Nordic Walking Promoted Weight Loss in Overweight and Obese People: A Systematic Review for Future Exercise Prescription
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
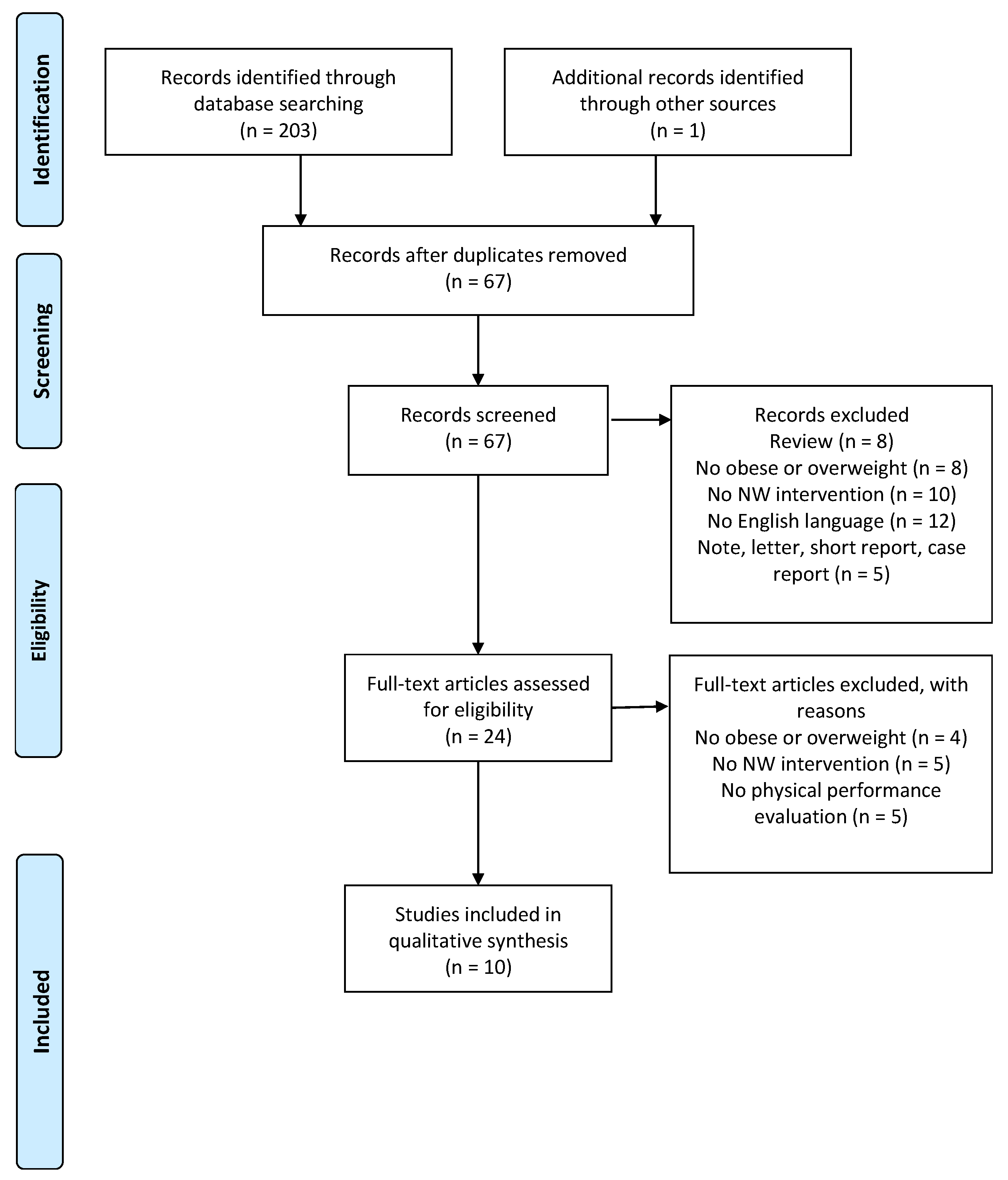
2.2. Literature Search
2.3. Inclusion and Exclusion Criteria
2.4. Study Quality Assessment
2.5. Data Extraction and Synthesis
3. Results
3.1. Description of the Study
3.2. Anthropometric Parameters and Body Composition
3.3. Cardiovascular Parameters and Aerobic Capacity
3.4. Blood Sample and Glucose Tolerance
4. Discussion
4.1. Anthropometric Parameters and Body Composition
4.2. Blood Sample and Glucose Tolerance
4.3. Cardiovascular Parameters and Aerobic Capacity
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stamatakis, E.; Gale, J.; Bauman, A.; Ekelund, U.; Hamer, M.; Ding, D. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J. Am. Coll. Cardiol. 2019, 73, 2062–2072. [Google Scholar] [CrossRef]
- Ekelund, U.; Brown, W.J.; Steene-Johannessen, J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.E.; Lee, I.M. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850 060 participants. Br. J. Sports Med. 2018. [Google Scholar] [CrossRef]
- WHO Obesity: Situation and Trends. Available online: https://www.who.int/gho/ncd/risk_factors/physical_activity/en/ (accessed on 6 May 2019).
- The American College of Sports Medicine; Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; Berrington de Gonzalez, A.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef]
- Kokkinos, P. Physical activity, health benefits, and mortality risk. ISRN Cardiol. 2012, 2012, 718789. [Google Scholar] [CrossRef]
- Haapanen, N.; Miilunpalo, S.; Pasanen, M.; Oja, P.; Vuori, I. Association between leisure time physical activity and 10-year body mass change among working-aged men and women. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 288–296. [Google Scholar] [CrossRef]
- Di Pietro, L.; Dziura, J.; Blair, S.N. Estimated change in physical activity level (PAL) and prediction of 5-year weight change in men: The Aerobics Center Longitudinal Study. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1541–1547. [Google Scholar] [CrossRef][Green Version]
- Figard-Fabre, H.; Fabre, N.; Leonardi, A.; Schena, F. Physiological and perceptual responses to Nordic walking in obese middle-aged women in comparison with the normal walk. Eur. J. Appl. Physiol. 2010, 108, 1141–1151. [Google Scholar] [CrossRef]
