Effects of Weight-Cutting Practices on Sleep, Recovery, and Injury in Combat Sports: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias
2.7.1. RoB 2
2.7.2. ROBINS-I
2.8. Data Synthesis Methods and Evidence Gap Map
3. Results
3.1. Study Selection
3.2. Study Characteristics

| Study | n | Sex | Sport | Age | Study Design | Weight-Cutting Method | Outcomes | Methods of Measurement | Findings |
|---|---|---|---|---|---|---|---|---|---|
| [19] | 21 | M/F | Muay Thai | 24.62 ± 5.42 | Single-arm repeated-measures trial (baseline, post-RWL) | RWL: −1000 kcal/day, low CHO (<30 g/day) for 3 days; RWG: High CHO (4.5 g/kg/day) for 8 h | Creatinine | Blood samples (ADVIA 1800, Centaur XP) | No differences found between pre–post assessments |
| [20] | 11 | Not reported | Wrestling | 20.45 ± 2.69 | Repeated-measures design with three assessments: baseline (day 1, pre-RWL), post-RWL (day 4, after 4–5% weight loss), and post-12-h recovery (day 4) | Self-directed rapid weight loss (4–5% body weight) over 4 days via energy and fluid restriction | Leg fatigue index (%), arm fatigue index (%), and heart rate recovery | Wingate anaerobic test | Rapid weight loss increased fatigue index |
| [21] | 17 | M | Mixed martial arts | 27.4 ± 5.3 | Pre–post design with two groups: no rapid weight loss (NWL, weigh-in ~30 min pre-fight) and rapid weight loss (RWL, weigh-in 24 h pre-fight, ~10% body mass loss in 1 week) | Dietary restriction with sauna use (self-reported); details of restriction not provided | Creatinine and cortisol | Venous blood samples (10 mL) pre- and post-match | Rapid weight loss increased muscle damage |
| [22] | 20 | M | Judo | Not reported | Randomized pre–post design with three assessments: baseline (T1), pre-competition (T2), post-competition (T3) | Self-determined 5% body weight loss in 1 week via energy (33%) and fluid (~22%) restriction | Cortisol and perceived fatigue | Blood samples | ~5% weight loss increased perceived fatigue and cortisol levels |
| [18] | 155 | M/F | Mixed martial arts and Muay Thai | Not reported | 14-month cross-sectional study with post-competition questionnaires | 4.9–6.1% rapid weight loss during 7 days; details of restriction not provided | Self-reported injury status | Online questionnaires: Weight management (1 day post-competition) and injury status (5–7 days post-competition) | RWL−24 h (3.0% ± 1.9%) increased injury odds by 1.2 per 1% weight loss (p = 0.044); 49% reported injuries |
| [23] | 11 | M | Judo | Not reported | Pre–post design with assessments during weight maintenance (T1) and after 7-day food restriction (T2) | Self-selected 7-day food restriction and low-carbohydrate diet | Perceived fatigue | POMS scale | 7-day food restriction significantly increased perceived fatigue |
| [28] | 39 | M | Judo | EG: 22.38 ± 1.84 CG: 22.01 ± 1.72 | Randomized experimental design with two-week intervention; assessments pre- and post-intervention | EG: 10% body weight reduction (5% weekly) via food restriction (6.7 MJ/day), sauna (6 × 10 min), and plastic clothing (4 sessions) | Perceived recovery (RESTQ-sport) | Psychophysiological recovery questionnaire | EG showed reduced perceived recovery (p < 0.01); CG significantly increased perceived recovery |
