Can Plantar Pressure Distribution During Gait Be Estimated from Quiet Stance in Healthy Individuals?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Assessment Protocol
2.3. Plantar Pressure Evaluation
2.4. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. CoP Sway and Total Plantar Pressure Distribution During Quiet Stance
3.3. Spatial-Temporal Variables of Gait and Total Plantar Pressure Distribution
3.4. Peak Pressures of the Foot Areas During Quiet Stance and Gait
3.5. Differences in Peak Pressures of the Foot Areas Between Gait and Quiet Stance
3.6. Relationships Between Quiet Stance, Gait, and BMI
4. Discussion
4.1. Plantar Pressures During Quiet Stance
4.2. Plantar Pressures During Gait
4.3. Relationship Between Quiet Stance and Gait
4.4. Comparison with Literature Findings
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CoP | Center of pressure |
| CoM | Center of mass |
| EO | Eyes open |
| kPa | Kilopascal |
| T1 | Big toe |
| T2,3,4,5 | Toes 2 to 5 |
| M1 | Metatarsal 1 |
| M2 | Metatarsal 2 |
| M3 | Metatarsal 3 |
| M4 | Metatarsal 4 |
| M5 | Metatarsal 5 |
| MF | Midfoot |
| MH | Medial half of the heel |
| LH | Lateral half of the heel |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| BMI | Body mass index |
References
- Zulkifli, S.S.; Loh, W.P. A state-of-the-art review of foot pressure. Foot Ankle Surg. 2020, 26, 25–32. [Google Scholar] [CrossRef]
- Cleland, L.D.; Rowland, H.M.; Mazzà, C.; Saal, H.P. Complexity of spatio-temporal plantar pressure patterns during everyday behaviours. J. R. Soc. Interface 2023, 20, 20230052. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, M.W.; McPoil, T.G. Velocity of the center of pressure during walking. J. Am. Podiatr. Med. Assoc. 2000, 90, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A.; Patla, A.E.; Frank, J.S. Assessment of balance control in humans. Med. Prog. Technol. 1990, 16, 31–51. [Google Scholar]
- Winter, D.A. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Orlin, M.N.; McPoil, T.G. Plantar pressure assessment. Phys. Ther. 2000, 80, 399–409. [Google Scholar] [CrossRef]
- Morton, D.J. Structural factors in static disorders of the foot. Am. J. Surg. 1930, 9, 315–328. [Google Scholar] [CrossRef]
- Boulton, A.J.; Hardisty, C.A.; Betts, R.P.; Franks, C.I.; Worth, R.C.; Ward, J.D.; Duckworth, T. Dynamic foot pressure and other studies as diagnostic and management aids in diabetic neuropathy. Diabetes Care 1983, 6, 26–33. [Google Scholar] [CrossRef]
- Vossen, L.E.; Bus, S.A.; Van Netten, J.J. Unlocking the multidimensionality of plantar pressure measurements for the evaluation of footwear in people with diabetes. J. Biomech. 2025, 180, 112502. [Google Scholar] [CrossRef]
- Jandova, S.; Pazour, J.; Janura, M. Comparison of Plantar Pressure Distribution During Walking After Two Different Surgical Treatments for Calcaneal Fracture. J. Foot Ankle Surg. 2019, 58, 260–265. [Google Scholar] [CrossRef]
- Dürr, C.; Apinun, J.; Mittlmeier, T.; Rammelt, S. Foot Function After Surgically Treated Intraarticular Calcaneal Fractures: Correlation of Clinical and Pedobarographic Results of 65 Patients Followed for 8 Years. J. Orthop. Trauma 2018, 32, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Mirando, M.; Conti, C.; Zeni, F.; Pedicini, F.; Nardone, A.; Pavese, C. Gait Alterations in Adults after Ankle Fracture: A Systematic Review. Diagnostics 2022, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Crosbie, J.; Hunt, A.; Ouvrier, R. The effect of pes cavus on foot pain and plantar pressure. Clin. Biomech. 2005, 20, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Seguín, L.M.; Diaz Mancha, J.A.; Sánchez Rodríguez, R.; Escamilla Martínez, E.; Gómez Martín, B.; Ramos Ortega, J. Comparison of plantar pressures and contact area between normal and cavus foot. Gait Posture 2014, 39, 789–792. [Google Scholar] [CrossRef]
