Abstract
Protein Kinase Cs (PKCs) are a family of 10 isoenzymes with critical roles in cell physiological processes like proliferation, differentiation, apoptosis. Muscular dystrophies are a heterogenous group of genetic degenerative diseases that affect skeletal and cardiac muscles. In the development of muscular dystrophies, several transduction pathways have been studied. A possible link between muscular dystrophies and PKCs have been recently proposed. After a brief description of the possible transduction pathways that are involved in the development of these genetic diseases, we summarize recent evidence on the role of PKC proteins in muscular dystrophies, with the aim to review possible candidates in molecular therapy of these pathologies.
1. Introduction
Muscular dystrophies are a group of degenerative diseases that affect skeletal (including diaphragm) and cardiac muscles. Although several studies have evidenced many genetic mutations involved in these pathologies, the signaling pathways, possibly implicated, have not been definitively clarified. In this review, we will describe the most important pathways involved in muscular dystrophies focusing on the role of Protein Kinase C (PKC) isoforms in muscular dystrophy pathology.
Muscular Dystrophies and Signal Transduction
Muscular dystrophies are a group of neurodegenerative genetic diseases that induce muscle atrophy and weakness. More than 30 types of muscular dystrophies have been described [1]. The first to be described was Duchenne muscular dystrophy (DMD) at the beginning of the 19th century [2]. DMD and Becker muscular dystrophy (BMD) have a combined prevalence of 1 in 7250 males [3]. The diseases are caused by genetic mutations in the X-linked dystrophin gene that induces a progressive degeneration of skeletal and cardiac muscle leading to premature death. Muscle pathology in DMD and BMD is characterized by different levels of atrophy and inflammation. Moreover, as the disease proceeds, the muscle tissue is substituted by fibro-adipose tissue [1]. Although many efforts and studies have been made until now, there is no cure for muscular dystrophies. Therapeutic strategies adopted to ameliorate dystrophic symptoms have been recently reviewed in [4,5,6]. Most of these strategies go through a molecular biology approach that includes replacement and editing of the dystrophin mutated gene. Although all these approaches show important challenges such as low efficiency and long term toxic effect of plasmid transfection, they represent the most promising pathways for therapy of muscular dystrophies.
Myotonic dystrophy (DM) is the most common adult-onset muscular dystrophy. The symptoms of DM are systemic, affecting multiple organs, such as the skeletal muscle, heart, brain, and endocrine systems. This pathology causes several disorders like muscle wasting, arrhythmia, dilated cardiomyopathy, dementia, sleeplessness, and insulin resistance [7]. DM type 1 (DM1) is an autosomal dominant inherited disorder caused by a CTG repeat expansion in the 3′-untransrated region (UTR) of the DMPK gene. In DM1 patients the systemic symptoms are due to dysregulation of two splicing factors: muscle blind-like 1 (MBNL1) and CUG triplet repeat RNA-binding protein (CUGBP1) that provoke aberrant splicing events in various genes [8,9]. Current therapeutic approaches concern the identification of molecular agents able to reduce the toxicity of the repetitive RNA both by increasing its degradation or avoiding its production. Pre-clinical models, by preferential knockdown of mutated RNAs showed encouraging results as reviewed in [10,11].
Primary molecules involved in molecular signaling of muscular dystrophies are part of the dystrophin-associated protein complex (DAPC) [12,13,14]. DAPC is a transmembrane multiprotein complex that connects intracellular proteins with the extracellular matrix components like collagen and laminin. The signals are transduced inside the muscle fibers by many players. For example, DAPC regulates calmodulin-dependent protein kinases that are involved in the activation of downstream pathways like phosphatidylinositide-3 kinase (PI3K/Akt) inducing muscle hypertrophy and attenuating disease progression [12]. In fact, activation of Akt has been proposed to mitigate pathology and to increase strength of dystrophic patients [15,16,17]. On the other side, DAPC activates the system calcineurin/nuclear factor of activated T-cells (NFAT) that is responsible for cytoskeleton utrophin upregulation. Utrophin is important for fiber type specialization and hypertrophy [18]. Consequently, also NFAT pathway activation could represent a possible way to contrast muscular dystrophies. More recently, the positive effect of calcineurin activation has been demonstrated also in DM1 mice [19].