| [24] | 20 | M | Judo | 24 ± 5 | Randomized pre–post design with two groups (WL, WS); assessments at baseline, pre-competition (T0), and after five fights (F1–F5) | ≥3% body mass loss in 1 week via restrictive diet; details of restriction not provided | Cortisol and perceived fatigue | Blood samples | Weight loss group had significant decreases in cortisol levels across 5 fights; control group had no significant differences |
| [25] | 24 | M | Wrestling | Weight loss group: 19.40 ± 0.74 No weight loss group: 19.22 ± 0.67 | Pre–post design comparing weight loss (WL) and non-weight loss (NWL) groups; assessments at national camp start and pre-competition weigh-in | WL: Food/fluid restriction 1–2 weeks pre-competition | CK | Blood samples | WL group had higher CK, LDH, and ALT (p < 0.05) post-test; no C-RP differences |
| [17] | 219 | M/F | Taekwondo | 17.83 ± 0.38 | Prospective cohort study over 2019 | Details of restriction not provided | Injury rate | Daily injury report forms | RWL showed high injury rates during weight loss periods |
| [29] | 12 | M | Wrestling | 24.30 ± 5.10 | Crossover study with initial measurement (IM), RWL + HISST (P1), and HISST alone (P2) | Self-chosen RWL methods to reduce 5% body mass in 3 days; details of restriction not provided | CK | Blood samples (8 mL) | RWL caused greater increases in muscle damage markers (Mb, CK, ALD, AST, ALT, LDH) |
| [26] | 18 | M | Brazilian Jiu-jitsu | L-CHO: 30.1 ± 7.5 A-CHO: 22.9 ± 3.4 | Randomized clinical trial with 30-day low (L-CHO) vs. adequate (A-CHO) carbohydrate diets | Caloric restriction aiming 5% body mass loss; L-CHO: 2–3 g/kg/day; A-CHO: 4–6 g/kg/day | Cortisol | Immunoassay analyzer | No differences between time points and between groups |
| [27] | 14 | M | Wrestling | 17.79 ± 0.75 | Randomized pre–post design with fast weight loss (FWL) and short-term weight loss (SHWL) groups | Fast WL: Severe food/water restriction and thick clothing (24 h); Slow WL: 4–5% calorie reduction and 10-day exercise | Cortisol | Saliva samples | No significant changes were found between both groups and between pre–post assessments |
| [9] | 8 | M | MMA, BJJ, and Muay Thai | 21.62 ± 1.4 | Pre–post design with assessments 14 days and 1 day before weigh-in | Details of restriction not provided | Perceived fatigue | Brums Mood Scale | RWL significantly increased perceived fatigue |
| [30] | 18 | M | Judo | 25.3 ± 5.4 | Crossover study with exercise-only (4 days) and RWL (3 days) phases | Increased activity, plastic suit training, caloric deficit, reduced fluid intake, and sauna | CK | Blood samples (2 mL EDTA, 8 mL serum) | RWL significantly increased CK and ALD |
| [16] | 10 | F | Taekwondo | 21.3 ± 1.2 | Longitudinal study conducted at 28, 14, 7, and 1 days pre-competition, and at 1, 7, and 21 days post-competition | ≥2 kg weight loss in 7-to-1 or 1-to-11 day periods; details of restriction not provided | Salivary cortisol | Saliva samples (Salivette, ELISA) | RWL showed a significant cortisol increase 7 days post-competition period only |
