A Fully Replicable Exercise Program for Individuals with Sleep-Disordered Breathing: Protocol Design and Training Load Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants and Procedures
2.3. Exercise Program
- (i)
- Upper body push:
- −
- Resistance band chest press. Performed standing with a resistance band anchored behind, pushing forward to activate the chest and triceps.
- −
- Wall push-up. A beginner-friendly push-up variation performed against the wall to develop upper body strength with minimal load.
- −
- Dumbbell chest press on a Bosu ball. Lying on a Bosu ball, pressing dumbbells upward, to strengthen the chest and improve core stability.
- −
- Knee push-up. A modified push-up, performed with knees on the ground to reduce body weight load while training the chest and arms.
- −
- Triceps dip on a chair. Using a stable chair, lower and raise the body with arms to work the triceps and shoulders.
- −
- Standard push-up. A full-body push-up from plank position, strengthening chest, shoulders, arms, and core.
- (ii)
- Upper body pull:
- −
- Renegade row with dumbbells. Performed from a plank position by pulling one dumbbell toward the chest while stabilizing the body with the opposite arm, targeting back, shoulders, and core.
- −
- Seated resistance band row. Sitting on the floor with legs extended, pull resistance bands toward the torso, engaging the back and biceps.
- −
- Standing resistance band row. While standing, pull a resistance band anchored in front of the body toward the chest, emphasizing scapular retraction and engaging the upper back and biceps.
- −
- Bent-over row with resistance band. Performed in a hinged position, pull the band handles toward the waist while keeping the back straight and elbows close, strengthening the upper back and lats.
- −
- Upright row with resistance band. Standing on the band, pull it upward toward the collarbones with elbows high to target the shoulders and traps.
- (iii)
- Knee-dominant:
- −
- Resistance band squat walk. Performed in a squat position while taking small lateral steps with a resistance band around the thighs to activate the glutes and quads.
- −
- Step-up with dumbbells. Involves stepping onto a raised surface with one leg while holding dumbbells, then returning to the starting position to strengthen the quadriceps and glutes.
- −
- Bosu ball squat. Squat on a Bosu ball to develop balance and lower body strength, focusing on the knees and core.
- −
- Forward lunge. Step forward and lower the back knee toward the ground, emphasizing knee flexion and muscle engagement in the front leg.
- −
- Bulgarian split squat. With the rear foot elevated, lower the body into a single-leg squat to target the quads and improve unilateral strength and stability.
- (iv)
- Hip-dominant:
- −
- Resistance band deadlift. With feet on the band, hinge at the hips to lift against the resistance, engaging glutes and hamstrings.
- −
- Banded sumo deadlift. A wide-stance deadlift using resistance bands to target the posterior chain, especially the glutes.
- −
- Glute bridge. Lying on the floor, lifting hips through glute activation, can be performed with both feet planted or with one leg extended to increase difficulty.
- −
- Single-leg Romanian deadlift with dumbbells. While balancing on one leg, hinge at the hips and lower the dumbbells. Targeting the hamstrings and glutes while enhancing balance, and posterior chain stability.
- −
- Standing hip extension with band. Push one leg backward against the resistance band to isolate glute activation.
- −
- Bosu ball hamstring bridge. Lying down with heels on a Bosu ball, lift hips to strengthen the hamstrings and glutes.
- (v)
- Core-specific:
- −
- Side crunch on Bosu ball. Lying sideways on a Bosu ball, perform crunches to activate the obliques through lateral trunk flexion.
- −
- Side plank with crunch. From a side plank position, bring the top elbow and knee together to engage the obliques and hip stabilizers.
- −
- Mountain climber on Bosu. In a plank position with hands on a Bosu ball, alternate driving knees toward the chest to engage the core through dynamic movement.
- −
- Plank on Bosu ball. Hold a forearm plank with elbows on a Bosu ball to challenge core stability and shoulder control.