- Shim, J.M.; Kwon, H.Y.; Kim, H.R.; Kim, B.I.; Jung, J.H. Comparison of the Effects of Walking with and without Nordic Pole on Upper Extremity and Lower Extremity Muscle Activation. J. Phys. Ther. Sci. 2013, 25, 1553–1556. [Google Scholar] [CrossRef]
- Bullo, V.; Gobbo, S.; Vendramin, B.; Duregon, F.; Cugusi, L.; Di Blasio, A.; Bocalini, D.S.; Zaccaria, M.; Bergamin, M.; Ermolao, A. Nordic Walking Can Be Incorporated in the Exercise Prescription to Increase Aerobic Capacity, Strength, and Quality of Life for Elderly: A Systematic Review and Meta-Analysis. Rejuvenat. Res. 2018, 21, 141–161. [Google Scholar] [CrossRef]
- Cugusi, L.; Solla, P.; Serpe, R.; Carzedda, T.; Piras, L.; Oggianu, M.; Gabba, S.; Di Blasio, A.; Bergamin, M.; Cannas, A.; et al. Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation 2015, 37, 245–254. [Google Scholar] [CrossRef]
- Willson, J.; Torry, M.R.; Decker, M.J.; Kernozek, T.; Steadman, J.R. Effects of walking poles on lower extremity gait mechanics. Med. Sci. Sports Exerc. 2001, 33, 142–147. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- van Tulder, M.W.; Assendelft, W.J.; Koes, B.W.; Bouter, L.M. Method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group for Spinal Disorders. Spine (Phila Pa 1976) 1997, 22, 2323–2330. [Google Scholar] [CrossRef]
- Gobbo, S.; Bergamin, M.; Sieverdes, J.C.; Ermolao, A.; Zaccaria, M. Effects of exercise on dual-task ability and balance in older adults: A systematic review. Arch. Gerontol. Geriatr. 2014, 58, 177–187. [Google Scholar] [CrossRef]
- Vendramin, B.; Bergamin, M.; Gobbo, S.; Cugusi, L.; Duregon, F.; Bullo, V.; Zaccaria, M.; Neunhaeuserer, D.; Ermolao, A. Health Benefits of Zumba Fitness Training: A Systematic Review. PM R 2016, 8, 1181–1200. [Google Scholar] [CrossRef]
- Kucio, C.; Narloch, D.; Kucio, E.; Kurek, J. The application of Nordic walking in the treatment hypertension and obesity. Fam. Med. Prim. Care Rev. 2017, 19, 144–148. [Google Scholar] [CrossRef]
- Pilch, W.; Tyka, A.; Cebula, A.; Sliwicka, E.; Pilaczynska-Szczesniak, L.; Tyka, A. Effects of a 6-week Nordic walking training on changes in 25(OH)D blood concentration in women aged over 55. J. Sports Med. Phys. Fitness 2017, 57, 124–129. [Google Scholar]
- Hagner-Derengowska, M.; Kaluzny, K.; Hagner, W.; Kochanski, B.; Plaskiewicz, A.; Borkowska, A.; Bronisz, A.; Budzynski, J. The influence of a ten-week Nordic walking training-rehabilitation program on the level of lipids in blood in overweight and obese postmenopausal women. J. Phys. Ther. Sci. 2015, 27, 3039–3044. [Google Scholar] [CrossRef]