| [31] | 16 | M | Freestyle Wrestling | 20.2 ± 3.2 | Observational cohort study with assessments at T1 (slow RWL start), T2 (rapid RWL start), and T3 (pre-competition) | Slow RWL (30 days): Diet adjustment and aerobic exercise; Rapid RWL (7 days): Water restriction, high-intensity intervals, and sauna | CK, sleep quality, and fatigue | Blood samples (Beckman Coulter, Hitachi) and POMS questionnaire | CK and fatigue increased from T1 to T2, and decreased from T2 to T3; sleep quality was not affected |
3.3. Risk of Bias in Studies
3.4. Results of Individual Studies
3.5. Evidence Gap Map
4. Discussion
4.1. Participant Characteristics
4.2. Prevalent Weight-Cutting Characteristics
4.3. Methodological Approaches and Analyzed Outcomes
4.4. Reported Findings
4.5. Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RWL | rapid weight loss |
| CK | creatine kinase |
| HPA | hypothalamic–pituitary–adrenal |
References
- Barley, O.R.; Chapman, D.W.; Abbiss, C.R. Weight Loss Strategies in Combat Sports and Concerning Habits in Mixed Martial Arts. Int. J. Sports Physiol. Perform. 2018, 13, 933–939. [Google Scholar] [CrossRef]
- Barley, O.; Chapman, D.; Abbiss, C. The Current State of Weight-Cutting in Combat Sports. Sports 2019, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Brechney, G.C.; Cannon, J.; Goodman, S.P. Effects of Weight Cutting on Exercise Performance in Combat Athletes: A Meta-Analysis. Int. J. Sports Physiol. Perform. 2022, 17, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Reale, R.; Slater, G.; Burke, L.M. Weight Management Practices of Australian Olympic Combat Sport Athletes. Int. J. Sports Physiol. Perform. 2018, 13, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Song, Y.; Artioli, G.G.; Gee, T.I.; French, D.N.; Zheng, H.; Lyu, M.; Li, Y. The Practice of Weight Loss in Combat Sports Athletes: A Systematic Review. Nutrients 2024, 16, 1050. [Google Scholar] [CrossRef]
- Dunican, I.C.; Eastwood, P.; Murray, K.; Caldwell, J.A.; Reale, R. The Effect of Water Loading for Acute Weight Loss Following Fluid Restriction on Sleep Quality and Quantity in Combat Sports Athletes. Sleep Med. 2019, 64, S99–S100. [Google Scholar] [CrossRef]
- Thomas, C.; Langan-Evans, C.; Germaine, M.; Artukovic, M.; Jones, H.; Whitworth-Turner, C.; Close, G.L.; Louis, J. Case Report: Effect of Low Energy Availability and Training Load on Sleep in a Male Combat Sport Athlete. Front. Sports Act. Living 2023, 4, 981755. [Google Scholar] [CrossRef]
- Dunican, I.; Galpin, A.; Turner, M.; Reale, R. Sleep Behaviours and Nutritional Knowledge in Amateur and Professional Combat Sport Athletes. Sleep Adv. 2024, 5, A62. [Google Scholar] [CrossRef]
- do Nascimento-Carvalho, B.; Mayta, M.A.C.; Izaias, J.E.; Doro, M.R.; Scapini, K.; Caperuto, E.; Grilletti, J.V.F.; Sanches, I.C. Cardiac Sympathetic Modulation Increases after Weight Loss in Combat Sports Athletes. Rev. Bras. Med. Esporte 2018, 24, 413–417. [Google Scholar] [CrossRef]
- Yang, W.H.; Heine, O.; Pauly, S.; Kim, P.; Bloch, W.; Mester, J.; Grau, M. Rapid Rather than Gradual Weight Reduction Impairs Hemorheological Parameters of Taekwondo Athletes through Reduction in RBC-NOS Activation. PLoS ONE 2015, 10, e0123767. [Google Scholar] [CrossRef]
- Thomas, S.; Gonzalez, A.M.; Ghigiarelli, J.J. The Relationship between Weight Cutting and the Female Athlete Triad in Combat Sport Athletes. Int. J. Kinesiol. Sports Sci. 2021, 9, 9–14. [Google Scholar] [CrossRef]
