Long-Term Cumulative Effects of Repeated Concussions in Cyclists: A Neurophysiological and Sensorimotor Study
Abstract
1. Introduction
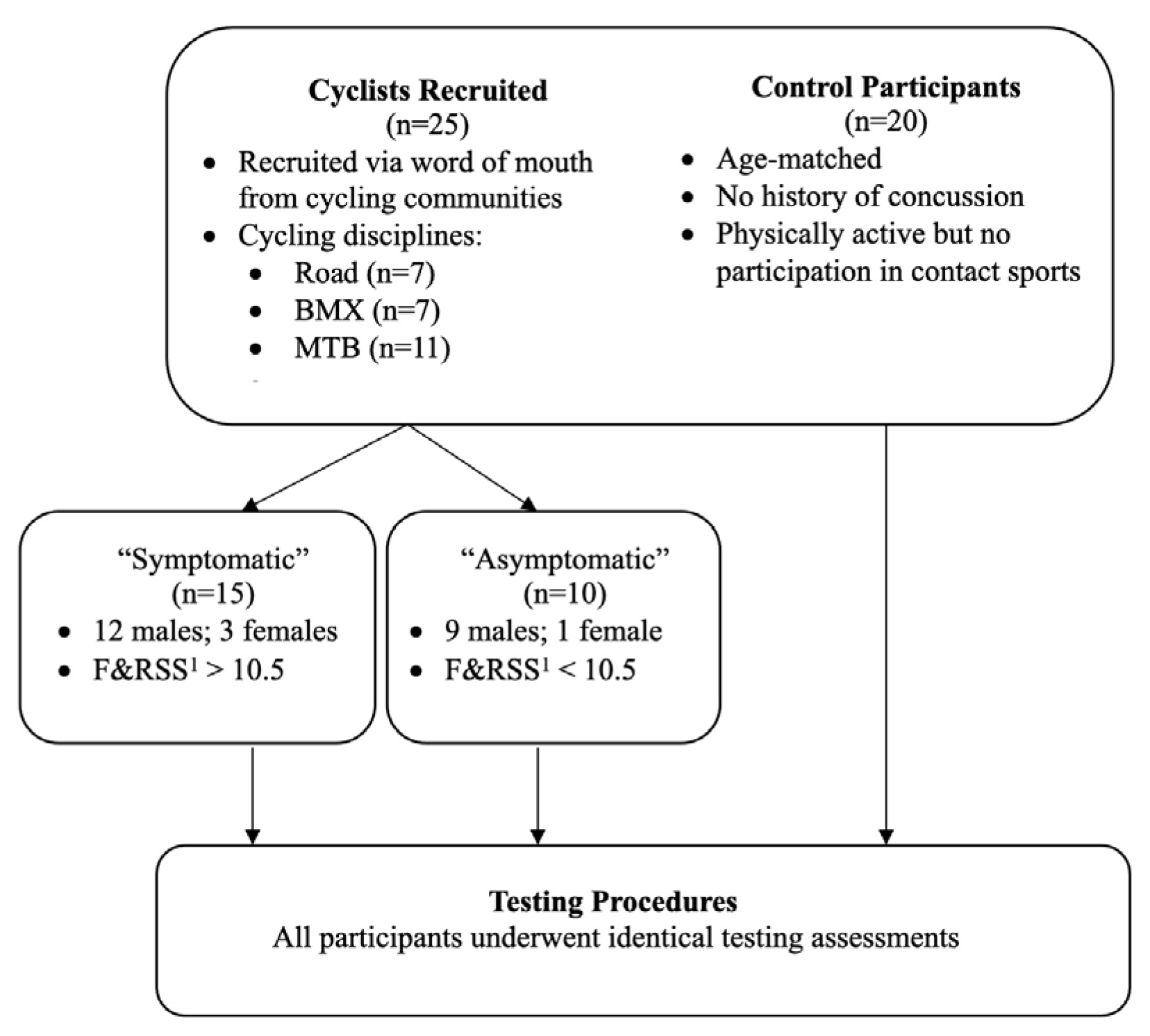
2. Materials and Methods
2.1. Symptom Assessment
2.2. Cognitive and Motor Testing
2.3. Sensorimotor Testing