- −
- Medicine ball woodchopper. Perform a rotational movement from high to low with a medicine ball to activate the entire core, especially the obliques.
- −
- Plank on stability ball. Place forearms on a stability ball while holding a plank position, engaging deep abdominal muscles for balance and control.
- (vi)
- Medicine ball throw:
- −
- Medicine ball rotational throw. Rotate the torso and explosively throw the medicine ball sideways against a wall to train rotational power.
- −
- Medicine ball overhead throw. Squat down holding the ball, then extend the hips and arms to throw it vertically overhead, targeting total-body power.
- −
- Medicine ball forward throw. Raise the ball overhead and throw it straight forward, engaging the core and upper body in an explosive motion.
- −
- Medicine ball scoop toss. From a squat position, explosively extend and toss the ball upward in a scooping motion to train hip and arm coordination.
- (vii)
- Aerobic-specific exercises:
- −
- Basic step-up. Step up and down on a platform repeatedly to elevate heart rate and improve lower-body endurance.
- −
- Jumping jacks. Perform full-body jumping movements by spreading the legs and raising the arms overhead, then returning to the starting position to promote cardiovascular fitness and coordination.
- −
- High knees. Jog in place while lifting the knees as high as possible to enhance aerobic capacity and coordination.
- −
- Step jump. Jump onto and off a raised platform in a continuous motion to improve power and cardiovascular fitness.
- −
- Resisted running with a band. One partner runs forward while the other holds a resistance band to increase sprint effort and conditioning.
2.4. Planning of Training Volume, Intensity, and Load
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHI | Apnea–Hypopnea Index |
| BMI | Body Mass Index |
| CERT | Consensus on Exercise Reporting Template |
| CI | Confidence interval |
| ES | Effect sizes |
| HR | Heart rate |
| HRQoL | Health-Related Quality of Life |
| OSA | Obstructive Sleep Apnea |
| RCT | Randomized Controlled Trial |
| RPE | Rating of Perceived Exertion |
| SDB | Sleep-Disordered Breathing |
| TRIMP | Training Impulse |
References
- Lobelo, F.; Stoutenberg, M.; Hutber, A. The Exercise is Medicine Global Health Initiative: A 2014 update. Br. J. Sports Med. 2014, 48, 1627–1633. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/336656 (accessed on 4 August 2025).