- Hammer, E.; Sanfilippo, J.L.; Johnson, G.; Hetzel, S. Association of In-Competition Injury Risk and the Degree of Rapid Weight Cutting Prior to Competition in Division I Collegiate Wrestlers. Br. J. Sports Med. 2023, 57, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Andreato, L.V.; Silva, R.B.; Bueno, J.C.A.; Andrade, A. Effects of Weight Loss on Psychological Variables in Combat Sports Athletes: A Systematic Review. Sport Sci. Health 2025, 21, 1295–1307. [Google Scholar] [CrossRef]
- Januszko, P.; Lange, E. Nutrition, Supplementation and Weight Reduction in Combat Sports: A Review. AIMS Public Health 2021, 8, 485–498. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Tsai, M.-L.; Ko, M.-H.; Chang, C.-K.; Chou, K.-M.; Fang, S.-H. Impact of Intense Training and Rapid Weight Changes on Salivary Parameters in Elite Female Taekwondo Athletes. Scand. J. Med. Sci. Sports 2011, 21, 758–764. [Google Scholar] [CrossRef]
- Kim, C.; Park, K.J. Injuries and Rapid Weight Loss in Elite Adolescent Taekwondo Athletes: A Korean Prospective Cohort Study. Phys. Med. Rehabil. Kurortmed. 2021, 31, 184–193. [Google Scholar] [CrossRef]
- Doherty, C.S.; Barley, O.R.; Fortington, L.V. Is There a Relationship between Rapid Weight Changes and Self-Reported Injury in Combat Sports Athletes? A 14-Month Study of 24 Combat Sports Events. J. Sci. Med. Sport 2025, 28, 465–474. [Google Scholar] [CrossRef]
- Cannataro, R.; Cione, E.; Gallelli, L.; Marzullo, N.; Bonilla, D.A. Acute Effects of Supervised Making Weight on Health Markers, Hormones and Body Composition in Muay Thai Fighters. Sports 2020, 8, 137. [Google Scholar] [CrossRef]
- Cengiz, A. Effects of Self-Selected Dehydration and Meaningful Rehydration on Anaerobic Power and Heart Rate Recovery of Elite Wrestlers. J. Phys. Ther. Sci. 2015, 27, 1441–1444. [Google Scholar] [CrossRef]
- Coswig, V.S.; Fukuda, D.H.; Del Vecchio, F.B. Rapid Weight Loss Elicits Harmful Biochemical and Hormonal Responses in Mixed Martial Arts Athletes. Int. J. Sport. Nutr. Exerc. Metab. 2015, 25, 480–486. [Google Scholar] [CrossRef]
- Degoutte, F.; Jouanel, P.; Bègue, R.J.; Colombier, M.; Lac, G.; Pequignot, J.M.; Filaire, E. Food Restriction, Performance, Biochemical, Psychological, and Endocrine Changes in Judo Athletes. Int. J. Sports Med. 2006, 27, 9–18. [Google Scholar] [CrossRef]
- Filaire, E.; Maso, F.; Degoutte, F.; Jouanel, P.; Lac, G. Food Restriction, Performance, Psychological State and Lipid Values in Judo Athletes. Int. J. Sports Med. 2001, 22, 454–459. [Google Scholar] [CrossRef]
- Isacco, L.; Degoutte, F.; Ennequin, G.; Pereira, B.; Thivel, D.; Filaire, E. Rapid Weight Loss Influences the Physical, Psychological and Biological Responses during a Simulated Competition in National Judo Athletes. Eur. J. Sport. Sci. 2020, 20, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Isik, O.; Yildirim, I.; Ersoz, Y.; Koca, H.B.; Dogan, I.; Ulutas, E. Monitoring of Pre-Competition Dehydration- Induced Skeletal Muscle Damage and Inflammation Levels among Elite Wrestlers. J. Back Musculoskelet. Rehabil. 2018, 31, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Maynard, D.d.C.; Matos, R.C.; Damasceno, I.C.; Brito, C.J.; Miarka, B.; Grigoletto, M.E.S.; Mendes-Netto, R.S. Low versus adequate carbohydrate diet in Brazilian jiu-jitsu athletes: Comparisons of hormonal biomarkers, physical and psychological. Arch Budo. 2018, 14. [Google Scholar]
