The Acute Effect of Warm-Up with Cold Water Immersion upon Calf Raise Performance, Muscle Tension, and Oxygen Saturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measurements
2.4. Statistical Analysis
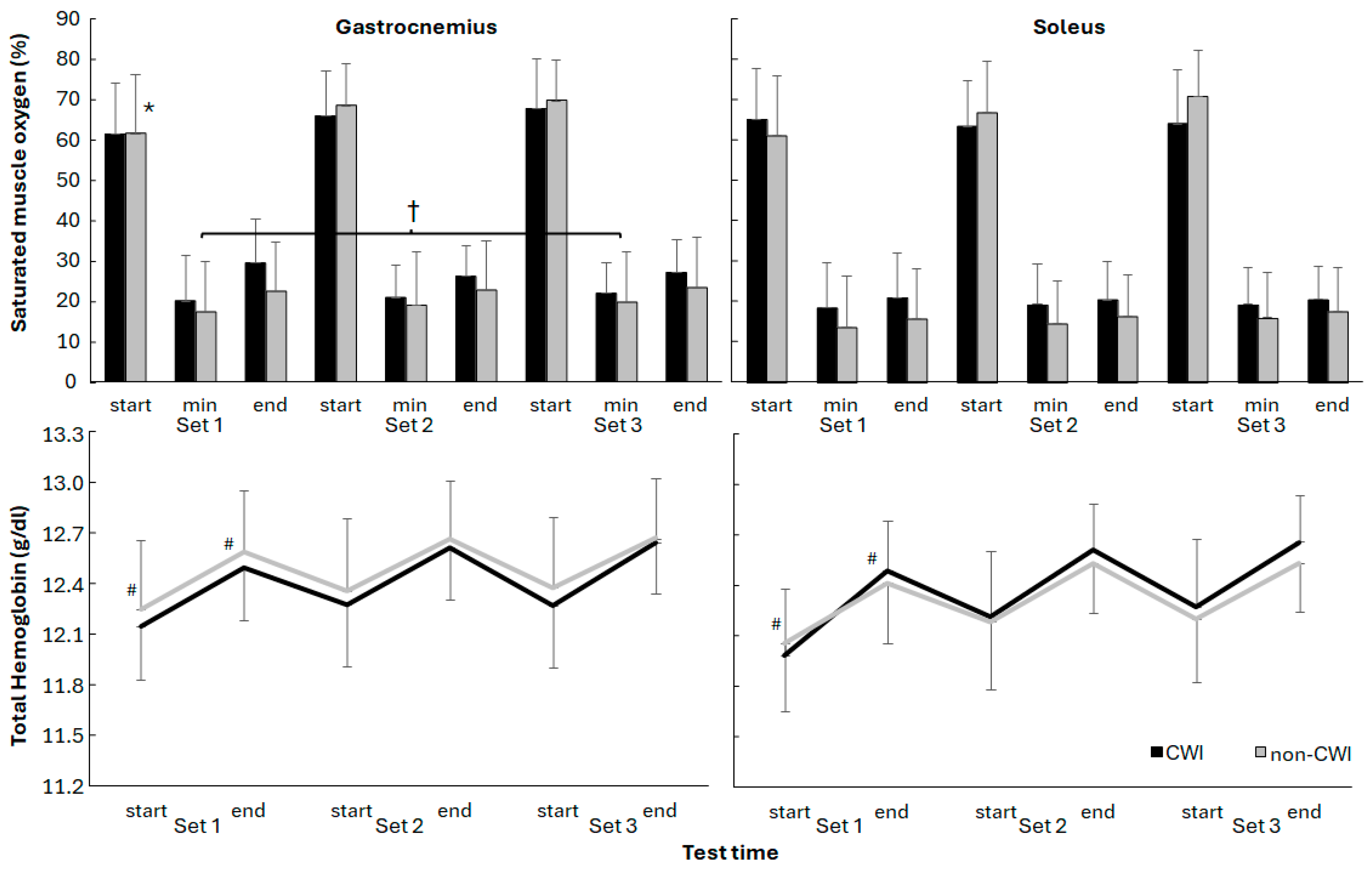
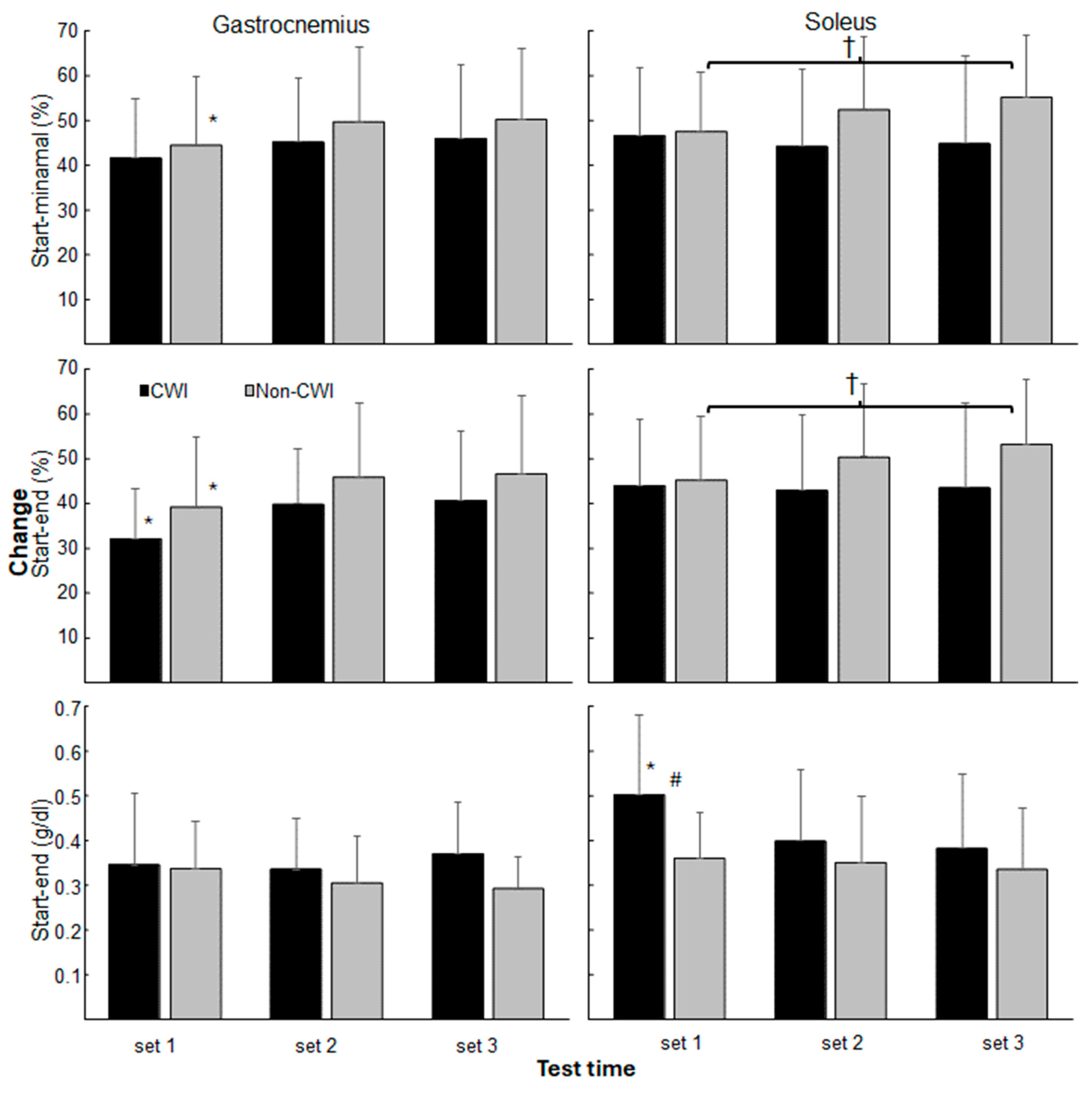
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaile, J.; O’Hagan, C.; Stefanovic, B.; Walker, M.; Gill, N.; Askew, C.D. Effect of cold water immersion on repeated cycling performance and limb blood flow. Br. J. Sports Med. 2011, 45, 825–829. [Google Scholar] [CrossRef]
- Ihsan, M.; Watson, G.; Lipski, M.; Abbiss, C.R. Influence of postexercise cooling on muscle oxygenation and blood volume changes. Med. Sci. Sports Exerc. 2013, 45, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Mawhinney, C.; Jones, H.; Joo, C.H.; Low, D.A.; Green, D.J.; Gregson, W. Influence of cold-water immersion on limb and cutaneous blood flow after exercise. Med. Sci. Sports Exerc. 2013, 45, 2277–2285. [Google Scholar] [CrossRef]
- Tseng, C.-Y.; Lee, J.-P.; Tsai, Y.-S.; Lee, S.-D.; Kao, C.-L.; Liu, T.-C.; Lai, C.-H.; Harris, M.B.; Kuo, C.-H. Topical cooling (icing) delays recovery from eccentric exercise–induced muscle damage. J. Strength Cond. Res. 2013, 27, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Vaile, J.; Halson, S.; Gill, N.; Dawson, B. Effect of cold water immersion on repeat cycling performance and thermoregulation in the heat. J. Sports Sci. 2008, 26, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Yamane, M.; Teruya, H.; Nakano, M.; Ogai, R.; Ohnishi, N.; Kosaka, M. Post-exercise leg and forearm flexor muscle cooling in humans attenuates endurance and resistance training effects on muscle performance and on circulatory adaptation. Eur. J. Appl. Physiol. 2006, 96, 572–580. [Google Scholar] [CrossRef]