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Kraus, W.; Powell, K.; Haskell, W.; Janz, K.; Campbell, W.; Jakicic, J.; Troiano, R.; Sprow, K.; Torres, A.; Piercy, K.L. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med. Sci. Sports Exerc. 2019, 51, 1270–1281. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Buchner, D.M.; Jakicic, J.M.; Powell, K.E.; Kraus, W.E.; Bloodgood, B.; Campbell, W.W.; Dietz, S.; Dipietro, L.; George, S.M.; et al. Physical activity to prevent and treat hypertension: A systematic review. Med. Sci. Sports Exerc. 2019, 51, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Crippa, A.; Woodcock, J.; Brage, S. Physical activity and incident type 2 diabetes mellitus: A systematic review and dose–response meta-analysis of prospective cohort studies. Diabetologia 2016, 59, 2527–2545. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical activity in cancer prevention and survival: A systematic review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Núñez-Cortés, R.; Salazar-Méndez, J.; Nijs, J. Physical activity as a central pillar of lifestyle modification in the management of chronic musculoskeletal pain: A narrative review. J. Funct. Morphol. Kinesiol. 2023, 8, 183. [Google Scholar] [CrossRef]
- Singh, B.; Olds, T.; Curtis, R.; Dumuid, D.; Virgara, R.; Watson, A.; Szeto, K.; O’Connor, E.; Ferguson, T.; Eglitis, E.; et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: An overview of systematic reviews. Br. J. Sports Med. 2023, 57, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Gregson, J.; Duarte, K.; Gueyffier, F.; Rossignol, P.; Zannad, F.; Pocock, S. Individualizing treatment choices in the systolic blood pressure intervention trial. J. Hypertens. 2018, 36, 428–435. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, Y.; Zhao, Y.; Ren, H. Effects of exercise on patients with obstructive sleep apnea: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 10845. [Google Scholar] [CrossRef] [PubMed]
- Randerath, W.; de Lange, J.; Hedner, J.; Ho, J.P.T.F.; Marklund, M.; Schiza, S.; Steier, J.; Verbraecken, J. Current and novel treatment options for obstructive sleep apnoea. ERJ Open Res. 2022, 8, 00126–02022. [Google Scholar] [CrossRef]
- Randerath, W.; Verbraecken, J.; de Raaff, C.A.L.; Hedner, J.; Herkenrath, S.; Hohenhorst, W.; Jakob, T.; Marrone, O.; Marklund, M.; McNicholas, W.T.; et al. European respiratory society guideline on non-CPAP therapies for obstructive sleep apnoea. Eur. Respir. Rev. 2021, 30, 210200. [Google Scholar] [CrossRef]
- Araújo, C.E.L.; Ferreira-Silva, R.; Gara, E.M.; Goya, T.T.; Guerra, R.S.; Matheus, L.; Toschi-Dias, E.; Rodrigues, A.G.; Barbosa, E.R.F.; Fazan, R., Jr.; et al. Effects of exercise training on autonomic modulation and mood symptoms in patients with obstructive sleep apnea. Braz. J. Med. Biol. Res. 2021, 54, e10543. [Google Scholar] [CrossRef]
- Berger, M.; Barthélémy, J.-C.; Hupin, D.; Raffin, J.; Dupré, C.; Labeix, P.; Costes, F.; Gaspoz, J.-M.; Roche, F. Benefits of supervised community physical activity in obstructive sleep apnoea. Eur. Respir. J. 2018, 52, 1801592. [Google Scholar] [CrossRef] [PubMed]