- Eghbal Moghanlou, A.; Salehi, V.; Akalan, C.; Akalin, H. The Impact of Fast and Short-Term Weight Loss on the Hormones and Performance of Iranian Young Elite Wrestlers; Unpublished Conference Paper; Atatürk University: Yakutiye, Turkey, October 2019. [Google Scholar]
- Fortes, L.S.; Lira, H.A.A.S.; Mendonça, L.C.V.; Paes, P.P.; Vianna, J.M.; Pérez, A.J. Effect of Body Weight Reduction on Stress and Recovery among Brazilian Judokas. Int. J. Sport. Exerc. Psychol. 2019, 17, 74–84. [Google Scholar] [CrossRef]
- Lukic-Sarkanovic, M.; Roklicer, R.; Trivic, T.; Manojlovic, M.; Gilic, B.; Milovancev, A.; Rossi, C.; Bianco, A.; Carraro, A.; Cvjeticanin, M.; et al. Acute Muscle Damage as a Metabolic Response to Rapid Weight Loss in Wrestlers. Biomed. Hum. Kinet. 2024, 16, 99–105. [Google Scholar] [CrossRef]
- Roklicer, R.; Lakicevic, N.; Stajer, V.; Trivic, T.; Bianco, A.; Mani, D.; Milosevic, Z.; Maksimovic, N.; Paoli, A.; Drid, P. The Effects of Rapid Weight Loss on Skeletal Muscle in Judo Athletes. J. Transl. Med. 2020, 18, 142. [Google Scholar] [CrossRef]
- Yu, L.; Lei, L.; Cheng, L. Influence of Slow and Rapid Weight Loss Periods on Physiological Performance, Mood State and Sleep Quality in Male Freestyle Wrestlers: A Study from Sichuan Province, China. Front. Psychol. 2024, 15, 1445810. [Google Scholar] [CrossRef]
- Palmer, A.K.; Jensen, M.D. Metabolic Changes in Aging Humans: Current Evidence and Therapeutic Strategies. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef] [PubMed]
- Amawi, A.; AlKasasbeh, W.; Jaradat, M.; Almasri, A.; Alobaidi, S.; Hammad, A.A.; Bishtawi, T.; Fataftah, B.; Turk, N.; Saoud, H.A.; et al. Athletes’ Nutritional Demands: A Narrative Review of Nutritional Requirements. Front. Nutr. 2024, 10, 1331854. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.; Rosenbloom, C. Fundamentals of Glycogen Metabolism for Coaches and Athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Wąsacz, W.; Rydzik, Ł.; Ouergui, I.; Koteja, A.; Ambroży, D.; Ambroży, T.; Ruzbarsky, P.; Rzepko, M. Comparison of the Physical Fitness Profile of Muay Thai and Brazilian Jiu-Jitsu Athletes with Reference to Training Experience. Int. J. Environ. Res. Public Health 2022, 19, 8451. [Google Scholar] [CrossRef]
- Andrews, R.R.; Anderson, K.R.; Fry, J.L. Sex-Specific Variation in Metabolic Responses to Diet. Nutrients 2024, 16, 2921. [Google Scholar] [CrossRef]
- Christensen, P.; Meinert Larsen, T.; Westerterp-Plantenga, M.; Macdonald, I.; Martinez, J.A.; Handjiev, S.; Poppitt, S.; Hansen, S.; Ritz, C.; Astrup, A.; et al. Men and Women Respond Differently to Rapid Weight Loss: Metabolic Outcomes of a Multi-centre Intervention Study after a Low-energy Diet in 2500 Overweight, Individuals with Pre-diabetes (PREVIEW). Diabetes Obes. Metab. 2018, 20, 2840–2851. [Google Scholar] [CrossRef]
- Susanto, A.; Burk, J.; Hocking, S.; Markovic, T.; Gill, T. Differences in Weight Loss Outcomes for Males and Females on a Low-Carbohydrate Diet: A Systematic Review. Obes. Res. Clin. Pract. 2022, 16, 447–456. [Google Scholar] [CrossRef]