- Malta, E.S.; Dutra, Y.M.; Broatch, J.R.; Bishop, D.J.; Zagatto, A.M. The effects of regular cold-water immersion use on training-induced changes in strength and endurance performance: A systematic review with meta-analysis. Sports Med. 2021, 51, 161–174. [Google Scholar] [CrossRef]
- Yamane, M.; Ohnishi, N.; Matsumoto, T. Does regular post-exercise cold application attenuate trained muscle adaptation? Int. J. Sports Med. 2015, 36, 647–653. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Wang, W.-Y.; Huang, Y.-M.; Wu, C.-M. Effect of acute interset foot cooling on lower limb strength training workout. Int. J. Sports Physiol. Perform. 2021, 16, 682–687. [Google Scholar] [CrossRef]
- Grahn, D.A.; Cao, V.H.; Heller, H.C. Heat extraction through the palm of one hand improves aerobic exercise endurance in a hot environment. J. Appl. Physiol. 2005, 99, 972–978. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Robergs, R.A.; Kravitz, L.R.; Gurney, B.A.; Mermier, C.M.; Schneider, S.M. Palm cooling delays fatigue during high-intensity bench press exercise. Med. Sci. Sports Exerc. 2010, 42, 1557–1565. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Robergs, R.A.; Mermier, C.M.; Schneider, S.M.; Gurney, A.B. Palm cooling and heating delays fatigue during resistance exercise in women. J. Strength Cond. Res. 2015, 29, 2261–2269. [Google Scholar] [CrossRef]
- Wu, C.-M.; Lee, M.-H.; Wang, W.-Y.; Cai, Z.-Y. Acute effects of intermittent foot cooling on 1 RM leg press strength in resistance-trained men: A pilot study. Int. J. Environ. Res. Public Health 2021, 18, 9594. [Google Scholar] [CrossRef]
- McMahon, G.; Kennedy, R.; Burden, A. No effect of interset palm cooling on acute bench press performance, electromyography amplitude, or spectral frequencies in resistance-trained men. J. Strength Cond. Res. 2023, 37, 555–563. [Google Scholar] [CrossRef]