Other signaling molecules eventually involved in muscular dystrophy are: nitric oxide (NO) that is produced following injury and promotes muscle growth through several mechanisms; activation of MMP2 and the release of hepatocyte growth factor (HGF); and generation of peroxynitrite through reaction with NOX4. The peroxynitrite increases intracellular calcium concentration and activates mTOR pathway promoting muscle growth. Finally, NO reacts with histone deacetylases (HDACs) reducing HDACs suppression of micro RNAs (miRNAs) that regulate muscle fiber growth [20].
Moreover, dysferlin and phosphatidylinositol 4,5-bisphosphate involved in the biogenesis of the T-tubule system [21] are considered as possible intermediate molecules in disease development.
Finally, another group of signal transducers involved in muscular dystrophies are mitogen-activated protein kinases (MAPKs) that includes extracellular signal-related kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p-38 MAPK. Several reports demonstrated activation of all these kinases in skeletal and cardiac muscle of Duchenne muscular dystrophies [22,23]. Interestingly, MAPKs are targets of PKC proteins.
On the other side, the pro-inflammatory molecule, NF-κB (upregulated in Duchenne and Emery–Dreifuss muscular dystrophies) is involved in muscle atrophy. In fact, NF-κB activation decreases skeletal muscle mass, but the target genes of NF-κB in this mechanism are still under study [12].
2. Protein Kinase C (PKC) Protein Family
PKC is a family of 10 isoenzymes (kinases) that transduce myriad of signals playing critical functions in cellular physiology (proliferation, differentiation, transformation). It is divided in three sub-families based on structural domains and mechanisms of activation:
- (i)
- conventional PKCs (α, β1, β2, γ) that are activated by diacylglycerol (DAG) and calcium (Ca2+);
- (ii)
- novel PKCs (δ, ε, θ, η) that are activated only by diacylglycerol (DAG);
- (iii)
- atypical PKCs (ξ, ι/λ) that are independent of second messengers.
All the members present the N-terminal regulatory domain and the C-terminal catalytic domain linked by a hinge. A general signaling pathway of PKC includes activation of Phospholipase Cβ by a G-protein. Phopholipase Cβ hydrolizes phosphatidyl inositol 4,5 bi-phosphate into diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). This last messenger induces the release of calcium from the endoplasmic reticulum. PKCs translocate to endo-membrane in response to DAG/Ca2+ binding that releases the auto-inhibitory conformation, thus activating its kinase function. The return of activated PKC to a quiescent state requires the removal of DAG through its phosphorylation by DAG kinases. Dynamics of PKC membrane interaction and conformational modifications have been recently reviewed by Igumenova [24].
PKC proteins are involved in many processes like cell proliferation, differentiation, apoptosis, and transformation [25,26]. PKC functions are exerted through interaction with several proteins. For example, PKC can interact with Ina D (a protein that re-direct PKC to its upstream activators, Phospholipase C), or PKC can translocate to a different subcellular compartment like cytoskeleton and interacts with actin or desmin. More frequently, PKC is directed towards substrate molecules that, in turn, activate other signaling proteins like NF-κB, NO in the immune system [27,28], and Ras/Raf MAPK pathway [29].
3. PKCs and Muscular Dystrophies
Since many molecules involved in muscular dystrophy development like NF-κB, NO, and MAP Kinases are common elements with PKCs transduction pathways, several studies addressed the question if PKCs could be involved in the muscular dystrophy pathologies. Here, we summarize latest findings on this concern.
In DM1 patients LIM domain binding 3 (LDB3) is aberrantly spliced and, consequently, shows different affinity for PKCα/βII through its C-terminal LIM domains [8]. Instead, PKCε binding affinity for LDB3 was not affected in DM1 patients, suggesting specific PKC isoforms involvement in DM1 disease. Moreover, hyperphosphorylation of CUGBP1 mediated by PKCα/βII has been observed in cardiac tissues obtained from DM1 mouse models and patients with DM1 patients [30].
Although pharmacologic inhibition of PKCs induces beneficial effects on cardiac conduction defects and arrhythmia in DM1 mice, the related mechanisms remain to be established [8,31].