- Bencker, C.; Gschwandtner, L.; Nayman, S.; Grikšienė, R.; Nguyen, B.; Nater, U.M.; Guennoun, R.; Sundström-Poromaa, I.; Pletzer, B.; Bixo, M.; et al. Progestagens and Progesterone Receptor Modulation: Effects on the Brain, Mood, Stress, and Cognition in Females. Front. Neuroendocrinol. 2025, 76, 101160. [Google Scholar] [CrossRef]
- Sipavičienė, S.; Daniusevičiutė, L.; Klizienė, I.; Kamandulis, S.; Skurvydas, A. Effects of Estrogen Fluctuation during the Menstrual Cycle on the Response to Stretch-Shortening Exercise in Females. Biomed. Res. Int. 2013, 2013, 243572. [Google Scholar] [CrossRef]
- Furrer, R.; Hawley, J.A.; Handschin, C. The Molecular Athlete: Exercise Physiology from Mechanisms to Medals. Physiol. Rev. 2023, 103, 1693–1787. [Google Scholar] [CrossRef]
- Kacprzak, B.; Stańczak, M.; Surmacz, J.; Hagner-Derengowska, M. Biophysics of ACL Injuries. Orthop. Rev. (Pavia) 2024, 16, 126041. [Google Scholar] [CrossRef]
- Anderson, N.; Robinson, D.G.; Verhagen, E.; Fagher, K.; Edouard, P.; Rojas-Valverde, D.; Ahmed, O.H.; Jederström, M.; Usacka, L.; Benoit-Piau, J.; et al. Under-Representation of Women Is Alive and Well in Sport and Exercise Medicine: What It Looks like and What We Can Do about It. BMJ Open Sport. Exerc. Med. 2023, 9, e001606. [Google Scholar] [CrossRef] [PubMed]
- Nuccio, R.P.; Barnes, K.A.; Carter, J.M.; Baker, L.B. Fluid Balance in Team Sport Athletes and the Effect of Hypohydration on Cognitive, Technical, and Physical Performance. Sports Med. 2017, 47, 1951–1982. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Montain, S.J. Fluid and Electrolyte Supplementation for Exercise Heat Stress. Am. J. Clin. Nutr. 2000, 72, 564S–572S. [Google Scholar] [CrossRef]
- Ocana, P.D.; Darabseh, M.Z.; Ishihara, K.; Aburub, A.; Zambolin, F.; Montgomery, G.; Mills, R.; Scorcelletti, M.; Cameron, J.; Ganse, B.; et al. Age-Related Declines in Muscle and Respiratory Function Are Proportionate to Declines in Performance in Master Track Cyclists. Eur. J. Appl. Physiol. 2021, 121, 3447–3457. [Google Scholar] [CrossRef]
- Baskerville, R.; Castell, L.; Bermon, S. Sports and Immunity, from the Recreational to the Elite Athlete. Infect. Dis. Now 2024, 54, 104893. [Google Scholar] [CrossRef]
- Samadi, M.; Bagheri, A.; Pasdar, Y.; Hozoori, M.; Moradi, S.; Karimi, S.; Chaghazardi, M. A Review of High-Risk Rapid Weight Loss Behaviors with Assessment of Food Intake and Anthropometric Measurements in Combat Sport Athletes. Asian J. Sports Med. 2019, in press. [CrossRef]
- Ozkan, I.; Ibrahim, C.H. Dehydration, Skeletal Muscle Damage and Inflammation before the Competitions among the Elite Wrestlers. J. Phys. Ther. Sci. 2016, 28, 162–168. [Google Scholar] [CrossRef]
- Powell, D.J.H.; Liossi, C.; Moss-Morris, R.; Schlotz, W. Unstimulated Cortisol Secretory Activity in Everyday Life and Its Relationship with Fatigue and Chronic Fatigue Syndrome: A Systematic Review and Subset Meta-Analysis. Psychoneuroendocrinology 2013, 38, 2405–2422. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Yamamoto, L.M.; Judelson, D.A.; Farrell, M.J.; Lee, E.C.; Armstrong, L.E.; Casa, D.J.; Kraemer, W.J.; Volek, J.S.; Maresh, C.M. Effects of Hydration State and Resistance Exercise on Markers of Muscle Damage. J. Strength Cond. Res. 2008, 22, 1387–1393. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2016; pp. 603–621. [Google Scholar]