2.4. Transcranial Magnetic Stimulation and Surface Electromyography
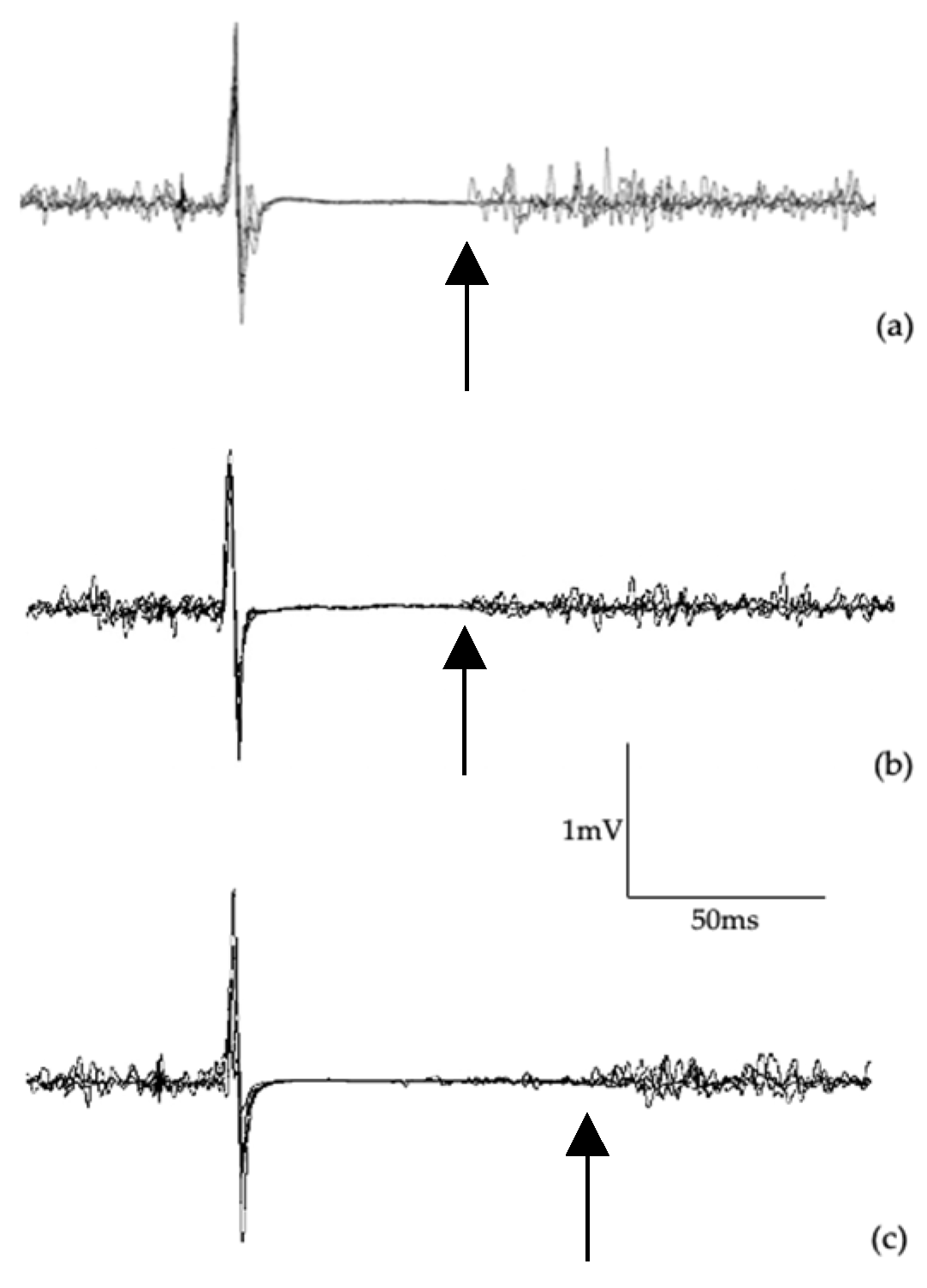
2.4.1. Single-Pulse TMS
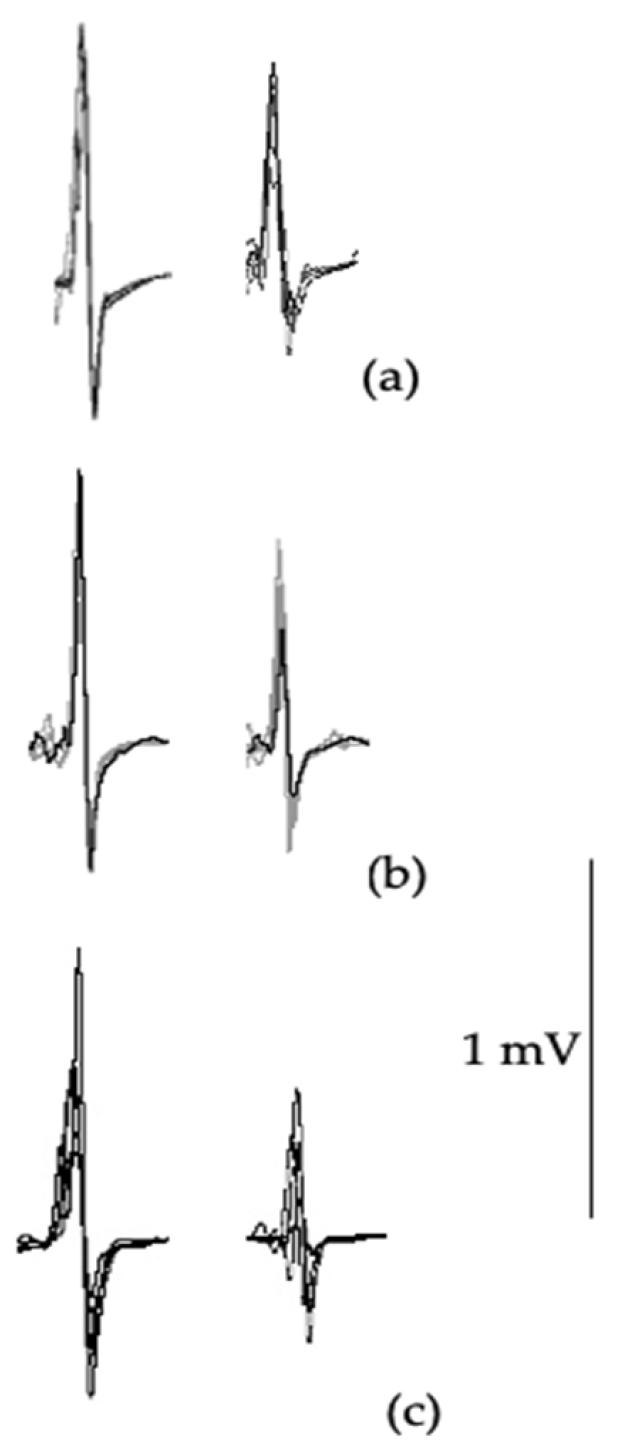
2.4.2. Paired-Pulse TMS
2.5. Statistical Analyses
3. Results
3.1. Cognitive and Motor Assessment
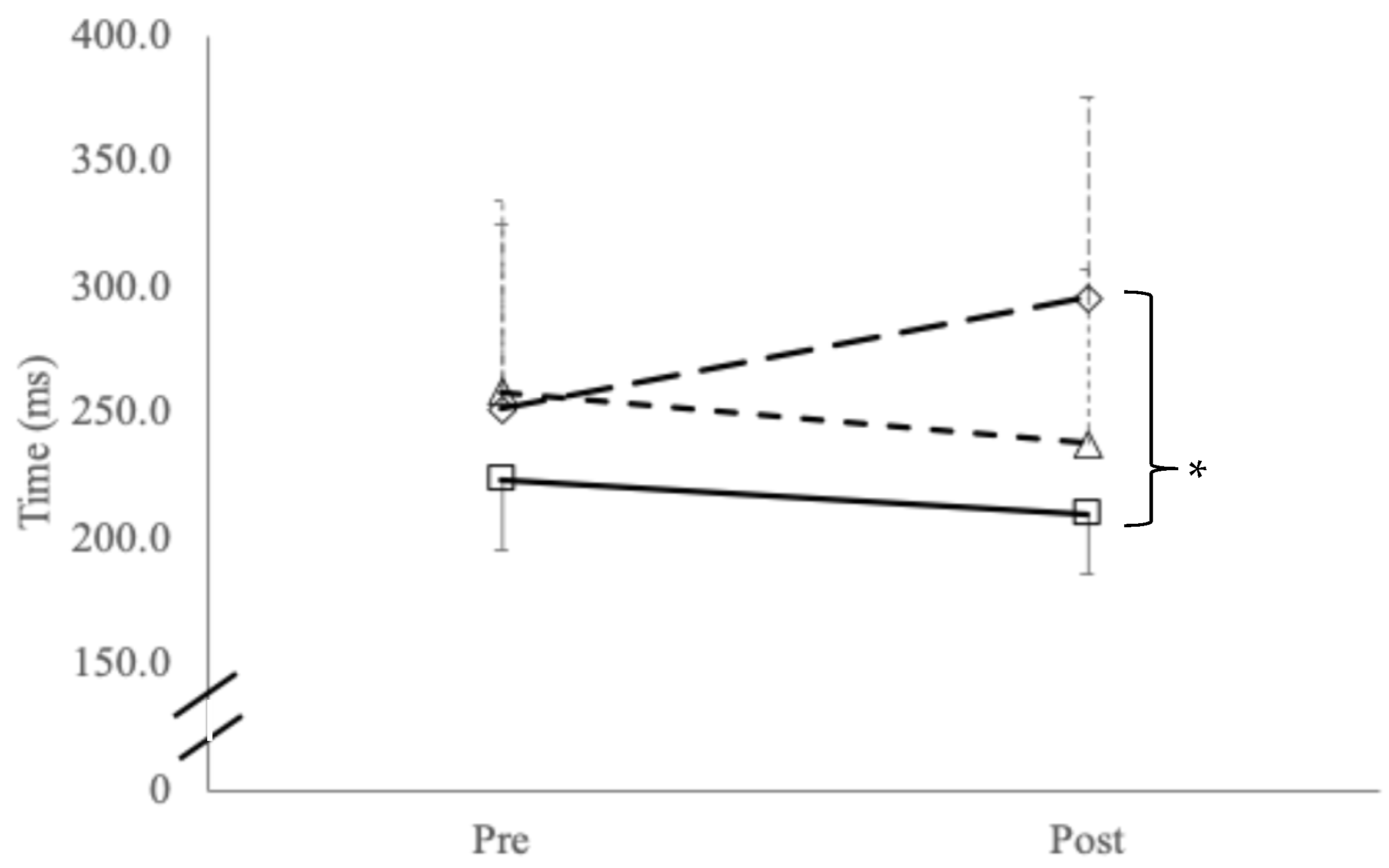
3.2. Sensorimotor Testing
3.3. Transcranial Magnetic Stimulation
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinberg, L. Concussion Epidemic Is Existential Threat to Football. Forbes, 2017. Available online: https://www.forbes.com/sites/leighsteinberg/2017/11/18/concussion-epidemic-is-existential-threat-to-football/ (accessed on 25 February 2025).
- Mark, D. Rugby League’s Existential Crisis as Concussion Threatens the Game’s Physicality. ABC Australia, 2021. Available online: https://www.abc.net.au/news/2021-06-05/rugby-leagues-existential-crisis-concussion/100192676 (accessed on 25 February 2025).
