Conservative Treatment of Chronic Achilles Tendinopathy: A Systematic Review
Abstract
1. Introduction
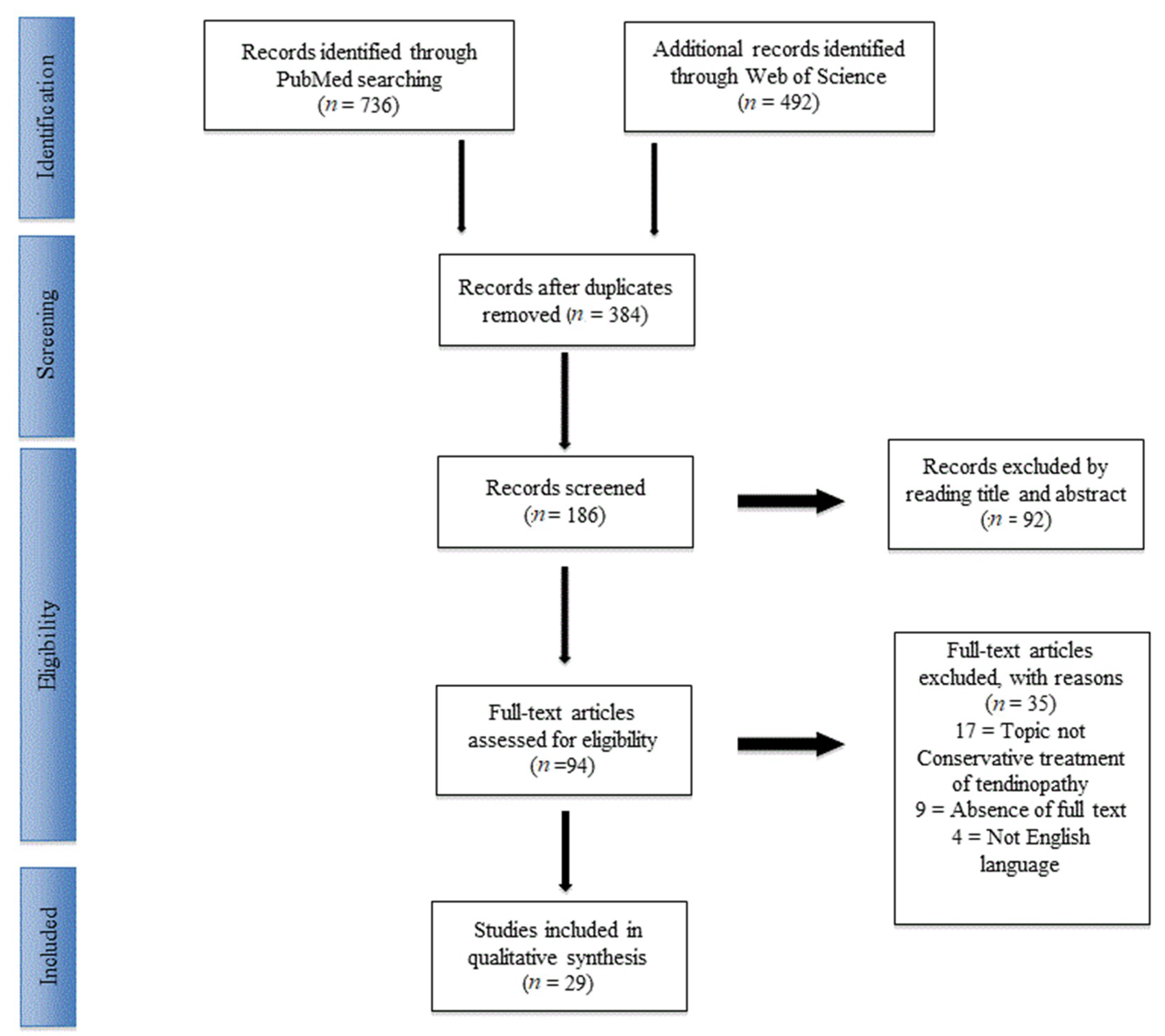
2. Materials and Methods
2.1. Study Selection
2.2. Inclusion and Exclusion Criteria
3. Results
3.1. Included Studies
3.2. Physical Exercise
3.3. Platelet Rich Plasma and Leukocyte- and Platelet-Rich Plasma
3.4. Extracorporeal Shockwave Therapy
3.5. Topical and Systemic Pharmacologic Therapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schepsis, A.A.; Jones, H.; Haas, A.L. Current concepts: Achilles tendon disorders in athletes. Am. J. Sports Med. 2002, 30, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Khan, K.M.; Purdam, C. Achilles tendinopathy. Man. Ther. 2002, 7, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Understanding and managing Achilles tendinopathy. Br. J. Hosp. Med. 2006, 67, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.R.; Longo, U.G.; Denaro, V.; Maffulli, N. Achilles tendon problems: Not just an orthopaedic issue. Disabil. Rehabil. 2008, 30, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.M.; Rozen, W.M.; Pan, W.R.; Ashton, M.W.; Richardson, M.D.; Taylor, G. The arterial anatomy of the achilles tendon: Anatomical study and clinical implications. Clin. Anat. 2009, 22, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Longo, U.G.; Maffulli, G.D.; Khanna, A.; Denaro, V. Achilles tendon ruptures in elite athletes. Foot Ankle Int. 2011, 32, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Magnan, B.; Bondi, M.; Pierantoni, S.; Samaila, E. The pathogenesis of Achilles tendinopathy: A systematic review. Foot Ankle Sur. 2014, 20, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.B.; Mann, R.A.; Wells, L. Epidemiological factors associated with rupture ofthe Achilles tendon. Contemp. Orthop. 1991, 23, 327–331. [Google Scholar]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, andobesity-related health risk factors. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef]
- Backman, C.; Boquist, L.; Friden, J.J.; Lorentzon, R.; Toolanen, G. Chronic achilles paratenonitis with tendinosis: An experimental model in the rabbit. J. Orthop. Res. 1990, 8, 541–547. [Google Scholar] [CrossRef]
- Wren, T.A.; Lindsey, D.P.; Beaupre, G.S.; Carter, D.R. Effects of creep and cyclic loading on the mechanical properties and failure ofhuman Achilles tendons. Ann. Biomed. Eng. 2003, 31, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Rompe, J.D.; Furia, J.P.; Maffulli, N. Mid-portion Achilles tendinopathy—current options for treatment. Disabil. Rehabil. 2008, 30, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, S.; Tol, J.L.; Weir, A.; Waarsing, J.H.; Verhaar, J.A.; de Vos, R.J. The tendon structure returns to asymptomatic values in nonoperatively treated Achilles tendinopathy but is not associated with symptoms: A prospective study. Amer J Sports Med 2015, 43, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Beyer, R.; Kongsgaard, M.; Hougs Kjær, B.; Øhlenschlæger, T.; Kjær, M.; Magnusson, S.P. Heavy slow resistance versus eccentric training as treatment for Achilles tendinopathy: A randomized controlled trial. Amer. J. Sports Med. 2015, 43, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Stasinopoulos, D.; Manias, P. Comparing two eccentric exercise programmes for the management of Achilles tendinopathy. A pilot trial. J. Bod. Mov. Ther. 2013, 17, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.M.; Pallister, I.; Evans, R.M.; Bodger, O.; Topliss, C.J.; Williams, P.; Beard, D.J. Intense pulsed light treatment of chronic mid-body Achilles tendinopathy: A double blind randomised