- Jones, C.; Gwenin, C. Cortisol Level Dysregulation and Its Prevalence—Is It Nature’s Alarm Clock? Physiol. Rep. 2021, 8, e14644. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Wideman, L. Exercise and the Cortisol Awakening Response: A Systematic Review. Sports Med. Open 2017, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Boyas, S.; Guével, A. Neuromuscular Fatigue in Healthy Muscle: Underlying Factors and Adaptation Mechanisms. Ann. Phys. Rehabil. Med. 2011, 54, 88–108. [Google Scholar] [CrossRef] [PubMed]
- Milovancev, A.; Ilic, A.; Miljkovic, T.; Petrovic, M.; Stojsic Milosavljevic, A.; Roklicer, R.; Trivic, T.; Manojlovic, M.; Rossi, C.; Bianco, A.; et al. Cardiac Biomarkers Alterations in Rapid Weight Loss and High-Intensity Training in Judo Athletes: A Crossover Pilot Study. J. Sports Med. Phys. Fit. 2024, 64, 1224–1233. [Google Scholar] [CrossRef]
- Ceylan, B.; Aydos, L.; Šimenko, J. Effect of Rapid Weight Loss on Hydration Status and Performance in Elite Judo Athletes. Biology 2022, 11, 500. [Google Scholar] [CrossRef]
- Cleary, M.A.; Sitler, M.R.; Kendrick, Z.V. Dehydration and Symptoms of Delayed-Onset Muscle Soreness in Normothermic Men. J. Athl. Train. 2006, 41, 36–45. [Google Scholar]
- Li, A.; Li, X.; Zhou, T.; Ma, H.; Heianza, Y.; Williamson, D.A.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; Qi, L. Sleep Disturbance and Changes in Energy Intake and Body Composition During Weight Loss in the POUNDS Lost Trial. Diabetes 2022, 71, 934–944. [Google Scholar] [CrossRef]
- Greenlund, I.M.; Carter, J.R. Sympathetic Neural Responses to Sleep Disorders and Insufficiencies. Am. J. Physiol.-Heart Circ. Physiol. 2022, 322, H337–H349. [Google Scholar] [CrossRef]
- Charest, J.; Grandner, M.A. Sleep and Athletic Performance. Sleep Med. Clin. 2020, 15, 41–57. [Google Scholar] [CrossRef]
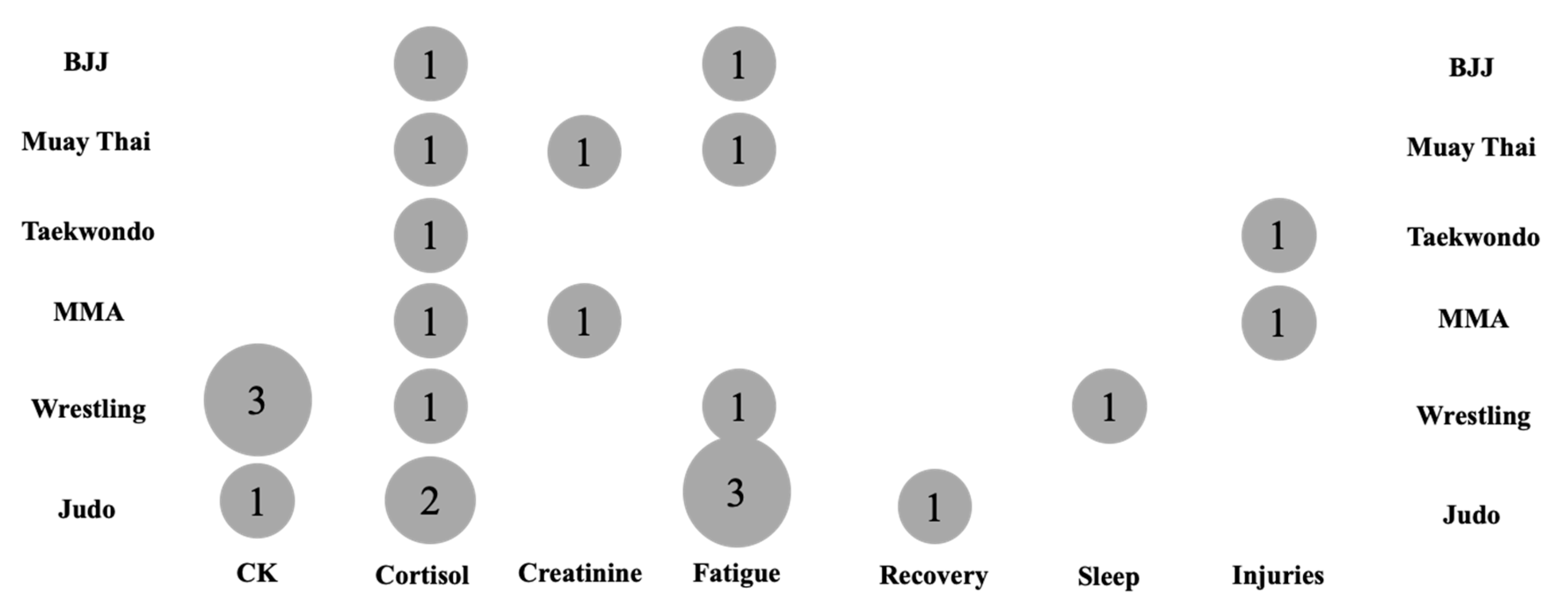
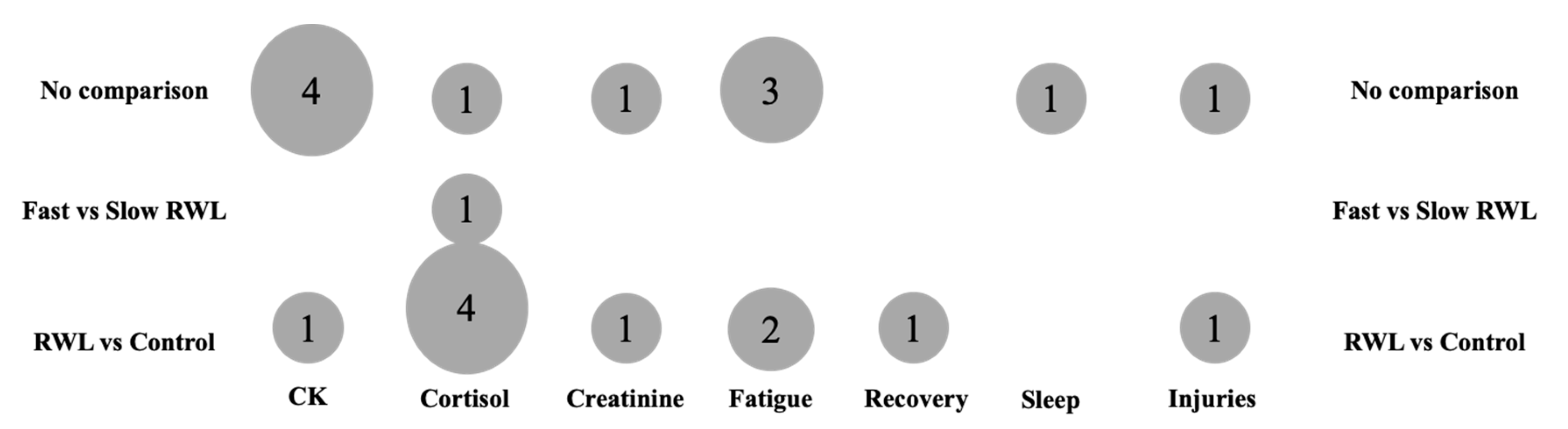
| Study | Outcome | Sex | Group | Baseline (Mean ± SD) | Post-RWL (Mean ± SD) |
|---|---|---|---|---|---|
| [19] | Creatinine (mg/dL) | M | N/A | 1.01 ± 0.10 | 1.03 ± 0.09 |
| F | N/A | 0.93 ± 0.03 | 0.93 ± 0.03 | ||
| [20] | Leg fatigue index (%) | M | N/A | 55.6 ± 4.4 | 60.6 ± 5.0 |
| Arm fatigue index (%) | NR | N/A | 64.9 ± 7.6 | 71.0 ± 8.2 | |
| HRR (Bpm) | NR | N/A | 68.0 ± 1.4 | 169.4 ± 6.9 | |
| [21] | Creatinine (µmol/L) | M | RWL | Pre-match: 69.0 ± 10.0 | Post-match: 79.0 ± 16.0 |
| Control | Pre-match: 101.6 ± 15.0 | Post-match: 142.0 ± 23.0 | |||
| Cortisol (nmol/L) | M | RWL | Pre-match: 499.9 ± 107.8 | Post-match: 731.6 ± 80.2 | |
| Control | Pre-match: 476 ± 184.4 | Post-match: 719.8 ± 125.1 | |||
| [22] | Cortisol (mmol/L) | M | RWL | 438.3 ± 33.7 | 505.9 ± 41.9 |
| Control | 496.4 ± 38.0 | 510.1 ± 44.4 | |||
| Perceived fatigue (A.U.) | M | RWL | 41.8 ± 0.9 | 51.3 ± 2.0 | |
| Control | 42.5 ± 1.7 | 51.3 ± 3.3 | |||
| [23] | Perceived fatigue (A.U.) | M | N/A | 47.1 ± 4.3 | 51.4 ± 5.0 |
| [28] | Perceived recovery (A.U.) | M | RWL | 101.40 ± 2.52 | 87.63 ± 2.47 |