- Manley, G.T.; Gardner, A.J.; Schneider, K.J.; Guskiewicz, K.M.; Bailes, J.; Cantu, R.C.; Castellani, R.J.; Turner, M.; Jordan, B.D.; Randolph, C.; et al. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 2017, 51, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.J.; Tommerdahl, M.; King, D. Neurophysiological abnormalities in individuals with persistent post-concussion symptoms. Neuroscience 2019, 408, 272–281. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. Persistent Post-concussion syndrome: Pathophysiology, diagnosis, current and evolving treatment strategies. Expert Rev. Neurother. 2025, 25, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Patricios, J.S.; Schneider, K.J.; Dvorak, J.; Ahmed, O.H.; Blauwet, C.; Cantu, R.C.; Davis, G.A.; Echemendia, R.J.; Makdissi, M.; McNamee, M.; et al. Consensus statement on concussion in sport: The 6th International Conference on Concussion in Sport–Amsterdam, October 2022. Br. J. Sports Med. 2023, 57, 695–711. [Google Scholar] [CrossRef] [PubMed]
- UK Government. UK Concussion Guidelines for Non-Elite (Grassroots) Sport; UK Government: London, UK, 2023. [Google Scholar]
- Swart, J.; Bigard, X.; Fladischer, T.; Palfreeman, R.; Riepenhof, H.; Jones, N.; Heron, N. Harrogate consensus agreement: Cycling-specific sport-related concussion. Sports Med. Health Sci. 2021, 3, 110–114. [Google Scholar] [CrossRef]
- Fallon, T.; Palmer, D.; Bigard, X.; Heron, N. Epidemiology of injury and illness across all the competitive cycling disciplines: A systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2025, 11, e002364. [Google Scholar] [CrossRef]
- Hardwicke, J.; Hurst, H.T. Concussion knowledge and attitudes amongst competitive cyclists. J. Sci. Cycl. 2020, 9, 53–56. [Google Scholar] [CrossRef]
- Clark, G.; Johnson, N.A.; Saluja, S.S.; Correa, J.A.; Delaney, J.S. Do mountain bikers know when they have had a concussion and, do they know to stop riding? Clin. J. Sport Med. 2021, 31, e414–e419. [Google Scholar] [CrossRef]
- Pearce, A.J.; Young, J.A.; Parrington, L.; Aimers, N. Do as I say: Contradicting beliefs and attitudes towards sports concussion in Australia. J. Sports Sci. 2017, 35, 1911–1919. [Google Scholar] [CrossRef]
- Baron, D.A.; Reardon, C.L.; Baron, S.H. Clinical Sports Psychiatry: An International Perspective; John Wiley & Sons: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Heron, N.; Elliott, J.; Jones, N.; Loosemore, M.; Kemp, S. Sports-related concussion (SRC) in road cycling: The RoadsIde heaD Injury assEssment (RIDE) for elite road cycling. Br. J. Sports Med. 2020, 54, 127–128. [Google Scholar] [CrossRef]
- Hurst, H.T.; Atkins, S.; Dickinson, B.D. The magnitude of translational and rotational head accelerations experienced by riders during downhill mountain biking. J. Sci. Med. Sport 2018, 21, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Hurst, H.T.; Rylands, L.; Atkins, S.; Enright, K.; Roberts, S.J. Profiling of translational and rotational head accelerations in youth BMX with and without neck brace. J. Sci. Med. Sport 2018, 21, 263–267. [Google Scholar] [CrossRef]
- Grimm, N. Australia’s First Female Olympic Cyclist Julie Speight Donating Brain for Medical Research. ABC News, 2021. Available online: https://www.abc.net.au/news/2021-03-16/cyclist-julie-speight-donating-brain-to-science-cte-research/13252276 (accessed on 25 February 2025).
- Roenigk, A. Doctors Say Late BMX Legend Dave Mirra Had CTE. ESPN, 2016. Available online: https://www.espn.com/action/story/_/id/15614274/bmx-legend-dave-mirra-diagnosed-cte (accessed on 25 February 2025).
- Pearce, A.J.; Hoy, K.; Rogers, M.A.; Corp, D.; Maller, J.J.; Drury, H.G.; Fitzgerald, P.B. The long-term effects of sports concussion on retired Australian football players: A study using transcranial magnetic stimulation. J. Neurotrauma 2014, 31, 1139–1145. [Google Scholar] [CrossRef]