- Hagner-Derengowska, M.; Kaluzny, K.; Kochanski, B.; Hagner, W.; Borkowska, A.; Czamara, A.; Budzynski, J. Effects of Nordic Walking and Pilates exercise programs on blood glucose and lipid profile in overweight and obese postmenopausal women in an experimental, nonrandomized, open-label, prospective controlled trial. Menopause 2015, 22, 1215–1223. [Google Scholar] [CrossRef]
- Wiklund, P.; Alen, M.; Munukka, E.; Cheng, S.M.; Yu, B.; Pekkala, S.; Cheng, S. Metabolic response to 6-week aerobic exercise training and dieting in previously sedentary overweight and obese pre-menopausal women: A randomized trial. J. Sport Health Sci. 2014, 3, 217–224. [Google Scholar] [CrossRef][Green Version]
- Trabka, B.; Zubrzycki, I.Z.; Ossowski, Z.; Bojke, O.; Clarke, A.; Wiacek, M.; Latosik, E. Effect of a MAST Exercise Program on Anthropometric Parameters, Physical Fitness, and Serum Lipid Levels in Obese Postmenopausal Women. J. Hum. Kinet. 2014, 42, 149–155. [Google Scholar] [CrossRef][Green Version]
- Fritz, T.; Caidahl, K.; Krook, A.; Lundstrom, P.; Mashili, F.; Osler, M.; Szekeres, F.L.; Ostenson, C.G.; Wandell, P.; Zierath, J.R. Effects of Nordic walking on cardiovascular risk factors in overweight individuals with type 2 diabetes, impaired or normal glucose tolerance. Diabetes Metab. Res. Rev. 2013, 29, 25–32. [Google Scholar] [CrossRef]
- Venojarvi, M.; Wasenius, N.; Manderoos, S.; Heinonen, O.J.; Hernelahti, M.; Lindholm, H.; Surakka, J.; Lindstrom, J.; Aunola, S.; Atalay, M.; et al. Nordic walking decreased circulating chemerin and leptin concentrations in middle-aged men with impaired glucose regulation. Ann. Med. 2013, 45, 162–170. [Google Scholar] [CrossRef]
- Figard-Fabre, H.; Fabre, N.; Leonardi, A.; Schena, F. Efficacy of Nordic walking in obesity management. Int. J. Sports Med. 2011, 32, 407–414. [Google Scholar] [CrossRef]
- Gram, B.; Christensen, R.; Christiansen, C.; Gram, J. Effects of nordic walking and exercise in type 2 diabetes mellitus: A randomized controlled trial. Clin. J. Sport Med. 2010, 20, 355–361. [Google Scholar]
- Sugiyama, K.; Kawamura, M.; Tomita, H.; Katamoto, S. Oxygen uptake, heart rate, perceived exertion, and integrated electromyogram of the lower and upper extremities during level and Nordic walking on a treadmill. J. Physiol. Anthropol. 2013, 32, 2. [Google Scholar] [CrossRef]
- Bouchard, C.; Blair, S.N.; Haskell, W. Physical Activity and Health, 2nd ed.; Human Kinetics 10%; Human Kinetics: Champaign, IL, USA, 2012. [Google Scholar]