Involvement of the novel PKCs was evidenced in lipodystrophic mice. In detail, controlled-release mitochondrial protonophore (CRMP) significantly reduced hypertriglyceridemia and insulin resistance in liver and skeletal muscle through downregulation of diacyglicerol content and PKCε and PKCθ activity [32]. Lipodystrophies are a complex disease characterized by loss of adipose tissue. Muscular defects include tight Achilles, ankle equinus, variation in fiber size, and atrophic fibers as reviewed in [33]. However, a definitive link between novel PKCs and lipodystrophy is still lacking.
Possible role of PKCs in Duchenne muscular dystrophy has been initially proposed by MacLennan et al. in 1991 [34]. Specifically, carbohydrate and protein metabolism, that was altered in dystrophic muscles, was suggested to be dependent on calcium activation of PKCs. Therefore, conventional PKCs seemed to be related to muscular dystrophy. Nevertheless, MacLennan also observed that low calcium concentration did not affect glycogen rate of muscle suggesting that PKC could exert its influence through mechanisms independent from calcium concentration and thus from conventional PKC activity.
Later, in 2002, Kumar et al. [35] showed the presence of the novel PKCθ in human muscle fibers. When specimens were collected from dystrophic muscles, PKCθ cytoplasmic levels were lower than in healthy muscle and the protein essentially localized to particulate fraction.
Madaro et al. in 2012 [36] showed that in double null mice pkcθ/mdx the muscle wasting was significantly reduced, while muscle regeneration was increased with respect to mdx dystrophic mice. This response was mainly associated with the lack of PKCθ in inflammatory cells, suggesting that PKCθ could represent a pharmacological target to improve the efficacy of therapy approach [37].
Concomitantly, in 2012, Yuretic et al. [38] found that depolarization of dystrophic human myoblasts induced expression of neuregulin-1beta (NRG-1) through conventional PKC isoform activity. Although there is some evidence of the involvement of utrophin in these signaling pathways, the mechanisms have not been definitively clarified.
On the other side, Di Marcantonio et al. [39] evidenced the role of PKCε in muscle stem cell differentiation, showing that fine tuning of this PKC isoform is important to regulate HMGA1 signaling in skeletal muscle differentiation, opening new perspectives for pharmacological therapy of muscle diseases.
Cardiac pathologies are common symptoms in patients affected by muscular dystrophies. Because of more severe problems due to muscle weakness, in these patients, cardiac disease is often under-estimated. Nevertheless, heart diseases are responsible for morbidity and mortality in a certain number of muscular dystrophies. Cardiac pathologies associated with muscular dystrophy include arrhythmia, conduction defects, myocardial hypertrophy, dilated cardiomyopathy, and heart failure [40]. Although with different penetrance all the muscular dystrophies manifest both cardiac conduction anomalies and myocardial hypertrophy. The molecular mechanisms that stand behind the cardiac disorders in muscular dystrophies are still unclear. However, the principal players include sarcolemmal proteins regulated by calcium and involved in cardiac contraction, cell signaling protein (like nitric oxide synthetase), and protein involved in the repair of muscle damage.
Conduction defects have been evidenced in Duchenne muscular dystrophy [41,42], Becker muscular dystrophy [43,44], myotonic dystrophy type 1 and 2 [45,46], Emery-Dreifuss muscular dystrophy [47], and limb girdle muscular dystrophy [48,49].
PKC isoforms are expressed in cardiac tissues and are involved in many aspects of cardiac physiology like heart rate and contraction/relaxation of myocardium [50,51]. A fundamental role of PKC isoforms has been shown in ischemic preconditioning [52,53]. Moreover, involvement of PKCs in cardiac arrhythmia [54] and heart failure [55] has been recently described. In detail, activation of the epsilon isoform of PKC prevented arrhythmia post ischemic injury [56]. On the other side, effects of PKCs have been demonstrated in regulation of connexin43, a principal component of the intercellular gap junction in heart and brain [57]. Finally, PKCs are involved in oxidative stress signaling. In fact, ROS activation of PKCs is important in cardiac hypertrophy and heart failure [58].