- Pearce, A.J.; King, D.; Kidgell, D.J.; Frazer, A.K.; Tommerdahl, M.; Suter, C.M. Assessment of Somatosensory and Motor Processing Time in Retired Athletes with a History of Repeated Head Trauma. J. Funct. Morphol. Kinesiol. 2022, 7, 109. [Google Scholar] [CrossRef]
- De Beaumont, L.; Lassonde, M.; Leclerc, S.; Théoret, H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery 2007, 61, 329–337. [Google Scholar] [CrossRef]
- De Beaumont, L.; Mongeon, D.; Tremblay, S.; Messier, J.; Prince, F.; Leclerc, S.; Lassonde, M.; Théoret, H. Persistent motor system abnormalities in formerly concussed athletes. J. Athl. Train. 2011, 46, 234–240. [Google Scholar] [CrossRef]
- Rabadi, M.H.; Jordan, B.D. The cumulative effect of repetitive concussion in sports. Clin. J. Sport Med. 2001, 11, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Pascual-Leone, A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.; Fried, P.J.; Pascual-Leone, A. Transcranial magnetic stimulation: Neurophysiological and clinical applications. In Handbook of Clinical Neurology; D’Esposito, M., Grafman, J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 163, pp. 73–92. [Google Scholar]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Orekhov, Y.; Ziemann, U. The role of GABAb receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 2006, 173, 86–93. [Google Scholar] [CrossRef]
- Scott, E.; Kidgell, D.J.; Frazer, A.K.; Pearce, A.J. The Neurophysiological Responses of Concussive Impacts: A Systematic Review and Meta-Analysis of Transcranial Magnetic Stimulation Studies. Front. Hum. Neurosci. 2020, 14, 306. [Google Scholar] [CrossRef]
- Tommerdahl, M.; Dennis, R.G.; Francisco, E.M.; Holden, J.K.; Nguyen, R.; Favorov, O.V. Neurosensory assessments of concussion. Mil. Med. 2016, 181, 45–50. [Google Scholar] [CrossRef]
- Slobounov, S.; Sebastianelli, W.; Moss, R. Alteration of posture-related cortical potentials in mild traumatic brain injury. Neurosci. Lett. 2005, 383, 251–255. [Google Scholar] [CrossRef]
- Johansson, B.; Berglund, P.; Rönnbäck, L. Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj. 2009, 23, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Echemendia, R.J.; Meeuwisse, W.; McCrory, P.; Davis, G.A.; Putukian, M.; Leddy, J.; Makdissi, M.; Sullivan, S.J.; Broglio, S.P.; Raftery, M.; et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5): Background and rationale. Br. J. Sports Med. 2017, 51, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.K.; Nguyen, R.H.; Francisco, E.M.; Zhang, Z.; Dennis, R.G.; Tommerdahl, M. A novel device for the study of somatosensory information processing. J. Neurosci. Methods 2012, 204, 215–220. [Google Scholar] [CrossRef]
- Wilson, S.A.; Lockwood, R.J.; Thickbroom, G.W.; Mastaglia, F.L. The muscle silent period following transcranial magnetic cortical stimulation. J. Neurol. Sci. 1993, 114, 216–222. [Google Scholar] [CrossRef]
- Wilson, S.A.; Thickbroom, G.W.; Mastaglia, F.L. Transcranial magnetic stimulation mapping of the motor cortex in normal subjects: The representation of two intrinsic hand muscles. J. Neurol. Sci. 1993, 118, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface Electromyography; Roessingh Research and Development: Enschede, The Netherlands, 1999. [Google Scholar]
- Chipchase, L.; Schabrun, S.; Cohen, L.; Hodges, P.; Ridding, M.; Rothwell, J.; Taylor, J.; Ziemann, U. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: An international consensus study. Clin. Neurophysiol. 2012, 123, 1698–1704. [Google Scholar] [CrossRef]
- Sandbrink, F. The MEP in clinical neurodiagnosis. In The Oxford Handbook of Transcranial Stimulation; Wasserman, E.M., Epstein, C.M., Ziemann, U., Walsh, V., Paus, T., Lisanby, S.H., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 237–283. [Google Scholar]