- Foster-Schubert, K.E.; Alfano, C.M.; Duggan, C.R.; Xiao, L.; Campbell, K.L.; Kong, A.; Bain, C.E.; Wang, C.Y.; Blackburn, G.L.; McTiernan, A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012, 20, 1628–1638. [Google Scholar] [CrossRef]
- Swift, D.L.; Johannsen, N.M.; Lavie, C.J.; Earnest, C.P.; Church, T.S. The role of exercise and physical activity in weight loss and maintenance. Prog. Cardiovasc. Dis. 2014, 56, 441–447. [Google Scholar] [CrossRef]
- Klein, S.; Allison, D.B.; Heymsfield, S.B.; Kelley, D.E.; Leibel, R.L.; Nonas, C.; Kahn, R.; Association for Weight, M.; Obesity, P.; Naaso, T.O.S.; et al. Waist circumference and cardiometabolic risk: A consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am. J. Clin. Nutr. 2007, 85, 1197–1202. [Google Scholar]
- Ross, R.; Janssen, I.; Dawson, J.; Kungl, A.M.; Kuk, J.L.; Wong, S.L.; Nguyen-Duy, T.B.; Lee, S.; Kilpatrick, K.; Hudson, R. Exercise-induced reduction in obesity and insulin resistance in women: A randomized controlled trial. Obes. Res. 2004, 12, 789–798. [Google Scholar] [CrossRef]
- Bouaziz, W.; Schmitt, E.; Kaltenbach, G.; Geny, B.; Vogel, T. Health benefits of endurance training alone or combined with diet for obese patients over 60: A review. Int. J. Clin. Pract. 2015, 69, 1032–1049. [Google Scholar] [CrossRef]
- Lopes, S.; Mesquita-Bastos, J.; Alves, A.J.; Ribeiro, F. Exercise as a tool for hypertension and resistant hypertension management: Current insights. Integr. Blood Press Control. 2018, 11, 65–71. [Google Scholar] [CrossRef]
- Fagard, R.H. Exercise characteristics and the blood pressure response to dynamic physical training. Med. Sci. Sports Exerc. 2001, 33, S484–S492. [Google Scholar] [CrossRef]
| Citation | Randomization Procedure | Similarity of Study Groups | Inclusion or Exclusion Criteria | Dropouts | Blinding | Compliance | Intention-To-Treat Analysis | Timing of Outcomes Assessment | Follow-Up | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Kucio et al. (2017) [19] | − | − | + | + | − | − | − | − | − | 2/9 |
| Pilch et al. (2017) [20] | − | − | − | − | − | − | − | + | − | 1/9 |
| Derengowska et al. (2015) [21] | − | − | − | − | − | − | − | − | − | 0/9 |
| Derengowska et al. (2015) [22] | − | − | + | + | − | − | − | − | − | 2/9 |
| Wiklund et al. (2014) [23] | + | − | − | + | + | − | + | + | − | 5/9 |
| Trabka et al. (2013) [24] | + | − | + | + | − | − | − | − | − | 3/9 |
| Fritz et al. (2013) [25] | + | − | + | + | − | − | + | − | − | 4/9 |