4. Conclusions
Muscular dystrophies are serious pathologies that affect both skeletal and cardiac muscles with more evident effects in voluntary muscles. Interestingly, women carrying mutation in the dystrophin gene can develop cardiac disease, like males, without skeletal muscle defects, suggesting that different pathways could be involved in skeletal and cardiac muscles. While the molecular mechanisms that stand behind skeletal muscle weakness and atrophy are better described in the literature, the cardiac pathologies related to muscular dystrophies are less characterized. PKCs are involved in many aspects of cell physiology like proliferation, differentiation, and apoptosis of many tissues, including muscular ones. Several signal transduction systems have been shown involved in muscular dystrophies. There are pathways engaged in “protective” role like phosphatidylinositide-3 kinase (PI3K/Akt), calcineurin/nuclear factor of activated T-cells (NFAT), NO, and MAPKs that stimulate muscle hypertrophy, contrasting the pathological muscle mass decrease. Interestingly, NO and MAPKs are possible targets of PKCs. On the other side there are pathways, like NF-kB, that promotes muscle atrophy and disease progression. Also, NF-kB is a possible target of PKCs.
Thus, PKC family seems to be involved in progression of the disease development. In fact, activation of PKCα/βII is important in advancement of DM1 disease and PKCθ is involved in development of DMD. In myocardium the positive involvement of PKCs to determine arrhythmia and dilated cardiomyopathy have been demonstrated.
Although many aspects remain elusive, we formulated a schematic model that summarizes the data present in the literature and reported in Figure 1. Since we wanted to center the figure on the role of PKCs we did not report, in the figure, the other possible mechanisms. As evidenced by the number of question marks present in the figure, possible other players, involved in the development of muscular dystrophies, need to be clarified and ordered. Thus, with the aim of finding possible therapeutic targets, more investigations will be necessary to understand the molecular processes behind muscular dystrophies.
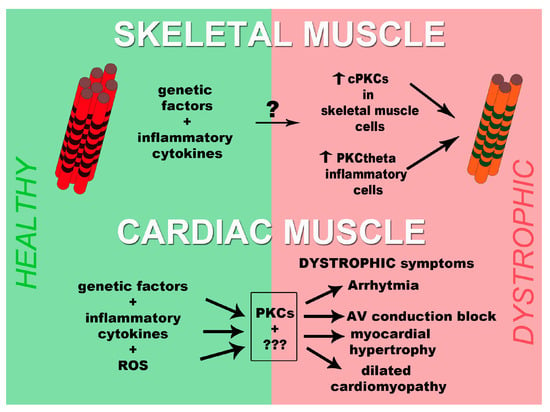
Figure 1.
Scheme of PKCs involvement in the development of muscular dystrophies both in skeletal muscle and heart. In skeletal muscle (upper part of the figure), genetic factors and inflammatory cytokines, through unknown mechanisms, activate conventional PKCs in skeletal muscle cells and PKCθ in inflammatory cells. These events will contribute to the processing of the pathology. In myocardium (lower part of the figure), PKCs together with unknown elements transduce the signal originated by genetic factors, inflammatory cytokines, and reactive oxygen species (ROS) leading to the development of dystrophic cardiac symptoms like arrhythmia, AV conduction block, myocardial hypertrophy, and dilated cardiomyopathy. PKCs, Protein kinases C; cPKC, conventional PKCs; ROS, reactive oxygen species.
Acknowledgments
We wish to thank Cristina Micheloni and Luciana Cerasuolo (University of Parma) for administrative support. We apologize if other contributors in the field were inadvertently omitted.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilson, K.; Faelan, C.; Patterson-Kane, J.C.; Rudmann, D.G.; Moore, S.A.; Frank, D.; Charleston, J.; Tinsley, J.; Young, G.D.; Milici, A.J. Duchenne and Becker muscular dystrophies: A review of animal models, clinical end points, and biomarker quantification. Toxicol. Pathol. 2017, 45, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Tyler, K.L. Origins and early descriptions of “Duchenne muscular dystrophy”. Muscle Nerve 2003, 28, 402–422. [Google Scholar] [CrossRef] [PubMed]
- Romitti, P.A.; Zhu, Y.; Puzhankara, S.; James, K.A.; Nabukera, S.K.; Zamba, G.K.; Ciafaloni, E.; Cunniff, C.; Druschel, C.M.; Mathews, K.D.; et al. Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics 2015, 135, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Razak, H.; Malerba, A.; Dickson, G. Advances in gene therapy for muscular dystrophies. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Saada, Y.B.; Dib, C.; Lipinski, M.; Vassetzky, Y.S. Genome- and cell-based strategies in therapy of muscular dystrophies. Biochemistry 2016, 81, 678–690. [Google Scholar] [PubMed]