- Wilson, S.A.; Thickbroom, G.W.; Mastaglia, F.L. Comparison of the magnetically mapped corticomotor representation of a muscle at rest and during low-level voluntary contraction. Electroencephalogr. Clin. Neurophysiol./Electromyogr. Mot. Control 1995, 97, 246–250. [Google Scholar] [CrossRef]
- Di Virgilio, T.G.; Hunter, A.; Wilson, L.; Stewart, W.; Goodall, S.; Howatson, G.; Donaldson, D.I.; Ietswaart, M. Evidence for acute electrophysiological and cognitive changes following routine soccer heading. eBioMedicine 2016, 13, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Škarabot, J.; Mesquita, R.N.; Brownstein, C.G.; Ansdell, P. Myths and Methodologies: How loud is the story told by the transcranial magnetic stimulation-evoked silent period? Exp. Physiol. 2019, 104, 635–642. [Google Scholar] [CrossRef]
- Orth, M.; Rothwell, J.C. The cortical silent period: Intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin. Neurophysiol. 2004, 115, 1076–1082. [Google Scholar] [CrossRef]
- Pearce, A.J.; Kidgell, D.J.; Frazer, A.K.; King, D.; Buckland, M.E.; Tommerdahl, M. Corticomotor correlates of somatosensory reaction time and variability in individuals with post concussion symptoms. Somatosens. Mot. Res. 2020, 37, 14–21. [Google Scholar] [CrossRef]
- McGinley, M.; Hoffman, R.L.; Russ, D.W.; Thomas, J.S.; Clark, B.C. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp. Gerontol. 2010, 45, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.J.; Rist, B.; Fraser, C.L.; Cohen, A.; Maller, J.J. Neurophysiological and cognitive impairment following repeated sports concussion injuries in retired professional rugby league players. Brain Inj. 2018, 32, 498–505. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Petit, K.M.; Savage, J.L.; Bretzin, A.C.; Anderson, M.; Covassin, T. The sport concussion assessment tool-5 (SCAT5): Baseline assessments in NCAA division I collegiate student-athletes. Int. J. Exerc. Sci. 2020, 13, 1143. [Google Scholar] [CrossRef]
- Tucker, R.; Falvey, E.; Fuller, G.W.; Hislop, M.; Patricios, J.; Raftery, M. Sport concussion assessment tool: Baseline and clinical reference limits for concussion diagnosis and management in elite rugby Union. J. Sci. Med. Sport 2021, 24, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.; Seppelt, B.; Mehler, B.; Reimer, B.; Rosenholtz, R. Rapid holistic perception and evasion of road hazards. J. Exp. Psychol. Gen. 2020, 149, 490. [Google Scholar] [CrossRef]
- Reneker, J.C.; Babl, R.; Flowers, M.M. History of concussion and risk of subsequent injury in athletes and service members: A systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2019, 42, 173–185. [Google Scholar] [CrossRef]
- McPherson, A.L.; Nagai, T.; Webster, K.E.; Hewett, T.E. Musculoskeletal injury risk after sport-related concussion: A systematic review and meta-analysis. Am. J. Sports Med. 2019, 47, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.L.; Shirley, M.B.; Schilaty, N.D.; Larson, D.R.; Hewett, T.E. Effect of a concussion on anterior cruciate ligament injury risk in a general population. Sports Med. 2020, 50, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.H.; Kotlier, J.L.; Fathi, A.; Castonguay, J.; Thompson, A.A.; Bolia, I.K.; Petrigliano, F.A.; Liu, J.N.; Weber, A.E.; Gamradt, S.C. NCAA football players are at higher risk of upper extremity injury after first-time concussion. Phys. Sportsmed. 2024, 52, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.L.; Duenkel, N.; Dunlop, R.; Russell, G. Evaluation of single-leg standing following anterior cruciate ligament surgery and rehabilitation. Phys. Ther. 1994, 74, 245–252. [Google Scholar] [CrossRef]
