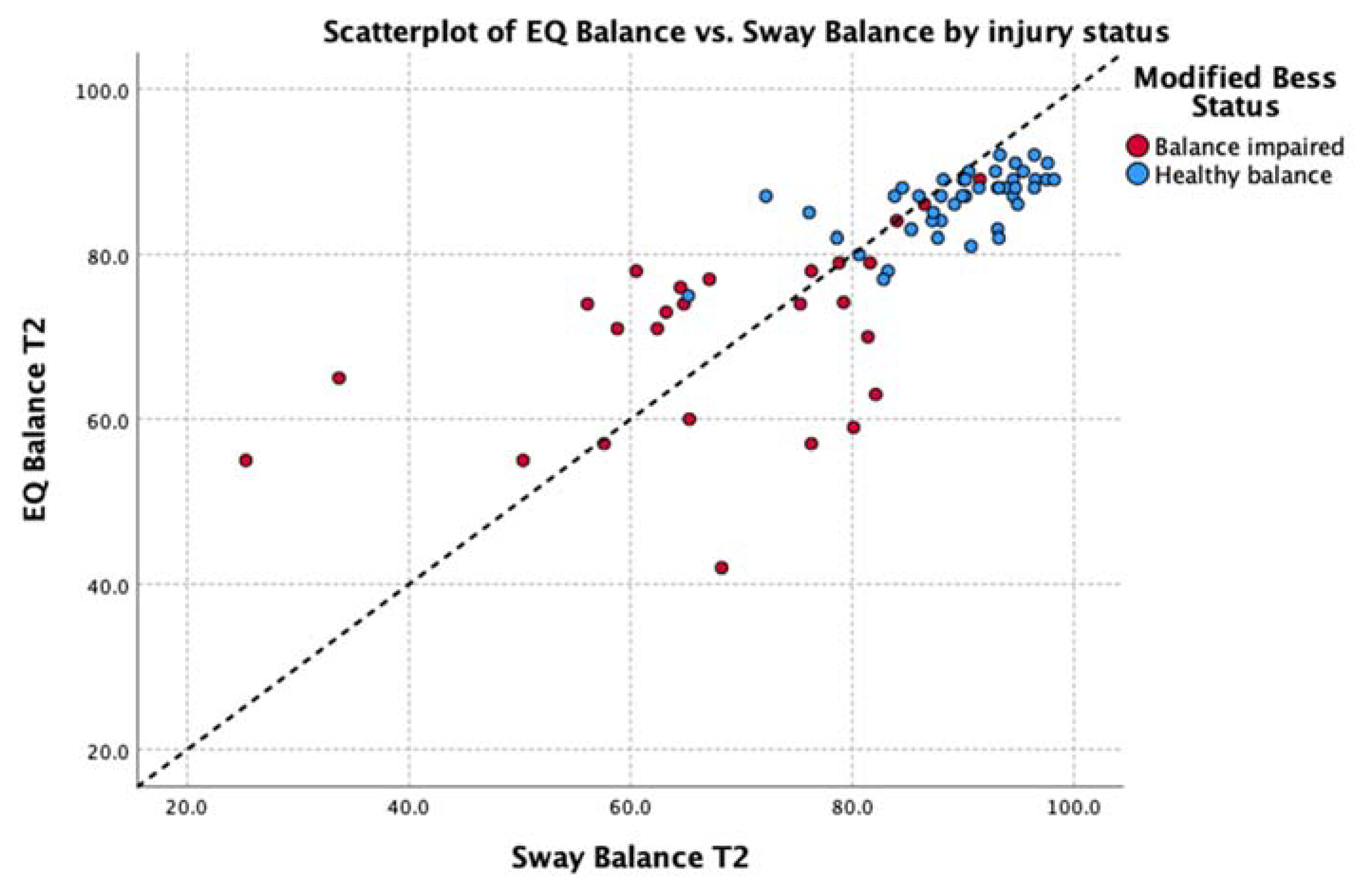
3.2. Analysis 1: Relationship between Measures
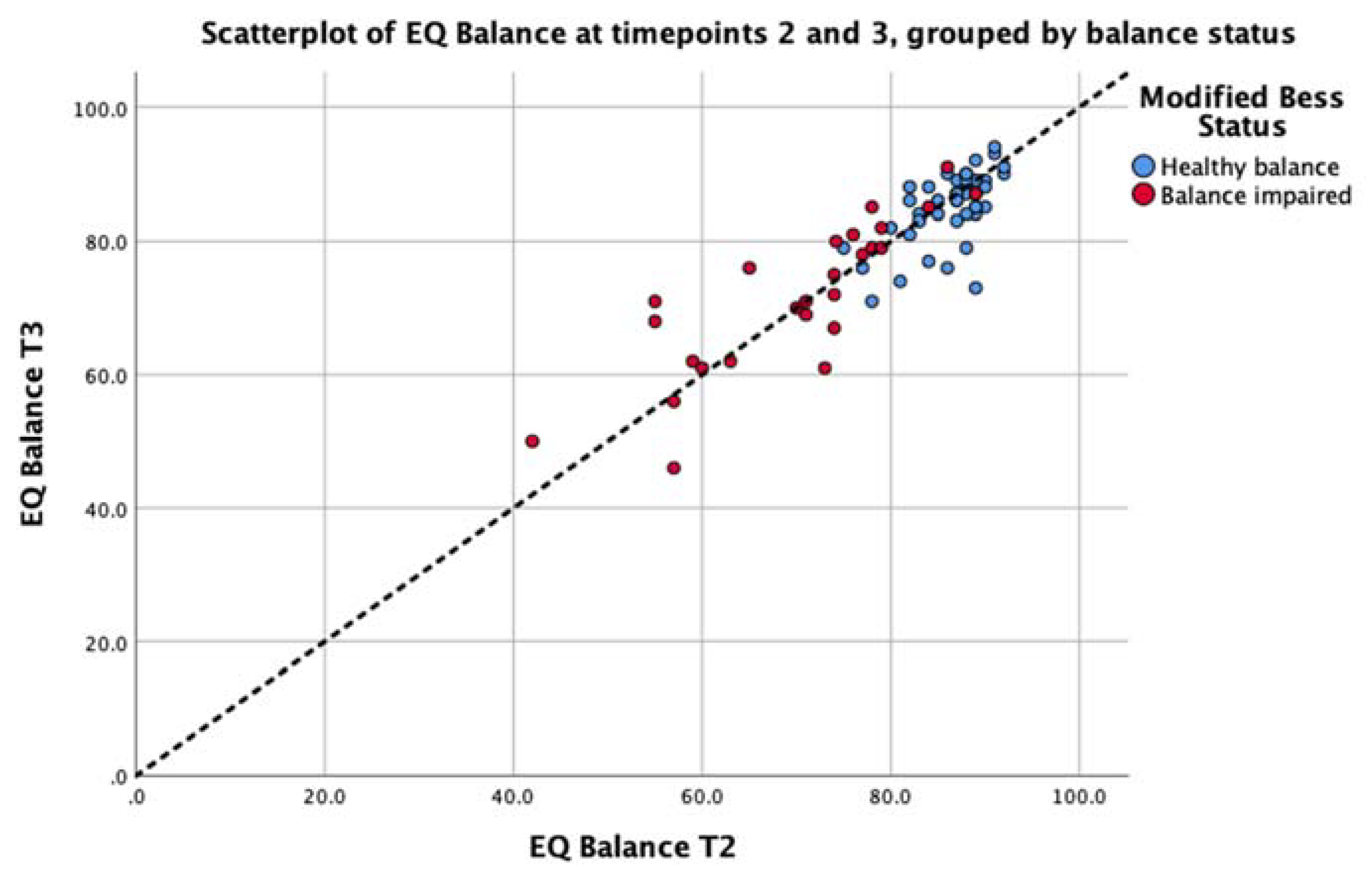
3.3. Analysis 2: Test-Retest Analysis of the EQ Balance Task
3.4. Post-Hoc Analysis: Group Comparison
4. Discussion
4.1. Future Directions
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pollock, A.S.; Durward, B.R.; Rowe, P.J.; Paul, J.P. What is balance? Clin. Rehabil. 2000, 14, 402–406. [Google Scholar] [CrossRef]
- Rose, D.J. Balance, posture, and locomotion. In Physical Dimensions of Aging, 2nd ed.; Spirduso, W., Francis, K., MacRae, P., Eds.; Physical Dimensions: Englewood, CO, USA, 2005; pp. 131–151. [Google Scholar]
- Rogers, M.E.; Rogers, N.L.; Takeshima, N. Balance training in older adults. Aging Health 2005, 1, 475–486. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; Perrin, D.H. Research and clinical applications of assessing balance. J. Sport Rehabil. 1996. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Shumway-Cook, A. Concepts and methods for assessing postural instability. J. Aging Phys. Act. 1996, 4, 214–233. [Google Scholar] [CrossRef]
- Davlin, C.D. Dynamic balance in high level athletes. Percept. Mot. Skill 2004, 98, 1171–1176. [Google Scholar] [CrossRef]
- Alexander, N.B. Postural control in older adults. J. Am. Geriatr. Soc. 1994, 42, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.; Salvatore, A.; Powell, D.; Reed-Jones, R. Reliability and validity evidence of multiple balance assessments in athletes with a concussion. J. Athl. Train. 2014, 49, 540–549. [Google Scholar] [CrossRef]
- Massingale, S.; Alexander, A.; Erickson, S.; McQueary, E.; Gerkin, R.; Kisana, H.; Silvestri, B.; Schodrof, S.; Nalepa, B.; Pardini, J. Comparison of uninjured and concussed adolescent athletes on the concussion balance test (COBALT). J. Neurol. Phys. 2018, 42, 149–154. [Google Scholar] [CrossRef]