- Fridgeirsdottir, K.Y.; Murphy, C.J.; Islind, A.S.; Árnadóttir, B.S.; Hrubos Strøm, H.; Arnardottir, E.S.; Saavedra, J.M. Effects of exercise and a lifestyle app on sleep disordered breathing, physical health, and quality of life. ERJ Open Res. 2025, 11, 01134–02024. [Google Scholar] [CrossRef]
- Goya, T.T.; Ferreira-Silva, R.; Macedo Gara, E.; Guerra, R.S.; Barbosa, E.R.F.; Toschi-Dias, E.; Cunha, P.J.; Negrão, C.E.; Lorenzi-Filho, G.; Ueno-Pardi, L.M. Exercise training reduces sympathetic nerve activity and improves executive performance in individuals with obstructive sleep apnea. Clinics 2021, 76, e2786. [Google Scholar] [CrossRef]
- Guerra, R.S.; Goya, T.T.; Silva, R.F.; Lima, M.F.; Barbosa, E.R.F.; Alves, M.J.D.N.N.; Rodrigues, A.G.; Lorenzi-Filho, G.; Negrão, C.E.; Ueno-Pardi, L.M. Exercise training increases metaboreflex control in patients with obstructive sleep apnea. Med. Sci. Sports Exerc. 2019, 51, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Jurado-García, A.; Molina-Recio, G.; Feu-Collado, N.; Palomares-Muriana, A.; Gómez-González, A.M.; Márquez-Pérez, F.L.; Jurado-Gamez, B. Effect of a graduated walking program on the severity of obstructive sleep apnea syndrome: A randomized clinical trial. Int. J. Environ. Res. Public Health 2020, 17, 6334. [Google Scholar] [CrossRef] [PubMed]
- Lins-Filho, O.; Germano-Soares, A.H.; Aguiar, J.L.P.; de Almedia, J.R.V.; Felinto, E.C.; Lyra, M.J.; Leite, D.B.; Moura, M.A.S.; Kline, C.E.; Pedrosa, R.P. Effect of high-intensity interval training on obstructive sleep apnea severity: A randomized controlled trial. Sleep Med. 2023, 112, 316–321. [Google Scholar] [CrossRef]
- Lins-Filho, O.L.; Pedrosa, R.P.; Gomes, J.M.L.; Moraes, S.L.D.; Vasconcelos, B.C.E.; Lemos, C.A.A.; Pellizzer, E.P. Effect of exercise training on subjective parameters in patients with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. 2020, 69, 1–7. [Google Scholar] [CrossRef]
- Servantes, D.M.; Javaheri, S.; Kravchychyn, A.C.P.; Storti, L.J.; Almeida, D.R.; de Mello, M.T.; Cintra, F.D.; Tufik, S.; Bittencourt, L. Effects of exercise training and CPAP in patients with heart failure and OSA: A preliminary study. Chest 2018, 154, 808–817. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Sakkas, G.K.; Hadjigeorgiou, G.M.; Manconi, M.; Bargiotas, P. Unfolding the role of exercise in the management of sleep disorders. Eur. J. Appl. Physiol. 2024, 124, 2547–2560. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.I.; Viljoen, W.; Bosch, A.N.; Pearce, A.J.; Sayers, M. General principles of training. In Olympic Textbook of Medicine in Sport, 3rd ed.; Schwellnus, M.P., Ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 1–18. [Google Scholar]