placebo-controlled trial. Bone Joint J. 2013, 95, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, T.; Jud, H.M.; Fröhlich, V.; Mündermann, A.; Grau, S. Whole-body vibration versus eccentric training or a wait-and-see approach for chronic Achilles tendinopathy: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2013, 43, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.M.; Zhong, L.W.; Xu, S.W.; Jiang, H.R.; Shen, J. Acupuncture for chronic Achilles tendnopathy: A randomized controlled study. Chin. J. Integ. Med. 2013, 19, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Yelland, M.J.; Sweeting, K.R.; Lyftogt, J.A.; Ng, S.K.; Scuffham, P.A.; Evans, K.A. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: A randomised trial. Br. J. Sports Med. 2011, 45, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Tumilty, S.; Mani, R.; Baxter, G.D. Photobiomodulation and eccentric exercise for Achilles tendinopathy: A randomized controlled trial. Lasers Me.d Sci 2016, 31, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Praet, S.; Purdam, C.; Welvaert, M.; Vlahovich, N.; Lovell, G.; Burke, L.; Gaida, J.E.; Manzanero, S.; Hughes, D.; Waddington, G. Oral Supplementation of Specific Collagen Peptides Combined with Calf-Strengthening Exercises Enhances Function and Reduces Pain in Achilles Tendinopathy Patients. Nutrients 2019, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- McCormack, J.R.; Underwood, F.B.; Slaven, E.J.; Cappaert, T.A. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports health 2016, 8, 230–237. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, S.; de Vos, R.J.; Van Schie, H.T.; Verhaar, J.A.; Weir, A.; Tol, J.L. One-year follow-up of a randomised controlled trial on added splinting to eccentric exercises in chronic midportion Achilles tendinopathy. Br. J. Sports Med. 2010, 44, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Verrall, G.; Schofield, S.; Brustad, T.; Physio, D. Chronic Achilles tendinopathy treated with eccentric stretching program. Foot Ankle Int. 2011, 32, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Meeuwisse, W.; Patel, C.; Wiseman, D.A.; Wiley, J.P. The limited effectiveness of a home-based eccentric training for treatment of Achilles tendinopathy. Clin. Inv. Med. 2013, 36, 197–206. [Google Scholar] [CrossRef]
- Yu, J.; Park, D.; Lee, G. Effect of eccentric strengthening on pain, muscle strength, endurance, and functional fitness factors in male patients with achilles tendinopathy. Am. J. Phys. Med. Rehab. 2013, 92, 68–76. [Google Scholar] [CrossRef]
- Stevens, M.; Tan, C.W. Effectiveness of the Alfredson protocol compared with a lower repetition-volume protocol for midportion Achilles tendinopathy: a randomized controlled trial. J. Orthop. Sports Phys. Ther. 2014, 44, 59–67. [Google Scholar] [CrossRef]
- Van der Plas, A.; de Jonge, S.; de Vos, R.J.; van der Heide, H.J.L.; Verhaar, J.A.N.; Weir, A.; Tol, J.L. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br. J. Sports Med. 2012, 46, 214–218. [Google Scholar] [CrossRef]
- Deans, V.M.; Miller, A.; Ramos, J. A prospective series of patients with chronic Achilles tendinopathy treated with autologous-conditioned plasma injections combined with exercise and therapeutic ultrasonography. J. Foot Ankle Surg. 2012, 51, 706–710. [Google Scholar] [CrossRef]