- Murray, N.G.; Reed-Jones, R.J.; Szekely, B.J.; Powell, D.W. Clinical assessments of balance in adults with concussion: An update. Semin. Speech Lang. 2019, 40, 48–56. [Google Scholar] [CrossRef]
- King, L.A.; Horak, F.B.; Mancini, M.; Pierce, D.; Priest, K.C.; Chesnutt, J.; Sullivan, P.; Chapman, J.C. Instrumenting the balance error scoring system for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch. Phys. Med. Rehabil. 2014, 95, 353–359. [Google Scholar] [CrossRef]
- Rogers, M.E.; Rogers, N.L.; Takeshima, N.; Islam, M.M. Methods to assess and improve the physical parameters associated with fall risk in older adults. Prev. Med. 2003, 36, 255–264. [Google Scholar] [CrossRef]
- Bell, D.R.; Guskiewicz, K.M.; Clark, M.A.; Padua, D.A. Systematic review of the balance error scoring system. Sports Health 2011, 3, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Blosch, C.; Schäfer, R.; De Marées, M.; Platen, P. Comparative analysis of postural control and vertical jump performance between three different measurement devices. PLoS ONE 2019, 14, e0222502. [Google Scholar] [CrossRef] [PubMed]
- Goble, D.J.; Manyak, K.A.; Abdenour, T.E.; Rauh, M.J.; Baweja, H.S. An initial evaluation of the btracks balance plate and sports balance software for concussion diagnosis. Int. J. Sports Phys. 2016, 11, 149–155. [Google Scholar]
- Merchant-Borna, K.; Jones, C.M.C.; Janigro, M.; Wasserman, E.B.; Clark, R.A.; Bazarian, J.J. Evaluation of nintendo Wii balance board as a tool for measuring postural stability after sport-related concussion. J. Athl. Train. 2017, 52, 245–255. [Google Scholar] [CrossRef]
- Paillard, T.; Noé, F. Techniques and methods for testing the postural function in healthy and pathological subjects. Biomed Res. Int. 2015. [Google Scholar] [CrossRef]