| Venojärvi et al. (2013) [26] | + | − | + | + | − | + | − | − | − | 4/9 |
| Fabre et al. (2011) [27] | − | + | + | − | − | + | − | − | − | 3/9 |
| Gram et al. (2010) [28] | + | − | + | + | − | + | − | − | + | 5/9 |
| Study | Subjects and Grouping | Training Modality, Program, and Intensity | Duration and Frequency |
|---|---|---|---|
| Kucio et al. (2017) [19] | 26 M 47–66 y.o NW (15) CG (11) | Supervised NW Warm up: 10’ Main part: week 1, 30’ of NW at 3 km/h. week 2–4, 40’ of NW at 5 km/h. HR 40–70% of HMmax | 4 weeks 5 d/w 40–50 min |
| Pilch et al. (2018) [20] | 17 F mean 57 y.o. NW (17) | Supervised NW Warm-up: 15’ of walking and dynamic stretching Main part: 60’ of NW on grass and natural surface at 60–70% of HRmax Cool-down: 10–15’ of stretching and relaxing exercises | 6 weeks 3 d/w 90 min |
| Derengowska et al. (2015) [21] | 32 F 50–68 y.o. NW (32) | Supervised NW Warm-up | 10 weeks 5 d/w 60 min |
| Derengowska et al. (2015) [22] | 89 F 50–75 y.o. NW (69) CG (20) | Supervised and no supervised NW Warm-up and cool-down (3 supervised and 3 no supervised) | 10 weeks 6 d/w 60 min |
| Wiklund et al. (2014) [23] | 90 F 20–50 y.o. NW (45) DI (45) | Supervised NW Week 1: 60% HRmax Week 2–3: 65% HRmax Week 4–5: 70% HRmax Week 6: 75% HRmax DI Reduction of portion size, control meal rhythm, change the composition of food (light margarine, vegetable oil, low-fat milk and meat, vegetables, fruits, increase fiber intake). Drink water, light beverage, mild juice, coffee and tea. | 6 weeks 3–4 d/w 30–60 min |
| Trabka et al. (2013) [24] | 46 F NW (25) DI (21) | Supervised NW Warm-up: 10 min Main part: 40 min of NW and 20 min of strength training. HR increased progressively from 50% to 80% of HRres, 10% every 2 weeks. Strength exercise was performed in 3 sets. Repetitions were 15 squats, 30 heel-raises, sit-ups until exhaustion, and 15 push-ups on knees. Cool-down: 10 min of stretching DI No high-fat and high-glycemic food Eating 5 times per day, no after 7.00 p.m. or 3 h before sleep Drinking at least 1.5 L of water | 10 weeks 3 d/w 80 min |
| Fritz et al. (2013) [25] | 213 (118 F, 95 M) 45–69 y.o. NW-NGT (87) NW-IGT (14) NW-T2DM (20) CG-NGT (75) CG-IGT (21) CG-T2DM (30) | No supervised NW Participants were instructed to increase their weekly level of physical activity by 5 h of NW. | 4 months 5 h/w |
| Venojärvi et al. (2013) [26] | 79 M 40–65 y.o. NW (39) CG (40) | Supervised NW Warm-up: walking and stretching of the main muscle group for 5’. Main part: weeks 1–4 at 55% of HRres; weeks 5–8 at 65% of HRres; weeks 9–12 at 75% of HRres. Cool-down: stretching of the main muscle group for 5’. | 12 weeks 3 d/w 60 min |