- De Vos, R.J.; Weir, A.; van Schie, H.T.; Bierma-Zeinstra, S.M.; Verhaar, J.A.; Weinans, H.; Tol, J.L. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA 2010, 303, 144–149. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, S.; de Vos, R.J.; Weir, A.; van Schie, H.T.; Bierma-Zeinstra, S.M.; Verhaar, J.A.; Tol, J.L. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: A double-blind randomized placebo-controlled trial. Amer. J. Sports Med. 2011, 39, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Di Matteo, B.; Di Martino, A.; Tesei, G.; Pelotti, P.; Marcacci, M. Platelet-rich plasma injections for the treatment of refractory Achilles tendinopathy: results at 4 years. Blood Transf. 2014, 12, 533. [Google Scholar]
- Guelfi, M.; Pantalone, A.; Vanni, D.; Abate, M.; Guelfi, M.G.; Salini, V. Long-term beneficial effects of platelet-rich plasma for non-insertional Achilles tendinopathy. Foot Ankle Surg. 2015, 21, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Njawaya, M.M.; Moses, B.; Martens, D.; Orchard, J.J.; Driscoll, T.; Negrine, J.; Orchard, J.W. Ultrasound Guidance Does Not Improve the Results of Shock Wave for Plantar Fasciitis or Calcific Achilles Tendinopathy: A Randomized Control Trial. Clin. J. Sport Med. 2018, 28(1), 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pavone, V.; Cannavò, L.; Di Stefano, A.; Testa, G.; Costarella, L.; Sessa, G. Low-energy extracorporeal shock-wave therapy in the treatment of chronic insertional Achilles tendinopathy: A case series. Bio. Med. Res. Int. 2016, 2016, 7123769. [Google Scholar] [CrossRef] [PubMed]
- Rompe, J.D.; Furia, J.; Maffulli, N. Eccentric loading compared with shock wave treatment for chronic insertional achilles tendinopathy: a randomized, controlled trial. JBJS 2008, 90, 52–61. [Google Scholar] [CrossRef]
- Vulpiani, M.C.; Trischitta, D.; Trovato, P.; Vetrano, M.; Ferretti, A. Extracorporeal shockwave therapy (ESWT) in Achilles tendinopathy. A long-term follow-up observational study. J. Sports Med. Phys. Fit 2009, 49, 171. [Google Scholar]
- Saxena, A.; Ramdath, S., Jr.; O’halloran, P.; Gerdesmeyer, L.; Gollwitzer, H. Extra-corporeal pulsed-activated therapy (“EPAT” sound wave) for Achilles tendinopathy: a prospective study. J. Foot Ankle Surg. 2011, 50, 315–319. [Google Scholar] [CrossRef]
- Taylor, J.; Dunkerley, S.; Silver, D.; Redfern, A.; Talbot, N.; Sharpe, I.; Guyver, P. Extracorporeal shockwave therapy (ESWT) for refractory Achilles tendinopathy: a prospective audit with 2-year follow up. Foot 2016, 26, 23–29. [Google Scholar] [CrossRef]
- Wetke, E.; Johannsen, F.; Langberg, H. Achilles tendinopathy: A prospective study on the effect of active rehabilitation and steroid injections in a clinical setting. Scand. J. Med. Sci Sports 2015, 25, e392–e399. [Google Scholar] [CrossRef] [PubMed]
- Lynen, N.; De Vroey, T.; Spiegel, I.; Van Ongeval, F.; Hendrickx, N.J.; Stassijns, G. Comparison of peritendinous hyaluronan injections versus extracorporeal shock wave therapy in the treatment of painful Achilles’ tendinopathy: a randomized clinical efficacy and safety study. Arch. Phys. Med. Rehab. 2017, 98, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Maquirriain, J.; Kokalj, A. Management of acute Achilles tendinopathy: Effect of etoricoxib on pain control and leg stiffness. Geor. Med. News 2013, 222, 36–43. [Google Scholar]
- Maffulli, N.; Longo, U.G. How do eccentric exercises work in tendinopathy? Rheumatology 2008, 47, 1444–1445. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Aaboe, J.; Bliddal, H.; Langberg, H. Biomechanical characteristics of the eccentric Achilles tendon exercise. J. Biomec. 2009, 42, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, D.J.; Mischke, J.J.; Strazzulla, R.L. Eccentric Exercise for Achilles Tendinopathy: A Narrative Review and Clinical Decision-Making Considerations. J. Funct. Morphol. Kinesiol. 2019, 4, 34. [Google Scholar] [CrossRef]