- Zhong, R.; Rau, P.L.P. Are cost-effective technologies feasible to measure gait in older adults? A systematic review of evidence-based literature. Arch. Gerontol. Geriatr. 2020, 87, 103970. [Google Scholar] [CrossRef]
- Ghislieri, M.; Gastaldi, L.; Pastorelli, S.; Tadano, S.; Agostini, V. Wearable inertial sensors to assess standing balance: A systematic review. Sensors (Basel) 2019, 19, 4075. [Google Scholar] [CrossRef]
- Hinman, M.R. Factors affecting reliability of the biodex balance system: A summary of four studies. J. Sport Rehabil. 2016, 9, 240–252. [Google Scholar] [CrossRef]
- Karimi, N.; Ebrahimi, I.; Kahrizi, S.; Torkaman, G. Reliability of postural balance evaluation using the biodex balance system in subjects with and without low back pain. JPMI J. Postgrad. Med. Inst. 2008, 22, 2. [Google Scholar]
- Patterson, J.A.; Amick, R.Z.; Thummar, T.; Rogers, M.E. Validation of measures from the smartphone: A pilot study. Int. J. Sports Phys. 2014, 9, 135–139. [Google Scholar]
- Hunt, T.N.; Ferrara, M.S.; Bornstein, R.A.; Baumgartner, T.A. The reliability of the modified balance error scoring system. Clin. J. Sport Med. 2009, 19, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Marcinak, J.; Hibyan, S.; Webbe, F. Normative values of major SCAT2 and SCAT3 components for a college athlete population. Appl. Neuropsychol. 2015, 22, 132–140. [Google Scholar] [CrossRef]
- Snyder, A.R.; Bauer, R.M. A normative study of the sport concussion assessment tool (SCAT2) in children and adolescents. Clin. Neuropsychol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bin Zahid, A.; Hubbard, M.E.; Dammavalam, V.M.; Balser, D.Y.; Pierre, G.; Kim, A.; Kolecki, R. Assessment of acute head injury in an emergency department population using SCAT3. Appl. Neuropsychol. 2018, 25, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intra-class correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Cornbleet, P.J.; Gochman, N. Incorrect least-squares regression coefficients in method-comparison analysis. Clin. Chem. 1979, 25, 432–438. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 36 (Suppl. 2), 7–11. [Google Scholar] [CrossRef]