- Bompa, T.O.; Buzzichelli, C. Periodization: Theory and Methodology of Training, 6th ed.; Human Kinetics: Champaign, IL, USA, 2019. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Wollesen, B.; Herden, M.; Lamberti, N.; Giannaki, C.D. Defining and reporting exercise intensity in interventions for older adults: A modified Delphi process. Eur. Rev. Aging Phys. Act. 2024, 21, 3. [Google Scholar] [CrossRef]
- Papale, O.; Festino, E.; Di Rocco, F.; Foster, C.; Prestanati, I.; Serafini, S.; Izzicupo, P.; Cortis, C.; Fusco, A. The impact of a multidimensional physical activity intervention on glycemic control in type 1 diabetes: A preliminary study. J. Funct. Morphol. Kinesiol. 2023, 8, 163. [Google Scholar] [CrossRef]
- Mujika, I. Intense training: The key to optimal performance before and during the taper. Scand. J. Med. Sci. Sports 2010, 20 (Suppl. S2), 24–31. [Google Scholar] [CrossRef]
- Halson, S.L. Monitoring training load to understand fatigue in athletes. Sports Med. 2014, 44 (Suppl. S2), S139–S147. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A.V. Physical Performance and Perceived Exertion; C.W.K. Gleerup: Lund, Sweden, 1962. [Google Scholar]
- Bordoli, C.; Varley, I.; Sharpe, G.R.; Johnson, M.A.; Hennis, P.J. Effects of oral lactate supplementation on acid–base balance and prolonged high-intensity interval cycling performance. J. Funct. Morphol. Kinesiol. 2023, 8, 139. [Google Scholar] [CrossRef]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshall, P.; Dodge, C. A new approach to monitoring exercise training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar] [CrossRef]
- Milani, J.G.P.O.; Milani, M.; Verboven, K.; Cipriano, G., Jr.; Hansen, D. Exercise intensity prescription in cardiovascular rehabilitation: Bridging the gap between best evidence and clinical practice. Front. Cardiovasc. Med. 2024, 11, 1380639. [Google Scholar] [CrossRef] [PubMed]
- Agner, V.F.C.; Garcia, M.C.; Taffarel, A.A.; Mourão, C.B.; da Silva, I.P.; da Silva, S.P.; Peccin, M.S.; Lombardi, I., Jr. Effects of concurrent training on muscle strength in older adults with metabolic syndrome: A randomized controlled clinical trial. Arch. Gerontol. Geriatr. 2018, 75, 158–164. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Kristjánsdóttir, H.; Gunnarsson, S.B.; García Hermoso, A. Effects of 2 physical exercise programs (circuit training and brisk walk) carried out during working hours on multidimensional components of workers’ health: A pilot study. Int. J. Occup. Med. Environ. Health 2021, 34, 39–51. [Google Scholar] [CrossRef]
- Timmons, J.F.; Minnock, D.; Hone, M.; Cogan, K.E.; Murphy, J.C.; Egan, B. Comparison of time-matched aerobic, resistance, or concurrent exercise training in older adults. Scand. J. Med. Sci. Sports 2018, 28, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.; Hope, S. Advanced Circuit Training: A Complete Guide to Progressive Planning for Fitness Training, 3rd ed.; A & C Black: London, UK, 2008. [Google Scholar]