- Mah, J.K. Current and emerging treatment strategies for Duchenne muscular dystrophy. Neuropsychiatr. Dis. Treat. 2016, 12, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Harper, P.S.; van Engelen, B.G.; Eymard, B.; Rogers, M.; Wilcox, D. 99th ENMC international workshop: Myotonic dystrophy: Present management, future therapy. Neuromuscul. Disord. 2002, 12, 596–599. [Google Scholar] [CrossRef]
- Yamashita, Y.; Matsuura, T.; Kurosaki, T.; Amakusa, Y.; Kinoshita, M.; Ibi, T.; Sahashi, K.; Ohno, K. LDB3 splicing abnormalities are specific to skeletal muscles of patients with myotonic dystrophy type 1 and alter its PKC binding affinity. Neurobiol. Dis. 2014, 69, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Osborne, R.J.; Thornton, C.A. RNA-dominant diseases. Hum. Mol. Genet. 2006, 15, R162–R169. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.A.; Wang, E.; Carrell, E.M. Myotonic dystrophy: Approach to therapy. Curr. Opin. Genet. Dev. 2017, 44, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, P.; Selma-Soriano, E.; Rapisarda, A.S.; Fernandez-Costa, J.M.; Perez-Alonso, M.; Artero, R. Myotonic dystrophy: Candidate small molecule therapeutics. Drug Discov. Today 2017, 22, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Kumar, A. Therapeutic targeting of signaling pathways in muscular dystrophy. J. Mol. Med. 2010, 88, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Rahimov, F.; Kunkel, L.M. The cell biology of disease: Cellular and molecular mechanisms underlying muscular dystrophy. J. Cell Biol. 2013, 201, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Ervasti, J.M.; Ohlendieck, K.; Kahl, S.D.; Gaver, M.G.; Campbell, K.P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 1990, 345, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Models Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kay, D.I.; Rudra, R.T.; Chen, B.M.; Hsu, N.; Izumiya, Y.; Martinez, L.; Spencer, M.J.; Walsh, K.; Grinnell, A.D.; et al. Myogenic Akt signaling attenuates muscular degeneration, promotes myofiber regeneration and improves muscle function indystrophin-deficient mdx mice. Hum. Mol. Genet. 2011, 20, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.S.; Casar, J.C.; Motohashi, N.; Vieira, N.M.; Eisenberg, I.; Marshall, J.L.; Gasperini, M.J.; Lek, A.; Myers, J.A.; Estrella, E.A.; et al. MicroRNA-486-dependent modulation of DOCK3/PTEN/AKT signaling pathways improves muscular dystrophy-associatedsymptoms. J. Clin. Investig. 2014, 124, 2651–2667. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.A.; Yutzey, K.E. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev. Biol. 2004, 266, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ravel-Chapuis, A.; Bélanger, G.; Côté, J.; Michel, R.N.; Jasmin, B.J. Misregulation ofcalcium-handling proteins promotes hyperactivation of calcineurin-NFAT signaling in skeletal muscle of DM1 mice. Hum. Mol. Genet. 2017, 26, 2192–2206. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Wehling-Henricks, M. Nitric oxide synthase deficiency and the pathophysiology of muscular dystrophy. J. Physiol. 2014, 592, 4627–4638. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, J.; Bersch, K.; Büssenschütt, R.; Drzymalski, M.; Liebetanz, D.; Nikolaev, V.O.; Wagner, S.; Maier, L.S.; Gärtner, J.; Klinge, L.; et al. Dysferlin mediates membrane tubulation and links T-tubule biogenesis to muscular dystrophy. J. Cell Sci. 2017, 130, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, S.M.; Walsh, G.S.; Balazsi, K.; Seale, P.; Sandoz, J.; Hierlihy, A.M.; Rudnicki, M.A.; Chamberlain, J.S.; Miller, F.D.; Megeney, L.A. Activation of JNK1 contributes to dystrophic muscle pathogenesis. Curr. Biol. 2001, 11, 1278–1282. [Google Scholar] [CrossRef]
- Nakamura, A.; Harrod, G.V.; Davies, K.E. Activation of calcineurin and stress activated protein kinase/p38-mitogen activated protein kinase in hearts of utrophin-dystrophin knockout mice. Neuromuscul. Disord. 2001, 11, 251–259. [Google Scholar] [CrossRef]