- Tyson, S.F.; Connell, L.A. How to measure balance in clinical practice. A systematic review of the psychometrics and clinical utility of measures of balance activity for neurological conditions. Clin. Rehabil. 2009, 23, 824–840. [Google Scholar] [CrossRef]
- Inness, E.L.; Sweeny, M.; Habib Perez, O.; Danells, C.; Chandra, T.; Foster, E.; Saverino, C.; Comper, P.; Bayley, M.; Mochizuki, G. Self-reported balance disturbance and performance-based balance impairment after concussion in the general population. J. Head Trauma Rehabil. 2018, 34, 37–46. [Google Scholar] [CrossRef]


| Cohort | Entire Cohort | Healthy | Concussed | Balance-Impaired |
|---|---|---|---|---|
| Age, continuous (units: mean years ± standard deviation) | 37.8 ± 14.8 | 35.6 ± 12.3 | 39.6 ± 16.4 | 45.6 ± 12.7 |
| Age, categorical (units: number of participants) | ||||
| ≤ 18 years | 12 | 3 | 9 | 2 |
| 18–65 years | 58 | 28 | 30 | 24 |
| ≥ 65 years | 0 | 0 | 0 | 0 |
| Sex Women Men | 45 25 | 16 15 | 29 10 | 18 8 |
| Grouping Variable | Spearman’s Rho | Coefficient of VAR. | Deming EquationEQ = B0 × Sway + B1 | T-Stat (p-val) Intercept ≠ 0 | T-stat (p-val) Slope ≠ 1 | ||
|---|---|---|---|---|---|---|---|
| Comparison by pose: all participants (n = 70) | |||||||
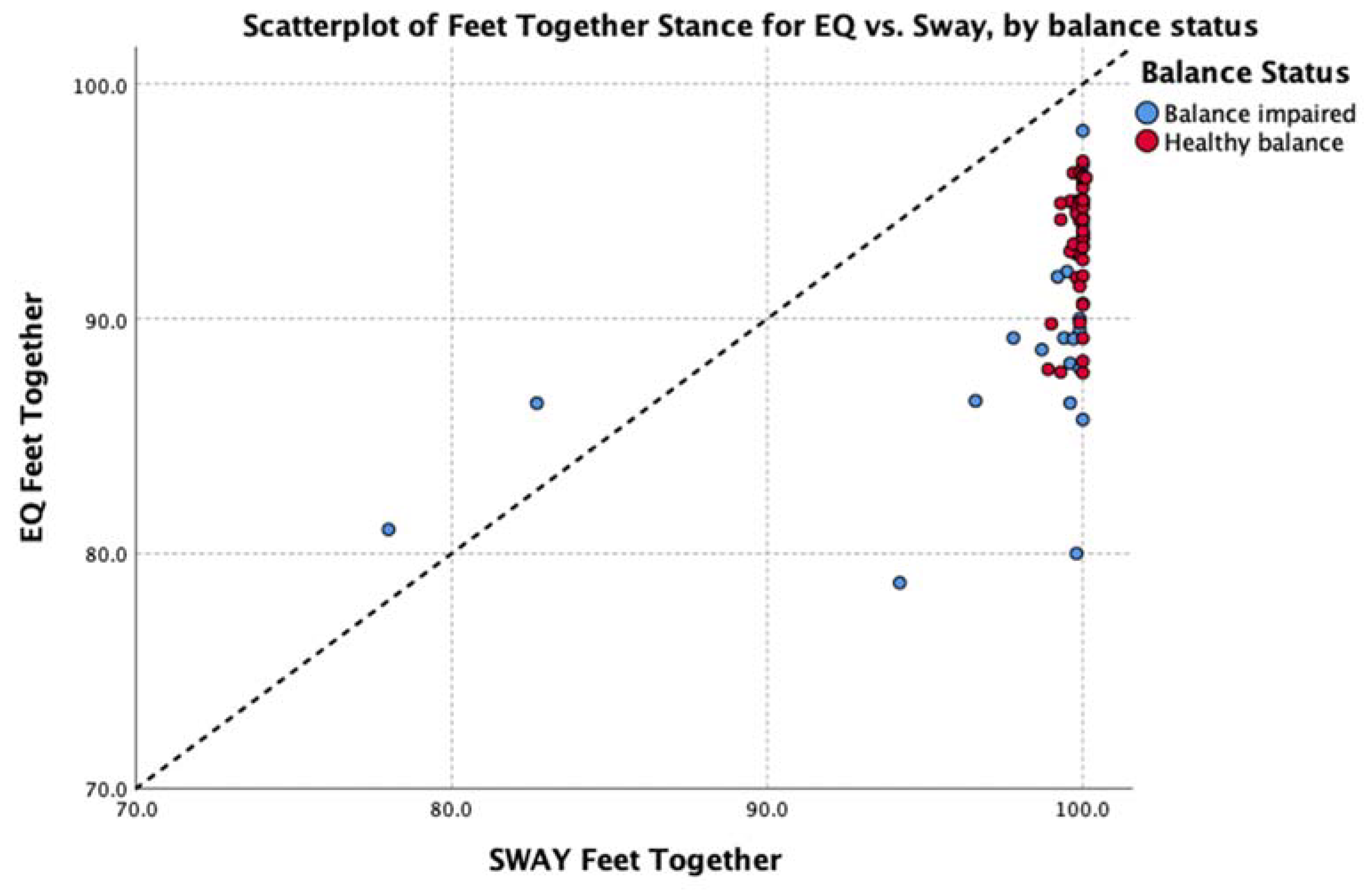
| Feet together | 0.49 | (p < 0.001) | EQ: 3.53 Sway: 2.01 | y = 0.87x + 6.02 | 0.06 (0.95) | 0.12 (0.90) | |
| Tandem left | 0.82 | (p < 0.001) | EQ: 12.35 Sway: 17.80 | y = 0.70x + 21.47 | 1.69 (0.09) | 2.22 (0.03)* | |
| Tandem right | 0.78 | (p < 0.001) | EQ: 13.06 Sway: 18.18 | y = 0.84x + 8.12 | 0.30 (0.77) | 0.54 (0.59) | |