- Barnett, A. Using recovery modalities between training sessions in elite athletes: Does it help? Sports Med. 2006, 36, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Banister, E.W.; Calvert, T.W.; Savage, M.V.; Bach, T. A systems model of training for athletic performance. Aust. J. Sports Med. 1975, 7, 57–61. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Ioannidis, J.P.A.; Greenland, S.; Hatlay, M.A.; Khoury, M.J.; Macleod, M.R.; Moher, D.; Schulz, K.F.; Tibshirani, R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014, 383, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Slade, S.C.; Finnegan, S.; Dionne, C.E.; Underwood, M.; Buchbinder, R. The Consensus on Exercise Reporting Template (CERT) applied to exercise interventions in musculoskeletal trials demonstrated good rater agreement and incomplete reporting. J. Clin. Epidemiol. 2018, 103, 120–130. [Google Scholar] [CrossRef]
- Hacke, C.; Schreiber, J.; Weisser, B. Application of the Templates TIDieR and CERT reveal incomplete reporting and poor replicability of exercise interventions for type 2 diabetes mellitus. Curr. Diabetes Rev. 2022, 18, e250821195838. [Google Scholar] [CrossRef]
- Raje, U.; Saumur, T.M.; Pesce de Souza, F.; Mathur, S.; Janaudis-Ferreira, T. Quality of the reporting of exercise interventions in solid organ transplant recipients: A systematic review. McGill J. Med. 2021, 19, 24. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Huang, L.; Bai, Y.; Wang, W.; Zhang, H. Effect of exercise interventions for sleep quality in patients. A systematic review and meta-analysis. Int. Urol. Nephrol. 2023, 55, 1193–1204. [Google Scholar] [CrossRef]
- Hansford, H.J.; Wewege, M.A.; Cashin, A.G.; Hagstrom, A.D.; Clifford, B.K.; McAuley, J.H.; Jones, M.D. If exercise is medicine, why don’t we know the dose? An overview of systematic reviews assessing reporting quality of exercise interventions in health and disease. Br. J. Sports Med. 2022, 56, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.E.; Crowley, E.P.; Ewing, G.B.; Burch, J.B.; Blair, S.N.; Dunsire, J.L.; Davis, J.M.; Youngstedt, S.D. The effect of exercise training on obstructive sleep apnea and sleep quality: A randomized controlled trial. Sleep 2011, 34, 1631–1640. [Google Scholar] [CrossRef]
- Mujika, I. The alphabet of sport science research starts with Q. Int. J. Sports Physiol. Perform. 2013, 8, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Gil-Moreno, G.; Palmi, J.; Prat-Subirana, J.A. Assessment of the subjective perception of fatigue in competition motorcyclists Rally-Raid Dakar. Accion Psicol. 2017, 14, 93–104. [Google Scholar] [CrossRef]
- Kong, Y.; Yu, B.; Guan, G.; Wang, Y.; He, H. Effects of sleep deprivation on sports performance and perceived exertion in athletes and non-athletes: A systematic review and meta-analysis. Front. Physiol. 2025, 16, 1544286. [Google Scholar] [CrossRef] [PubMed]