| Fabre et al. (2011) [27] | 23 F NW (12) WT (11) | Supervised and no supervised NW 4 weeks of learning of NW technique before the intervention Warm-up: 5–10’ Main part: 30’ of interval training, 6 bouts of 5 min (4’ at preferred walking speed followed by 1’ at maximal walking speed) Cool-down: 5–10’ 1 supervised and 2 unsupervised NW Supervised and no supervised WT Follow NW but without poles. 1 supervised and 2 unsupervised WT | 12 weeks 3 d/w 40–50 min |
| Gram et al. (2010) [28] | 44 (37 M, 31 F) 25–80 y.o. NW (22) CG (22) | Supervised NW Warm-up: 10’ Main part: 30’ (>40% of VO2max). Cool-down: 5’ | 4 months 1–2 d/w 45 min |
| Study | Group Comparison | Results |
|---|---|---|
| Kucio et al. (2017) [19] | NW vs. CG | Anthropometric parameters and body composition Weight (kg): ↓NW*; =CG BMI (kg/m2): ↓NW*; ↓CG Cardiovascular parameters and aerobic capacity M24-h SBP (mmHg): ↓NW; ↓CG M24-h DBP (mmHg): ↓NW; ↓CG M24-h MAP (mmHg): ↓NW; ↓CG M-daily SBP (mmHg): ↓NW; ↓CG M-daily DBP (mmHg)#: ↓NW; ↓CG M-daily MAP (mmHg): ↓NW; ↓CG M-nightly SBP (mmHg): ↓NW; ↓CG M-nightly DBP (mmHg): ↓NW; ↓CG M-nightly MAP (mmHg): ↓NW; ↓CG Time to exhaustion (min): ↑NW*; ↑CG* MET (mL/kg/min): ↑NW*; ↑CG Blood sample and glucose tolerance TC (mg%): ↓NW*; ↓CG LDL (mg%): ↓NW; ↓CG HDL (mg%): ↓NW; ↓CG TG (mg%): ↓NW*; ↑CG |
| Pilch et al. (2017) [20] | NW | Anthropometric parameters and body composition Weight (kg): ↓NW* BMI (kg/m2): ↓NW* BFM (%): ↓NW* LBM (kg): ↓NW Cardiovascular parameters and aerobic capacity HRrest (bpm): ↓NW* |
| Derengowska et al. (2015) [21] | NW | Anthropometric parameters and body composition Weight (kg): ↓NW* BMI (kg/m2): ↓NW* Blood sample and glucose tolerance TC (mg/dL): ↓NW* LDL (mg/dL): ↓NW* HDL (mg/dL): ↑NW* TG (mg/dL): ↓NW* |
| Derengowska et al. (2015) [22] | NW vs. CG | Anthropometric parameters and body composition Weight (kg): ↓NW*; ↑CG BMI (kg/m2)#: ↓NW*; ↑CG Blood sample and glucose tolerance TC (mg/dL): ↓NW*; ↑CG LDL (mg/dL): ↓NW*,**; ↑CG HDL (mg/dL): ↑NW*,**; ↓CG TG (mg/dL): ↓NW*; ↑CG nHDL (mg/dL): ↓NW*,**; ↑CG Glucose (mg/dL): ↓NW*,**; ↓CG |
| Wiklund et al. (2014) [23] | NW vs. DI | Anthropometric parameters and body composition Weight (kg)#: ↓NW*; ↓DI* BMI (kg/m2)#: ↓NW; ↓DI* BFM (kg)#: ↑NW; ↓DI VFA (cm2): ↓NW; ↓DI* FFM (kg): ↓NW; ↓DI* Cardiovascular parameters and aerobic capacity VO2max (mL/kg/min): ↑NW; ↑DI Blood sample and glucose tolerance TC (mmol/L): ↑NW; ↑DI LDL (mmol/L): ↑NW; ↑DI HDL (mmol/L): ↑NW; =DI TG (mmol/L): =NW; =DI Insulin (μU/L): ↓NW*; ↓DI Fasting glucose (mmol/L): ↓NW*,**; ↑DI HOMA-IR: ↓NW*,**; ↓DI |