- Caselli, A.; Pham, H.; Giurini, J.M.; Armstrong, D.G.; Veves, A. The forefoot-to-rearfoot plantar pressure ratio is increased in severe diabetic neuropathy and can predict foot ulceration. Diabetes Care 2002, 25, 1066–1071. [Google Scholar] [CrossRef]
- Periyasamy, R.; Anand, S. The effect of foot arch on plantar pressure distribution during standing. J. Med. Eng. Technol. 2013, 37, 342–347. [Google Scholar] [CrossRef]
- Perry, J.M.; Slac Thorofare, K.; Davids, J.R. Gait Analysis: Normal and Pathological Function. J. Pediatr. Orthop. 1992, 12, 815. [Google Scholar] [CrossRef]
- Han, T.R.; Paik, N.J.; Im, M.S. Quantification of the path of center of pressure (COP) using an F-scan in-shoe transducer. Gait Posture 1999, 10, 248–254. [Google Scholar] [CrossRef]
- Hughes, J.; Pratt, L.; Linge, K.; Clark, P.; Klenerman, L. Reliability of pressure measurements: The EM ED F system. Clin. Biomech. 1991, 6, 14–18. [Google Scholar] [CrossRef]
- Kelly, L.A.; Kuitunen, S.; Racinais, S.; Cresswell, A.G. Recruitment of the plantar intrinsic foot muscles with increasing postural demand. Clin. Biomech. 2012, 27, 46–51. [Google Scholar] [CrossRef]
- Schieppati, M.; Nardone, A.; Siliotto, R.; Grasso, M. Early and late stretch responses of human foot muscles induced by perturbation of stance. Exp. Brain Res. 1995, 105, 411–422. [Google Scholar] [CrossRef]
- Drăgulinescu, A.; Drăgulinescu, A.M.; Zincă, G.; Bucur, D.; Feieș, V.; Neagu, D.M. Smart Socks and In-Shoe Systems: State-of-the-Art for Two Popular Technologies for Foot Motion Analysis, Sports, and Medical Applications. Sensors 2020, 20, 4316. [Google Scholar] [CrossRef]
- Merry, K.; MacPherson, M.; Macdonald, E.; Ryan, M.; Park, E.J.; Sparrey, C.J. Differentiating Sitting, Standing, and Walking Through Regional Plantar Pressure Characteristics. J. Biomech. Eng. 2020, 142. [Google Scholar] [CrossRef]
- Penati, R.; Sozzi, S.; Mirando, M.; Nardone, A. Qualitative and quantitative differences in plantar pressure distribution during quiet stance and gait in healthy individuals. In Proceedings of the Congresso SIAMOC, online, 30 September 2021; p. 86. [Google Scholar]
- Vasileiou, K.; Barnett, J.; Thorpe, S.; Young, T. Characterising and justifying sample size sufficiency in interview-based studies: Systematic analysis of qualitative health research over a 15-year period. BMC Med. Res. Methodol. 2018, 18, 148. [Google Scholar] [CrossRef]
- De Blasiis, P.; Caravaggi, P.; Fullin, A.; Leardini, A.; Lucariello, A.; Perna, A.; Guerra, G.; De Luca, A. Postural stability and plantar pressure parameters in healthy subjects: Variability, correlation analysis and differences under open and closed eye conditions. Front. Bioeng. Biotechnol. 2023, 11, 1198120. [Google Scholar] [CrossRef]
- Fullin, A.; Caravaggi, P.; Picerno, P.; Mosca, M.; Caravelli, S.; De Luca, A.; Lucariello, A.; De Blasiis, P. Variability of Postural Stability and Plantar Pressure Parameters in Healthy Subjects Evaluated by a Novel Pressure Plate. Int. J. Environ. Res. Public Health 2022, 19, 2913. [Google Scholar] [CrossRef]
- Scoppa, F.; Gallamini, M.; Belloni, G.; Messina, G. Clinical stabilometry standardization: Feet position in the static stabilometric assessment of postural stability. Acta Med. Mediterr. 2017, 33, 707–713. [Google Scholar]
- Kim, J.; Kim, K.; Gubler, C. Comparisons of Plantar Pressure Distributions between the Dominant and Non-dominant Sides of Older Women during Walking. J. Phys. Ther. Sci. 2013, 25, 313–315. [Google Scholar] [CrossRef]