| Left single leg | 0.78 | (p < 0.001) | EQ: 16.62 Sway: 27.44 | y = 0.74x + 21.89 | 1.85 (0.07) | 1.69 (0.10) | |
| Right single leg | 0.79 | (p < 0.001) | EQ: 17.73 Sway:27.53 | y =0.47x + 42.50 | 6.19 (0.00)* | 6.16 (0.00)* | |
| Average of all poses | 0.85 | (p < 0.001) | EQ: 5.71 Sway: 9.02 | y = 0.76x + 18.36 | 1.36 (0.18) | 1.54 (0.13) | |
| Comparison by balance status (average score of all 5 poses) | |||||||
| healthy balance group (n = 44) | 0.67 | (p < 0.001) | EQ: 5.71 Sway: 9.02 | y = 0.54x + 37.75 | 3.47 (0.00)* | 3.87 (0.00)* | |
| impaired balance group (n = 26) | 0.50 | (p < 0.01) | EQ: 5.71 Sway: 9.02 | y = 0.80x + 15.46 | 0.45 (0.65) | 0.43(0.67) | |
| Comparison by sex (average score of all 5 poses) | |||||||
| Women (n = 45) | 0.80 | (p < 0.001) | EQ: 5.71 Sway: 9.02 | y = 0.44x + 47.64 | 3.23 (0.00)* | 3.50(0.00)* | |
| Men (n = 25) | 0.86 | (p < 0.001) | EQ: 5.71 Sway: 9.02 | y = 0.71x + 21.15 | 0.40 (0.69) | 0.38(0.71) | |
| Comparison by performance: split by Sway values above/below 80 (average score of all 5 poses) | |||||||
| Sway >= 80 (n = 47) | 0.72 | (p < 0.001) | EQ: 5.71 Sway: 9.02 | y = 1.70x - 67.63 | −2.00 (0.05) | 1.87 (0.07) | |
| Sway < 80 (n = 23) | 0.54 | (p < 0.01) | EQ: 5.71 Sway: 9.02 | y = 0.99x + 6.71 | 0.12 (0.90) | −0.01 (0.99) | |
| Pose | Healthy Balance (n = 44) Median (5% to 95% Range) | Impaired Balance (n = 26) Median (5% to 95% Range) | Mann-Whitney Z-Value (p-Value) |
|---|---|---|---|
| EQ Balance scores | |||
| Feet together | 94.0 (87.8 to 96.2) | 89.4 (79.2 to 97.5) | −3.30 (p < 0.001) |
| Tandem left | 91.4 (68.0 to 94.8) | 73.3 (31.2 to 92.8) | −5.04 (p < 0.000) |
| Tandem right | 90.0 (74.4 to 95.2) | 75.5 (42.7 to 94.0) | −4.86 (p < 0.000) |
| Left single leg | 83.2 (60.5 to 92.7) | 61.0 (25.0 to 85.3) | −5.16 (p < 0.000) |
| Right single leg | 81.6 (61.1 to 90.5) | 57.5 (11.4 to 82.3) | −5.54 (p < 0.000) |