- Scott, L.A.; Munteanu, S.E.; Menz, H.B. Effectiveness of orthotic devices in the treatment of Achilles tendinopathy: a systematic review. Sports Med. 2015, 45, 95–110. [Google Scholar] [CrossRef]
- Khan, K.M.; Cook, J.L.; Kannus, P.; Maffulli, N.; Bonar, S.F. Time to abandon the “tendinitis” myth: Painful, overuse tendon conditions have a non-inflammatory pathology. BMJ 2002, 324, 626–627. [Google Scholar] [CrossRef]
- Speed, C.A. Corticosteroid injections in tendon lesions. BMJ 2001, 323, 382–386. [Google Scholar] [CrossRef]
- Reeves, K.D. Prolotherapy: Present and future applications in soft-tissue pain and disability. Phys. Med. Rehab. Clin. 1995, 6, 917–926. [Google Scholar] [CrossRef]
- Jensen, K.T.; Rabago, D.P.; Best, T.M.; Patterson, J.J.; Vanderby, R., Jr. Response of knee ligaments toprolotherapy in a rat injury model. Am. J. Sports Med. 2008, 36, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Waugh, C.; Morrissey, D.; Jones, E.; Riley, G.; Langberg, H.; Screen, H. In vivo biological response to extracorporeal shockwave therapy in human tendinopathy: Response of tendinopathy to shockwave therapy. Eur. Cells Mat. 2015, 3, 268–280. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, W.; Chen, S.; Li, Y. Outcome of extracorporeal shock wave therapy for insertional achilles tendinopathy with and without haglund’s deformity. Bio. Med. Res. Int. 2016, 2016, 6315846. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Travers, M.; Gibson, W. Is heavy eccentric calf training superior to wait-and-see, sham rehabilitation, traditional physiotherapy and other exercise interventions for pain and function in mid-portion Achilles tendinopathy? Syst. Rev. 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Gavish, L.; Perez, L.; Gertz, S.D. Low-level laser irradiationmodulates matrix metalloproteinase activity and gene expressionin porcine aortic smooth muscle cells. Lasers Surg. Med. 2006, 38, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.; Pyatibrat, L.; Kalendo, G. Irradiation with He-Ne laserincreases A.T.P. level in cells cultivated in vitro. J Photochem. Photobiol. B. 1995, 27, 219–223. [Google Scholar] [CrossRef]
- Chughtai, M.; Newman, J.M.; Sultan, A.A.; Samuel, L.T.; Rabin, J.; Khlopas, A.; Mont, M.A. Astym® therapy: A systematic review. Ann. Trans. Med. 2019, 7, 70. [Google Scholar] [CrossRef] [PubMed]
| Ref | Authors (Year of Publication) | No. Of Patients | Treatment Groups | Results Summery |
|---|---|---|---|---|
| [15] | Beyer et al. (2015) | 58 | ECC vs. heavy slow resistance training (HSR) | Both groups had improvement in the short- and long-term ranges over the baseline (p < 0.05), but no differences between the treatments were recorded. |
| [16] | Stasinopoulos et al. (2013) | 41 | Alfredson ECC vs. Stanich ECC | Alfredson protocol was superior to Stanish model in reducing pain and improving function outcomes. Both groups had improvements at the 6-month follow-up over the baseline. |
| [17] | Hutchison et al. (2013) | 47 | Intense pulsed light vs. ECC | No differences between the groups at 1 year follow-up. |
| [18] | Hostmman et al. (2013) | 58 | Vibration traning vs. ECC vs. control | Pain reduction in the vibration training and ECC groups compared to the control. In the musculotendinous junction, ECC cohort had a 66.6% reduction in pain. The vibration-training group did not experience any change in pain at the musculotendinous junction, therefore, vibration training 0%, while the control group had an increase of the 73.3%. |
| [19] | Zhang et al. (2012) | 64 | Acupuncture vs. ECC | At the 8-week follow-up, there was superior improvement of the pain score acupuncture group compared to ECC (67.1 points and 48.5, respectively). |