- Walløe, L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature 2016, 3, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, F. Vasomotor responses in glabrous and nonglabrous skin during sinusoidal exercise. Med. Sci. Sports Exerc. 2002, 34, 767–772; discussion 773. [Google Scholar] [CrossRef] [PubMed]
- Grahn, D.; Dillon, J.; Heller, H. Heat loss through the glabrous skin surfaces of heavily insulated, heat-stressed individuals. J. Biomech. Eng. 2009, 131, 071005. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Waterworth, S.; Tallent, J.; Rogerson, M.; Morton, C.; Moran, J.; Southall-Edwards, R.; Cooper, C.E.; McManus, C. Cold-Water Immersion and Lower Limb Muscle Oxygen Consumption as Measured by Near-Infrared Spectroscopy in Trained Endurance Athletes. J. Athl. Train. 2024, 59, 317–324. [Google Scholar] [CrossRef]
- Jones, P.R.; Barton, C.; Morrissey, D.; Maffulli, N.; Hemmings, S. Pre-cooling for endurance exercise performance in the heat: A systematic review. BMC Med. 2012, 10, 166. [Google Scholar] [CrossRef]
- Tipton, M.J.; Collier, N.; Massey, H.; Corbett, J.; Harper, M. Cold water immersion: Kill or cure? Exp. Physiol. 2017, 102, 1335–1355. [Google Scholar] [CrossRef]
- Gregson, W.; Allan, R.; Holden, S.; Phibbs, P.; Doran, D.; Campbell, I.; Waldron, S.; Joo, C.H.; Morton, J.P. Postexercise cold-water immersion does not attenuate muscle glycogen resynthesis. Med. Sci. Sports Exerc. 2013, 45, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Vaile, J.; Halson, S.; Graham, S. Recovery review: Science vs. practice. J. Aust. Strength Cond. 2010, 18, 5–21. [Google Scholar]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; p. 174. [Google Scholar]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Piñero, A.; Burke, R.; Augustin, F.; Mohan, A.E.; DeJesus, K.; Sapuppo, M.; Weisenthal, M.; Coleman, M.; Androulakis-Korakakis, P.; Grgic, J. Throwing cold water on muscle growth: A systematic review with meta-analysis of the effects of postexercise cold water immersion on resistance training-induced hypertrophy. Eur. J. Sport Sci. 2024, 24, 177–189. [Google Scholar] [CrossRef]