| Average 5 poses | 87.0 (77.3 to 91.8) | 73.5 (46.6 to 88.0) | −6.08 (p < 0.000) |
| Sway Balance Scores | |||
| Feet together | 100.0 (99.1 to 100.0) | 99.8 (79.6 to 100.0) | −2.62 (p < 0.009) |
| Tandem left | 96.5 (83.9 to 99.6) | 79.0 (6.2 to 98.6) | −5.22 (p < 0.000) |
| Tandem right | 98.0 (80.4 to 99.9) | 82.9 (5.6 to 99.4) | −4.64 (p < 0.000) |
| Left single leg | 84.5 (13.7 to 97.5) | 61.4 (0.0 to 88.4) | −4.28 (p < 0.000) |
| Right single leg | 84.1 (39.2 to 97.3) | 59.3 (0.0 to 79.2) | −5.12 (p < 0.000) |
| Average 5 poses | 90.4 (73.2 to 97.6) | 67.7 (28.2 to 89.8) | −5.96 (p < 0.000) |
| Group | N | Range | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|---|---|
| Healthy | ||||||
| EQ Feet Together | 44 | 9.0 | 87.7 | 96.7 | 93.3 | 2.52 |
| SWAY Feet Together | 44 | 1.2 | 98.9 | 100.1 | 99.8 | 0.28 |
| Balance-impaired | ||||||
| EQ Feet Together | 26 | 19.3 | 78.8 | 98.0 | 89.6 | 4.88 |
| SWAY Feet Together | 26 | 22.0 | 78.0 | 100.0 | 97.9 | 5.35 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kis, M. Reliability of a New Test of Balance Function in Healthy and Concussion Populations. J. Funct. Morphol. Kinesiol. 2020, 5, 13. https://doi.org/10.3390/jfmk5010013
Kis M. Reliability of a New Test of Balance Function in Healthy and Concussion Populations. Journal of Functional Morphology and Kinesiology. 2020; 5(1):13. https://doi.org/10.3390/jfmk5010013
Chicago/Turabian StyleKis, Mihaly. 2020. "Reliability of a New Test of Balance Function in Healthy and Concussion Populations" Journal of Functional Morphology and Kinesiology 5, no. 1: 13. https://doi.org/10.3390/jfmk5010013
APA StyleKis, M. (2020). Reliability of a New Test of Balance Function in Healthy and Concussion Populations. Journal of Functional Morphology and Kinesiology, 5(1), 13. https://doi.org/10.3390/jfmk5010013