- Bullock-Saxton, J.E. Sensory changes associated with severe ankle sprain. Scand. J. Rehabil. Med. 1995, 27, 161–167. [Google Scholar] [CrossRef] [PubMed]

| Age (Years) | Number of Reported Concussions | Time Since Last Concussion (Years) | |
|---|---|---|---|
| Symptomatic (n = 15; 12 m, 3 f) | 29.3 (±4.6) | 6.9 1 (±5.1) | 2.7 (±1.7) |
| Asymptomatic (n = 10; 9 m, 1 f) | 26.7 (±3.6) | 3.3 (±1.4) | 1.5 (±0.8) |
| Controls (n = 20; 17 m; 3 f) | 25.8 (±6.2) | n/a 2 | n/a |
| Total F&RSS 1 (Max 44) | Reported Symptoms 2 (Max 22) | Severity of Symptoms 2 (Max 132) | |
|---|---|---|---|
| Symptomatic (n = 15; 12 m, 3f) | 18.2 3 (±3.8) | 10.7 3 (±3.9) | 23.5 3 (±12.7) |
| Asymptomatic (n = 10; 9 m, 1 f) | 6.2 (±3.4) | 3.3 (±3.0) | 4.4 (±4.1) |
| Controls (n = 20; 17 m; 3 f) | 6.3 (±2.9) | 3.3 (±3.2) | 3.7 (±3.6) |
| Immediate Recall (Max 30) | Delayed Recall (Max 10) | Concentration (Max 9) | |
|---|---|---|---|
| Symptomatic (n = 15; 12 m, 3 f) | 18.7 (±2.5) | 5.9 (±1.0) | 6.1 1 (±1.7) |
| Asymptomatic (n = 10; 9 m, 1 f) | 20.2 (±2.7) | 6.9 (±0.7) | 6.5 (±1.0) |
| Controls (n = 20; 17 m; 3 f) | 20.7 (±2.4) | 6.5 (±1.1) | 7.7 (±1.2) |
| Tandem Gait time (s) | Single Leg Stance—Eyes Closed (Errors) | Tandem Leg Stance—Eyes Closed (Errors) | |
|---|---|---|---|
| Symptomatic (n = 15; 12 m, 3 f) | 9.2 1 (±1.2) | 2.1 (±1.8) | 1.3 2 (±1.3) |
| Asymptomatic (n = 10; 9 m, 1 f) | 6.6 (±1.1) | 1.7 (±2.7) | 0.4 (±0.7) |
| Controls (n = 20; 17 m; 3 f) | 7.1 (±1.4) | 1.1 (±0.9) | 0.5 (±0.5) |
| Choice RT + (ms) | Choice RT Error (% Correct) | Sequential Amplitude Discrimination (μm) | Simultaneous Amplitude Discrimination (μm) | Temporal Order Judgement (ms) | Duration Discrimination (ms) | |
|---|---|---|---|---|---|---|
| Symptomatic (n = 15; 12 m, 3 f) | 467.0 (±118.8) | 94.0 (±5.5) | 49.2 (±37.3) | 80.5 (±46.4) | 42.1 1 (±25.2) | 65.3 (±38.5) |
| Asymptomatic (n = 10; 9 m, 1 f) | 448.3 (±84.7) | 90.0 (±5.8) | 37.4 (±29.2) | 54.5 (±23.3) | 25.9 (±15.4) | 64.2 (±46.3) |
| Controls (n = 20; 17 m; 3 f) | 423.3 (±82.1) | 93.0 (±4.8) | 43.5 (±33.5) | 55.0 (±34.5) | 25.8 (±11.3) | 53.5 (±20.1) |
| aMT * (% Maximal Stimulator Output) | Corticospinal Latency (ms) | cSP:MEP + Ratio | SICI # Ratio | LICI # Ratio | |||
|---|---|---|---|---|---|---|---|
| 130% aMT | 150% aMT | 170% aMT | |||||
| Symptomatic (n = 15; 12 m, 3 f) | 35.6 (±8.2) | 94.0 (±5.5) | 49.3 (±24.3) | 53.0 1 (±20.4) | 57.1 2 (±19.6) | 47.4 3 (±9.4) | 55.7 (±15.2) |
| Asymptomatic (n = 10; 9 m, 1 f) | 34.0 (±5.7) | 90.0 (±5.8) | 40.1 (±20.7) | 40.4 (±20.2) | 41.3 (±22.9) | 65.1 (±16.1) | 68.5 (±13.3) |
| Controls (n = 20; 17 m; 3 f) | 34.2 (±7.4) | 93.0 (±4.8) | 36.4 (±17.2) | 30.6 (±14.5) | 35.1 (±22.6) | 76.2 (±11.3) | 69.6 (±17.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearce, A.J.; King, D. Long-Term Cumulative Effects of Repeated Concussions in Cyclists: A Neurophysiological and Sensorimotor Study. J. Funct. Morphol. Kinesiol. 2025, 10, 414. https://doi.org/10.3390/jfmk10040414
Pearce AJ, King D. Long-Term Cumulative Effects of Repeated Concussions in Cyclists: A Neurophysiological and Sensorimotor Study. Journal of Functional Morphology and Kinesiology. 2025; 10(4):414. https://doi.org/10.3390/jfmk10040414
Chicago/Turabian StylePearce, Alan J., and Doug King. 2025. "Long-Term Cumulative Effects of Repeated Concussions in Cyclists: A Neurophysiological and Sensorimotor Study" Journal of Functional Morphology and Kinesiology 10, no. 4: 414. https://doi.org/10.3390/jfmk10040414
APA StylePearce, A. J., & King, D. (2025). Long-Term Cumulative Effects of Repeated Concussions in Cyclists: A Neurophysiological and Sensorimotor Study. Journal of Functional Morphology and Kinesiology, 10(4), 414. https://doi.org/10.3390/jfmk10040414