| [20] | Yelland et al.(2009) | 43 | Proloteraphy + ECC vs. ECC | At the short- and long-term follow-up, there was a superior increase amongproloteraphy + ECC compared to the others. At the 1 year follow-up, the proloteraphy + ECC group had an improvement of 86% for the functional score, with 73% for ECC. In combined treatment there was early recovery. |
| [21] | Tumilty et al. (2015) | 80 | Photobiomodulation +ECC vs. ECC | Photobiomodulation + ECC group showed statistically significant improvements over ECC only in functional score. |
| [22] | Praet et al (2019) | 18 | ECC + Oral supplementation (OS) | In the OS group, there was an early return to sport and improvements in functional outcome. |
| [23] | McCormack et al. (2016) | 16 | ECC vs. ECC + Astym Treatment | After 12-, 26- and 52-week follow-ups, Astym + ECC patients showed better outcomes than ECC groups for insertional tendinopathy. Both groups recorded significant improvements in pain over the baseline, but there was no difference between cohorts, except at the 12-week intervention period in the combined group. |
| [24] | de Jorge et al. (2010) | 58 | ECC vs. ECC + Night splint | There was an improvement in functional score in at the 3-month and 1-year follow-ups over the baseline in the ECC group and the ECC + night splint group. There was no significant difference found in increases in pain score. |
| [25] | Verral et al. (2011) | 190 | ECC | There was a reduction of pain after 12 weeks, in 6 to 14 months of treatment (p < 0.01 compared to the baseline). |
| [26] | Ram et al. (2013) | 45 | ECC vs. Control | There was superior patient satisfaction for the ECC group compared to the control. No statistically significant assessments at 12 weeks in groups of the satisfied and not satisfied patients were made, nor were improvements recorded at the subsequent follow-up. There was better color Doppler activity during their second ultrasound. |
| [27] | Yu et al, (2013) | 32 | ECC vs. COE | Both groups had improvements over the baseline (p < 0.05) |
| [28] | Stevens et al. (2014) | 28 | ECC vs. “Do-as-tolerated” protocol | Both groups presented clinically and statistically significant improvements at the 6-week follow-up over the baseline. |
| [29] | Van der Plas et al. (2011) | 46 | ECC | There was improvement of pain and functional scores after 1 year of treatment (p < 0.001), and at the 5-year follow-up (p < 0.01). 39.7% of the patients were completely pain-free at the follow-up and 48.3% had received one or more alternative treatments. |
| [30] | Deans et al. (2012) | 26 | PRP | There was improve quality of life and functional outcomes. 5 had worse symptoms, there was 1 rupture, and 2 found it difficult to work. |
| [31] | de Vos et al. (2010) | 54 | PRP vs. ECC+Placebo | At the 6-, 12-, and 24-week follow-ups, there was no significant difference in the improvement of functional outcomes between the 2 treatment groups. |
| [32] | de Jonge et al (2011) | 54 | PRP+ECC vs. ECC+Placebo | At the 6-month and 1-year follow-ups, there was not a significant difference in clinical or sonographic assessments after a PRP injection. In both groups, neovascularization continued decreasing after the increase at the 12-week follow-up. |
| [33] | Filardo et al. (2014) | 27 | L-PRP | 89% returned to sport and 93% were satisfied patients. In the unsatisfied patients, 1 patient had a corticosteroid injection and 2 patients had surgical intervention. |