| Trabka et al. (2013) [24] | NW vs. DI | Anthropometric parameters and body composition Weight (kg): ↓NW; ↓DI BMI (kg/m2): ↓NW; ↓DI WC (cm): ↓NW; ↑DI HC (cm): ↓NW; ↑DI WHR: ↑NW; ↓DI Cardiovascular parameters and aerobic capacity VO2max (mL/kg/min): ↑NW*; ↑DI Blood sample and glucose tolerance TC (mmol/L): ↑NW; ↓DI LDL (mmol/L): ↓NW; =DI* HDL (mmol/L): ↑NW; =DI TG (mmol/L): =NW; =DI |
| Fritz et al. (2013) [25] | NW vs. CG (NGT) | Anthropometric parameters and body composition Weight (kg): ↓NW*,**; ↓CG BMI (kg/m2): ↓NW*,**; ↓CG WC (cm): ↓NW*,**; ↓CG* Cardiovascular parameters and aerobic capacity SBP (mmHg): ↑NW; ↓CG DBP (mmHg): ↑NW; ↓CG Blood sample and glucose tolerance TC (mmol/L): =NW; =CG LDL (mmol/L): =NW; ↑CG HDL (mmol/L): =NW**; ↓CG* TG (mmol/L): =NW; =CG Fasting glucose (mmol/L): =NW; ↓CG* HOMA-IR: ↓NW; =CG OGTT 2h (mmol/L): ↓NW; ↑CG HbA1c (%): =NW; =CG HbA1c (mmol/L): =NW; ↑CG |
| NW vs. CG (IGT) | Anthropometric parameters and body composition Weight (kg): ↓NW; ↓CG BMI (kg/m2): ↓NW; ↓CG WC (cm): ↓NW*; ↓CG* Cardiovascular parameters and aerobic capacity SBP (mmHg): ↓NW; ↑CG DBP (mmHg): ↑NW; ↓CG Blood sample and glucose tolerance TC (mmol/L): ↑NW; ↓CG LDL (mmol/L): ↑NW; ↓CG HDL (mmol/L): =NW; ↓CG TG (mmol/L): ↓NW; ↓CG Fasting glucose (mmol/L): ↓NW; ↓CG HOMA-IR: ↑NW; ↑CG OGTT 2h (mmol/L): ↓NW*; ↓CG HbA1c (%): ↓NW**; ↑CG HbA1c (mmol/L): ↓NW; ↑CG | |
| NW vs. CG (T2DM) | Anthropometric parameters and body composition Weight (kg): ↓NW*; ↓CG BMI (kg/m2): ↓NW*; ↓CG WC (cm): ↓NW*; ↓CG Cardiovascular parameters and aerobic capacity SBP (mmHg): ↑NW; ↓CG DBP (mmHg): ↓NW; ↓CG Blood sample and glucose tolerance TC (mmol/L): ↓NW**; ↑CG* LDL (mmol/L): =NW; ↑CG* HDL (mmol/L): =NW; =CG TG (mmol/L)#: ↓NW; ↑CG Fasting glucose (mmol/L): ↓NW; ↓CG* HOMA-IR: ↑NW; ↓CG OGTT 2h (mmol/L): ↓NW*; ↓CG* HbA1c (%): ↓NW*; ↓CG HbA1c (mmol/L): ↓NW*; ↓CG | |
| Venojärvi et al. (2013) [26] | NW vs. CG | Anthropometric parameters and body composition Weight (kg): ↓NW**; ↓CG WC (cm): ↓NW; ↓CG BFM (%): ↓NW**; ↓CG FFM (kg): ↑NW; ↓CG Cardiovascular parameters and aerobic capacity SBP (mmHg): ↓NW; ↓CG DBP (mmHg): ↓NW; ↓CG 2-km UKK walk test: ↑NW**; ↑CG Blood sample and glucose tolerance TC (mmol/L): ↓NW; ↑CG LDL (mmol/L): ↓NW; ↑CG HDL (mmol/L): =NW; ↑CG TG (mmol/L): ↓NW; ↓CG Glucose (mmol/L): =NW; ↓CG Insulin (μIU/L)#: ↓NW; ↑CG Insulin 2h (μIU/L)#: ↓NW; ↓CG HOMA-IR#: ↓NW; ↑CG OGTT 2h (mmol/L): ↓NW; ↓CG HbA1c (%): =NW; ↑CG |