- Viana, B.F.; Pires, F.O.; Inoue, A.; Micklewright, D.; Santos, T.M. Correlates of mood and RPE during multi-lap off-road cycling. Appl. Psychophysiol. Biofeedback 2016, 41, 1–7. [Google Scholar] [CrossRef]
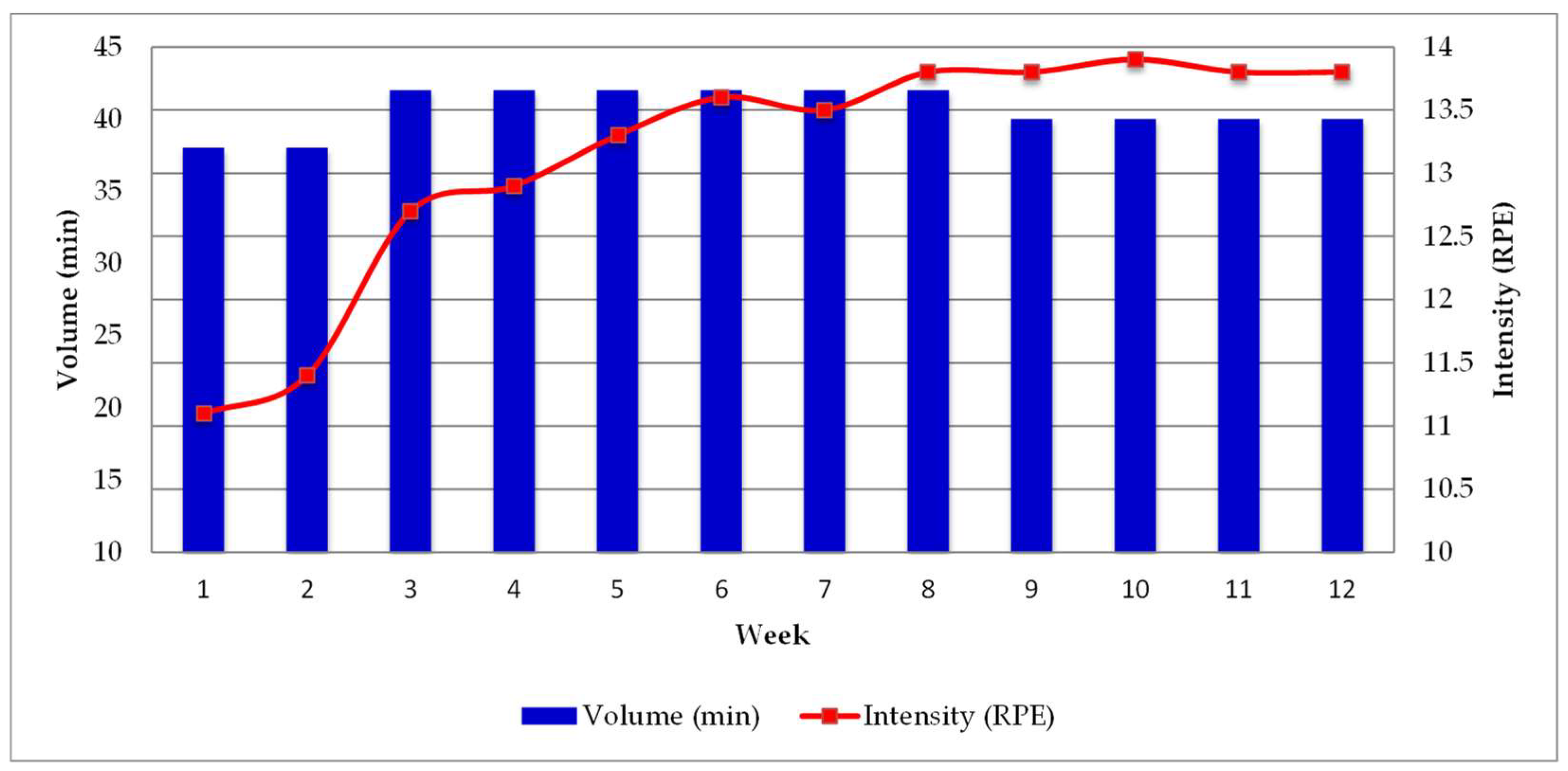
| Volume (min) | Intensity (RPE) | Training Load (Volume × Intensity) (AU) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | WU | CC | BW | CD | Total | WU | CC | BW | CD | Total | |
| 1–2 | 10 | 10 | 8 | 10 | 38 | 8 | 14 | 12 | 7 | 10.2 | 386.0 |
| 3–4 | 10 | 12 | 10 | 10 | 42 | 9 | 15 | 12 | 7 | 12.1 | 508.4 |
| 5–8 | 8 | 14 | 12 | 8 | 42 | 9 | 16 | 13 | 7 | 13.4 | 561.5 |
| 9–12 | 5 | 16 | 14 | 5 | 40 | 9 | 18 | 13 | 7 | 14.5 | 579.0 |
| Planned Intensity (RPE) | Performed Intensity (RPE) | Diff. (%) | Planned TL (UA) | Performed TL (UA) | Diff. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | WP | CC | BW | CD | Total | WP | CC | BW | CD | Total | ||||
| 1–2 | 8 | 14 | 12 | 7 | 10.2 | 9.4 | 15.3 | 10.9 | 9.5 | 11.3 | 10.8 | 386.0 | 428.9 | 11.1 |
| 3–4 | 9 | 15 | 12 | 7 | 12.1 | 9.2 | 15.3 | 11.9 | 9.1 | 12.8 | 5.8 | 508.4 | 536.5 | 5.5 |
| 5–8 | 9 | 16 | 13 | 7 | 13.4 | 9.3 | 15.9 | 12.4 | 8.8 | 13.6 | 1.5 | 561.5 | 570.4 | 1.6 |
| 9–12 | 9 | 18 | 13 | 7 | 14.5 | 9.1 | 16.8 | 11.8 | 8.7 | 13.8 | −4.8 | 579.0 | 550.4 | −4.9 |
| Mean | 8.8 | 15.8 | 12.5 | 7 | 12.5 | 9.2 | 15.8 | 11.7 | 9.0 | 12.9 | 3.2 | 508.7 | 521.5 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saavedra, J.M.; Fridgeirsdottir, K.Y.; Murphy, C.J.; Hrubos-Strøm, H.; Arnardottir, E.S. A Fully Replicable Exercise Program for Individuals with Sleep-Disordered Breathing: Protocol Design and Training Load Monitoring. J. Funct. Morphol. Kinesiol. 2025, 10, 311. https://doi.org/10.3390/jfmk10030311
Saavedra JM, Fridgeirsdottir KY, Murphy CJ, Hrubos-Strøm H, Arnardottir ES. A Fully Replicable Exercise Program for Individuals with Sleep-Disordered Breathing: Protocol Design and Training Load Monitoring. Journal of Functional Morphology and Kinesiology. 2025; 10(3):311. https://doi.org/10.3390/jfmk10030311
Chicago/Turabian StyleSaavedra, Jose M., Katrin Y. Fridgeirsdottir, Conor J. Murphy, Harald Hrubos-Strøm, and Erna S. Arnardottir. 2025. "A Fully Replicable Exercise Program for Individuals with Sleep-Disordered Breathing: Protocol Design and Training Load Monitoring" Journal of Functional Morphology and Kinesiology 10, no. 3: 311. https://doi.org/10.3390/jfmk10030311
APA StyleSaavedra, J. M., Fridgeirsdottir, K. Y., Murphy, C. J., Hrubos-Strøm, H., & Arnardottir, E. S. (2025). A Fully Replicable Exercise Program for Individuals with Sleep-Disordered Breathing: Protocol Design and Training Load Monitoring. Journal of Functional Morphology and Kinesiology, 10(3), 311. https://doi.org/10.3390/jfmk10030311