| Control | 100.97 ± 2.80 | 109.30 ± 2.71 | |||
| [24] | Cortisol | M | Weight loss | 603.2 ± 146.8 | 505.8 ± 118.4 |
| Weight stable | 535.6 ± 101.2 | 510.1 ± 140.5 | |||
| Perceived fatigue (A.U.) | M | Weight loss | 47.0 ± 5.0 | 53.0 ± 9.0 | |
| Weight stable | 46.0 ± 6.0 | 50.0 ± 5.0 | |||
| [25] | CK (U/L) | M | Weight loss | 158.3 ± 61.6 | 239.6 ± 115.7 |
| Non-weight loss | 120.1 ± 20.3 | 151.3 ± 33.6 | |||
| [29] | CK (U/L) | M | N/A | 168.9 ± 51.3 | 713.4 ± 194.6 |
| [26] | Cortisol (NR) | M | L-CHO | 13.5 ± 3.7 | 12.5 ± 4.3 |
| A-CHO | 13.5 ± 1.9 | 12.1 ± 2.1 | |||
| [27] | Cortisol (pg/mL) | M | Fast weight loss | 116.9 ± 48.5 | 150.2 ± 43.1 |
| Short-term weight loss | 135.0 ± 48.9 | 154.8 ± 46.7 | |||
| [9] | Perceived fatigue (A.U.) | M | N/A | 5.4 ± 1.5 | 11.8 ± 2.1 |
| [30] | CK (U/L) | M | N/A | 145.9 ± 64.8 | 386.8 ± 94.8 |
| [16] | Cortisol (ng /mL) | F | Weight loss | 53.7 ± 10.1 | 55.1 ± 10.4 |
| Non-weight loss | 45.1 ± 8.1 | 55.1 ± 8.1 | |||
| [31] | PSQI score (%) | M | N/A | 5.15 ± 1.83 | 5.52 ± 1.71 |
| Perceived fatigue (A.U.) | M | N/A | 10.0 ± 2.0 | 9.9 ± 2.9 | |
| CK (U/L) | M | N/A | 252.2 ± 61.0 | 263.2 ± 96.9 |
| Study | Outcome | Sex | Predictor/Group | Metric | Value (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| [18] | Injury status | M | RWL−24 h (%) | Odds Ratio | 1.19 (1.00–1.42) | 0.046 |
| F | Odds Ratio | 0.86 (0.68–1.10) | 0.231 | |||
| M | RWL−7 days (%) | Odds Ratio | 1.07 (0.97–1.18) | 0.198 | ||
| F | Odds Ratio | 0.92 (0.80–1.06) | 0.249 | |||
| [17] | Injury rate (%) | M | Weight loss period | Injuries/1000 AEs | 22.71 (17.41–29.11) | <0.001 |
| F | Weight loss period | Injuries/1000 AEs | 45.62 (37.16–55.43) | <0.001 | ||
| M | Normal Training | Injuries/1000 AEs | 10.86 (9.64–12.19) | <0.001 | ||
| F | Normal Training | Injuries/1000 AEs | 17.64 (15.94–19.46) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kużdżał, A.; Bilianskyi, O.; Wroński, Z.; Magoń, G.; Olaniszyn, G.; Hagner-Derengowska, M.; Michalska, A. Effects of Weight-Cutting Practices on Sleep, Recovery, and Injury in Combat Sports: A Scoping Review. J. Funct. Morphol. Kinesiol. 2025, 10, 319. https://doi.org/10.3390/jfmk10030319
Kużdżał A, Bilianskyi O, Wroński Z, Magoń G, Olaniszyn G, Hagner-Derengowska M, Michalska A. Effects of Weight-Cutting Practices on Sleep, Recovery, and Injury in Combat Sports: A Scoping Review. Journal of Functional Morphology and Kinesiology. 2025; 10(3):319. https://doi.org/10.3390/jfmk10030319
Chicago/Turabian StyleKużdżał, Adrian, Oleg Bilianskyi, Zbigniew Wroński, Grzegorz Magoń, Gracjan Olaniszyn, Magdalena Hagner-Derengowska, and Anna Michalska. 2025. "Effects of Weight-Cutting Practices on Sleep, Recovery, and Injury in Combat Sports: A Scoping Review" Journal of Functional Morphology and Kinesiology 10, no. 3: 319. https://doi.org/10.3390/jfmk10030319
APA StyleKużdżał, A., Bilianskyi, O., Wroński, Z., Magoń, G., Olaniszyn, G., Hagner-Derengowska, M., & Michalska, A. (2025). Effects of Weight-Cutting Practices on Sleep, Recovery, and Injury in Combat Sports: A Scoping Review. Journal of Functional Morphology and Kinesiology, 10(3), 319. https://doi.org/10.3390/jfmk10030319