- Van den Brande, P.; De Coninck, A.; Lievens, P. Skin microcirculation responses to severe local cooling. Int. J. Microcirc. 1997, 17, 55–60. [Google Scholar] [CrossRef]


| Before | Set 1 | Set 2 | Set 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Condition | CWI | Non-CWI | CWI | Non-CWI | CWI | Non-CWI | CWI | Non-CWI |
| Load | 94.5 ± 18.1 | 86.8 ± 26.3 | 98.0 ± 18.7 # | 87.6 ± 26.1 | 97.6 ± 18.6 # | 87.6 ± 26.1 | ||
| Repetitions | 23.7 ± 2.6 * | 21.5 ± 3.3 * | 16.3 ± 1.6 | 16.5 ± 1.6 | 15.6 ± 1.6 | 16.8 ± 3.2 | ||
| RPE † | 1.8 ± 1.4 | 2.7 ± 1.7 | 5.3 ± 1.6 | 5.8 ± 1.4 | 7.0 ± 1.1 | 7.6 ± 1.1 | 7.8 ± 1.4 | 8.2 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tillaar, R.v.d.; Lunde, P.; Mielniczek, M. The Acute Effect of Warm-Up with Cold Water Immersion upon Calf Raise Performance, Muscle Tension, and Oxygen Saturation. J. Funct. Morphol. Kinesiol. 2025, 10, 328. https://doi.org/10.3390/jfmk10030328
Tillaar Rvd, Lunde P, Mielniczek M. The Acute Effect of Warm-Up with Cold Water Immersion upon Calf Raise Performance, Muscle Tension, and Oxygen Saturation. Journal of Functional Morphology and Kinesiology. 2025; 10(3):328. https://doi.org/10.3390/jfmk10030328
Chicago/Turabian StyleTillaar, Roland van den, Patrick Lunde, and Milosz Mielniczek. 2025. "The Acute Effect of Warm-Up with Cold Water Immersion upon Calf Raise Performance, Muscle Tension, and Oxygen Saturation" Journal of Functional Morphology and Kinesiology 10, no. 3: 328. https://doi.org/10.3390/jfmk10030328
APA StyleTillaar, R. v. d., Lunde, P., & Mielniczek, M. (2025). The Acute Effect of Warm-Up with Cold Water Immersion upon Calf Raise Performance, Muscle Tension, and Oxygen Saturation. Journal of Functional Morphology and Kinesiology, 10(3), 328. https://doi.org/10.3390/jfmk10030328