| [34] | Guelfi et al. (2014) | 83 | L-PRP | At the final follow-up there was an improvement of functional outcome (p < 00.1 respect baseline). 91.6% were satisfied after 1 injection, the remaining 8.4% had a second PRP injection. No reported Achilles tendon ruptures. |
| [35] | Njawaya et al (2017) | 27 | US+ESWT vs. ESWT | Similar results were recorded in the 2 groups with, no major advantage seen in the addition of an ultrasound for guiding shock waves. |
| [36] | Pavone et al. (2016) | 40 | ESWT+ ECC vs. ECC | At the 12-month follow-up, 65.0% of patients did not complain about pain, 27.5% of patients got back to normal daily activities and sports despite residual pain, and 3 patients still complained about pain (VAS > 4). Statistically significant differences in the pain score and in the functional outcome results were observed as well as in the functional outcome. |
| [37] | Rompe et al. (2008) | 50 | ESWT vs. ECC | At the 4-month follow-up, no significant difference was seen in the functional assessments between the two groups. ESWT patients showed better outcomes than ECC subjects (p < 0.020) |
| [38] | Vulpiani et al. (2009) | 105 | ESWT | 60 days after the end of the treatment, there was a significant improvement over the baseline in pain score. |
| [39] | Saxena (2011) | 60 | ESWT | At least 1 year after treatment, there were significant improvements (78.38%) of satisfied patients treated with the low-energy radial shockwave devices. |
| [40] | Taylor et al. (2016) | 56 | ESWT | In both non- and insertional tendinopathies, there were improvements in the mean pain scores at rest and on activity. |
| [41] | Wetke et al. (2014) | 113 | GCS vs. training | There were good short-term effects, but no significant long-term effects. 26% of patients had training only, 58% had one supplementary injection, 14% had two injections, and 2% had three injections. 2 subjects had flare-ups more than 24 h after GCS injection |
| [42] | Lynen et al. (2016) | 59 | HA vs. ESWT | 90 days after the treatment, the HA group had greater treatment satisfaction than the standard ESWT in terms of pain (p = 0.0030). Similar findings for HA were also observed at 4 weeks (p = 0.0304) and 6 months (p = 0.0018). |
| [43] | Maquirriain et al. (2013) | 56 | Eterocoxib vs. Diclofenac | Over the 7-day treatment period, both groups had improvements over the baseline (p < 0.001). The analgesic effect averaged etoricoxib= 56.4% and diclofenac 50.6% (p = 0.64). The etericoxib groups recorded less side effects than those in the diclofenac group (0% and 14.2%, respectively, p = 0.037) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavone, V.; Vescio, A.; Mobilia, G.; Dimartino, S.; Di Stefano, G.; Culmone, A.; Testa, G. Conservative Treatment of Chronic Achilles Tendinopathy: A Systematic Review. J. Funct. Morphol. Kinesiol. 2019, 4, 46. https://doi.org/10.3390/jfmk4030046
Pavone V, Vescio A, Mobilia G, Dimartino S, Di Stefano G, Culmone A, Testa G. Conservative Treatment of Chronic Achilles Tendinopathy: A Systematic Review. Journal of Functional Morphology and Kinesiology. 2019; 4(3):46. https://doi.org/10.3390/jfmk4030046
Chicago/Turabian StylePavone, Vito, Andrea Vescio, Giuseppe Mobilia, Sara Dimartino, Giovanni Di Stefano, Annalisa Culmone, and Gianluca Testa. 2019. "Conservative Treatment of Chronic Achilles Tendinopathy: A Systematic Review" Journal of Functional Morphology and Kinesiology 4, no. 3: 46. https://doi.org/10.3390/jfmk4030046
APA StylePavone, V., Vescio, A., Mobilia, G., Dimartino, S., Di Stefano, G., Culmone, A., & Testa, G. (2019). Conservative Treatment of Chronic Achilles Tendinopathy: A Systematic Review. Journal of Functional Morphology and Kinesiology, 4(3), 46. https://doi.org/10.3390/jfmk4030046