- Igumenova, T.I. Dynamics and membrane interactions of protein kinase C. Biochemistry 2015, 54, 4953–4968. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.W.; Pula, G.; Hers, I.; Crosby, D.; Jones, M.L. PKC-interacting proteins: From function to pharmacology. Trends Pharmacol. Sci. 2004, 25, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, G.; Mirandola, P.; Carubbi, C.; Galli, D.; Vitale, M. Protein kinase C ε in hematopoiesis: Conductor or selector? Semin. Thromb. Hemost. 2013, 39, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.S.; Sutton, C.R.; Rao, S. Protein kinase C in the immune system: From signalling to chromatin regulation. Immunology 2015, 146, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, T.; Tuominen, R.K.; Moilanen, E. Protein kinase C and its inhibitors in the regulation of inflammation: Inducible nitric oxide synthase as an example. Basic Clin. Pharmacol. Toxicol. 2014, 114, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, A.; Balça-Silva, J.; Matias, D.; Lopes, M.C. PKC signaling in glioblastoma. Cancer Biol. Ther. 2013, 14, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu-Martinez, N.M.; Wang, G.S.; Cooper, T.A. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol. Cell 2007, 28, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Kuyumcu-Martinez, M.N.; Sarma, S.; Mathur, N.; Wehrens, X.H.; Cooper, T.A. PKC inhibition ameliorates the cardiac phenotype in a mouse model of myotonic dystrophy type 1. J. Clin. Investig. 2009, 119, 3797–3806. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, A.; Perry, R.J.; Camporez, J.P.G.; Jurczak, M.J.; Petersen, K.F.; Aspichueta, P.; Shulman, G.I. A controlled-release mitochondrial protonophore reverses hypertriglyceridemia, nonalcoholic steatohepatitis, and diabetes in lipodystrophic mice. FASEB J. 2017, 31, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Akinci, G.; Topaloglu, H.; Demir, T.; Danyeli, A.E.; Talim, B.; Keskin, F.E.; Kadioglu, P.; Talip, E.; Altay, C.; Yaylali, G.F.; et al. Clinical spectra of neuromuscular manifestations in patients with lipodystrophy: A multicenter study. Neuromuscul. Disord. 2017, 27, 923–930. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, P.A.; McArdle, A.; Edwards, R.H. Acute effects of phorbol esters on the protein-synthetic rate and carbohydrate metabolism of normal and mdx mouse muscles. Biochem. J. 1991, 275, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.V.; Shanmugasundaram, J.; Sundaram, C.; Anandaraj, M.P. Activity of novel protein kinase C and distribution of protein kinase C theta in subcellular fractions of normal and Duchenne muscular dystrophic muscle. Indian J. Biochem. Biophys. 2002, 39, 377–381. [Google Scholar] [PubMed]
- Madaro, L.; Pelle, A.; Nicoletti, C.; Crupi, A.; Marrocco, V.; Bossi, G.; Soddu, S.; Bouché, M. PKC theta ablation improves healing in a mouse model of muscular dystrophy. PLoS ONE 2012, 7, e31515. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, V.; Fiore, P.; Madaro, L.; Crupi, A.; Lozanoska-Ochser, B.; Bouché, M. Targeting PKCθ in skeletal muscle and muscle diseases: Good or bad? Biochem. Soc. Trans. 2014, 42, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Juretić, N.; Jorquera, G.; Caviedes, P.; Jaimovich, E.; Riveros, N. Electrical stimulation induces calcium-dependent up-regulation of neuregulin-1β in dystrophic skeletal muscle cell lines. Cell. Physiol. Biochem. 2012, 29, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Di Marcantonio, D.; Galli, D.; Carubbi, C.; Gobbi, G.; Queirolo, V.; Martini, S.; Merighi, S.; Vaccarezza, M.; Maffulli, N.; Sykes, S.M.; et al. PKCε as a novel promoter of skeletal muscle differentiation and regeneration. Exp. Cell Res. 2015, 339, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, N.J.; Ismail, H.; Zimetbaum, P.; Raynor, E.M. Cardiac involvement in the muscular dystrophies. Muscle Nerve 2017. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Comi, L.I.; Politano, L.; Bain, R.J. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int. J. Cardiol. 1990, 26, 271–277. [Google Scholar] [CrossRef]