| Fabre et al. (2011) [27] | NW vs. WT | Anthropometric parameters and body composition Weight (kg): ↓NW*; ↓WT* BMI (kg/m2): ↓NW; ↓WT BFM (%): ↓NW*; ↓WT* Skin-fold thickness (cm): ↓NW*; ↓WT* Cardiovascular parameters and aerobic capacity SBP (mmHg): ↓NW; ↓WT DBP (mmHg): ↓NW*; ↓WT* HR (bpm): ↑NW; ↑WT |
| Gram et al. (2010) [28] | NW vs. CG | Anthropometric parameters and body composition Weight (kg): ↓NW; ↓CG BMI (kg/m2): ↓NW; ↓CG WC (cm): ↓NW; ↑CG HC (cm)#: ↓NW; ↑CG BFM (kg): ↓NW**; ↑CG FFM (kg): ↓NW; ↓CG Cardiovascular parameters and aerobic capacity SBP (mmHg): ↓NW; ↓CG DBP (mmHg): ↓NW; ↓CG VO2max (L/min): ↑NW; ↑CG Blood sample and glucose tolerance TC (mmol/L): ↑NW; ↓CG LDL (mmol/L): =NW; ↓CG HDL (mmol/L): =NW; =CG TG (mmol/L): ↓NW; ↑CG HbA1c (%): ↓NW; ↑CG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobbo, S.; Bullo, V.; Roma, E.; Duregon, F.; Bocalini, D.S.; Rica, R.L.; Di Blasio, A.; Cugusi, L.; Vendramin, B.; Bergamo, M.; et al. Nordic Walking Promoted Weight Loss in Overweight and Obese People: A Systematic Review for Future Exercise Prescription. J. Funct. Morphol. Kinesiol. 2019, 4, 36. https://doi.org/10.3390/jfmk4020036
Gobbo S, Bullo V, Roma E, Duregon F, Bocalini DS, Rica RL, Di Blasio A, Cugusi L, Vendramin B, Bergamo M, et al. Nordic Walking Promoted Weight Loss in Overweight and Obese People: A Systematic Review for Future Exercise Prescription. Journal of Functional Morphology and Kinesiology. 2019; 4(2):36. https://doi.org/10.3390/jfmk4020036
Chicago/Turabian StyleGobbo, Stefano, Valentina Bullo, Enrico Roma, Federica Duregon, Danilo Sales Bocalini, Roberta Luksevicius Rica, Andrea Di Blasio, Lucia Cugusi, Barbara Vendramin, Manuele Bergamo, and et al. 2019. "Nordic Walking Promoted Weight Loss in Overweight and Obese People: A Systematic Review for Future Exercise Prescription" Journal of Functional Morphology and Kinesiology 4, no. 2: 36. https://doi.org/10.3390/jfmk4020036
APA StyleGobbo, S., Bullo, V., Roma, E., Duregon, F., Bocalini, D. S., Rica, R. L., Di Blasio, A., Cugusi, L., Vendramin, B., Bergamo, M., Cruz-Diaz, D., Alberton, C. L., Ermolao, A., & Bergamin, M. (2019). Nordic Walking Promoted Weight Loss in Overweight and Obese People: A Systematic Review for Future Exercise Prescription. Journal of Functional Morphology and Kinesiology, 4(2), 36. https://doi.org/10.3390/jfmk4020036