- Ishikawa, K. Cardiac involvement in progressive muscular dystrophy of the Duchenne type. Jpn. Heart J. 1997, 38, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Comi, L.I.; Politano, L.; Limongelli, F.M.; Nigro, V.; de Rimini, M.L.; Giugliano, M.A.; Petretta, V.R.; Passamano, L.; Restucci, B.; et al. Evaluation of the cardiomyopathy in Becker muscular dystrophy. Muscle Nerve 1995, 18, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Connuck, D.M.; Sleeper, L.A.; Colan, S.D.; Cox, G.F.; Towbin, J.A.; Lowe, A.M.; Wilkinson, J.D.; Orav, E.J.; Cuniberti, L.; Salbert, B.A.; et al. Pediatric cardiomyopathy registry study group. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: A comparative study from the pediatric cardiomyopathy registry. Am. Heart J. 2008, 155, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Bassez, G.; Lazarus, A.; Desguerre, I.; Varin, J.; Laforêt, P.; Bécane, H.M.; Meune, C.; Arne-Bes, M.C.; Ounnoughene, Z.; Radvanyi, H.; et al. Severe cardiac arrhythmias in young patients with myotonic dystrophy type 1. Neurology 2004, 63, 1939–1941. [Google Scholar] [CrossRef] [PubMed]
- Wahbi, K.; Meune, C.; Bécane, H.M.; Laforêt, P.; Bassez, G.; Lazarus, A.; Radvanyi-Hoffman, H.; Eymard, B.; Duboc, D. Left ventricular dysfunction and cardiac arrhythmias are frequent in type 2 myotonic dystrophy: A case control study. Neuromuscul. Disord. 2009, 19, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Gallina, M.; Merlini, L.; Bonne, G.; Toniolo, D.; Amati, S.; Biffi, M.; Martignani, C.; Frabetti, L.; Bonvicini, M.; et al. Clinical relevance of atrial fibrillation/flutter, stroke, pacemaker implant, and heart failure in Emery-Dreifuss muscular dystrophy: A long-term longitudinal study. Stroke 2003, 34, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Fanin, M.; Melacini, P.; Boito, C.; Pegoraro, E.; Angelini, C. LGMD2E patients risk developing dilated cardiomyopathy. Neuromuscul. Disord. 2003, 13, 303–309. [Google Scholar] [CrossRef]
- Melacini, P.; Fanin, M.; Duggan, D.J.; Freda, M.P.; Berardinelli, A.; Danieli, G.A.; Barchitta, A.; Hoffman, E.P.; DallaVolta, S.; Angelini, C. Heart involvement in muscular dystrophies due to sarcoglycan gene mutations. Muscle Nerve 1999, 22, 473–479. [Google Scholar] [CrossRef]
- Singh, R.M.; Cummings, E.; Pantos, C.; Singh, J. Protein kinase C and cardiac dysfunction: A review. Heart Fail Rev. 2017, 22, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Simonis, G.; Briem, S.K.; Schoen, S.P.; Bock, M.; Marquetant, R.; Strasser, R.H. Protein kinase C in the human heart: Differential regulation of the isoforms in aortic stenosis or dilated cardiomyopathy. Mol. Cell. Biochem. 2007, 305, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cohen, M.V.; Downey, J.M. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc. Drugs Ther. 2010, 24, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Budas, G.R.; Churchill, E.N.; Mochly-Rosen, D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol. Res. 2007, 55, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Mochly-Rosen, D.; Boutjdir, M. Regulation of cardiac excitability by protein kinase C isozymes. Front. Biosci. (Scholar Ed.) 2012, 4, 532–546. [Google Scholar] [CrossRef]
- Palaniyandi, S.S.; Sun, L.; Ferreira, J.C.; Mochly-Rosen, D. Protein kinase C in heart failure: A therapeutic target? Cardiovasc. Res. 2009, 82, 229–239. [Google Scholar] [CrossRef]
- Inagaki, K.; Hahn, H.S.; Dorn, G.W., II; Mochly-Rosen, D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation 2003, 108, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Alstrom, J.S.; Stroemlund, L.W.; Nielsen, M.S.; MacAulay, N. Protein kinase C-dependent regulation of connexin43 gap junctions and hemichannels. Biochem. Soc. Trans. 2015, 43, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Sag, C.M.; Wagner, S.; Maier, L.S. Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes. Free Radic. Biol. Med. 2013, 63, 338–349. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).