- Schieppati, M.; Hugon, M.; Grasso, M.; Nardone, A.; Galante, M. The limits of equilibrium in young and elderly normal subjects and in parkinsonians. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Hennig, E.M.; Sterzing, T. Sensitivity mapping of the human foot: Thresholds at 30 skin locations. Foot Ankle Int. 2009, 30, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Machado, Á.S.; Bombach, G.D.; Duysens, J.; Carpes, F.P. Differences in foot sensitivity and plantar pressure between young adults and elderly. Arch. Gerontol. Geriatr. 2016, 63, 67–71. [Google Scholar] [CrossRef] [PubMed]
- De Doncker, E.; Kowalski, C.; Coeur, P.L. Cinésiologie et Rééducation du Pied; Masson: Issy-les-Moulineaux, France, 1979. [Google Scholar]
- Ten Wolde, I.Y.; van Kouwenhove, L.; Dekker, R.; Hijmans, J.M.; Greve, C. Effects of rocker radii with two longitudinal bending stiffnesses on plantar pressure distribution in the forefoot. Gait Posture 2021, 90, 457–463. [Google Scholar] [CrossRef]
- Reints, R.; Hijmans, J.M.; Burgerhof, J.G.M.; Postema, K.; Verkerke, G.J. Effects of flexible and rigid rocker profiles on in-shoe pressure. Gait Posture 2017, 58, 287–293. [Google Scholar] [CrossRef]
- Pomarino, D.; Pomarino, A. Plantar Static Pressure Distribution in Healthy Individuals. Foot Ankle Spec. 2014, 7, 293–297. [Google Scholar] [CrossRef]
- Patel, H.M.; Balaganapathy, M. Normative values of static-dynamic plantar pressure and its cutoff values. Indian J. Med. Spec. 2024, 15, 4–11. [Google Scholar] [CrossRef]
- Tang, W.H.; Zhao, Y.N.; Cheng, Z.X.; Xu, J.X.; Zhang, Y.; Liu, X.M. Risk factors for diabetic foot ulcers: A systematic review and meta-analysis. Vascular 2024, 32, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Jonely, H.; Brismée, J.M.; Sizer, P.S.; James, C.R. Relationships between clinical measures of static foot posture and plantar pressure during static standing and walking. Clin. Biomech. 2011, 26, 873–879. [Google Scholar] [CrossRef]
- Tse, C.T.F.; Ryan, M.B.; Dien, J.; Scott, A.; Hunt, M.A. An exploration of changes in plantar pressure distributions during walking with standalone and supported lateral wedge insole designs. J. Foot Ankle Res. 2021, 14, 55. [Google Scholar] [CrossRef]
- Lalande, X.; Vie, B.; Weber, J.P.; Jammes, Y. Normal Values of Pressures and Foot Areas Measured in the Static Condition. J. Am. Podiatr. Med. Assoc. 2016, 106, 265–272. [Google Scholar] [CrossRef][Green Version]
- Arnold, J.B.; Causby, R.; Pod, G.D.; Jones, S. The impact of increasing body mass on peak and mean plantar pressure in asymptomatic adult subjects during walking. Diabet Foot Ankle 2010, 1, 5518. [Google Scholar] [CrossRef]
- Phethean, J.; Nester, C. The influence of body weight, body mass index and gender on plantar pressures: Results of a cross-sectional study of healthy children’s feet. Gait Posture 2012, 36, 287–290. [Google Scholar] [CrossRef]
- Yan, Y.; Ou, J.; Shi, H.; Sun, C.; Shen, L.; Song, Z.; Shu, L.; Chen, Z. Plantar pressure and falling risk in older individuals: A cross-sectional study. J. Foot Ankle Res. 2023, 16, 14. [Google Scholar] [CrossRef]
- Zammit, G.V.; Menz, H.B.; Munteanu, S.E. Reliability of the TekScan MatScan(R) system for the measurement of plantar forces and pressures during barefoot level walking in healthy adults. J. Foot Ankle Res. 2010, 3, 11. [Google Scholar] [CrossRef]
- Bus, S.A.; de Lange, A. A comparison of the 1-step, 2-step, and 3-step protocols for obtaining barefoot plantar pressure data in the diabetic neuropathic foot. Clin. Biomech. 2005, 20, 892–899. [Google Scholar] [CrossRef]
- McPoil, T.G.; Cornwall, M.W.; Dupuis, L.; Cornwell, M. Variability of plantar pressure data. A comparison of the two-step and midgait methods. J. Am. Podiatr. Med. Assoc. 1999, 89, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kernozek, T.W.; LaMott, E.E.; Dancisak, M.J. Reliability of an in-shoe pressure measurement system during treadmill walking. Foot Ankle Int. 1996, 17, 204–209. [Google Scholar] [CrossRef]
- Turcato, A.M.; Godi, M.; Giordano, A.; Schieppati, M.; Nardone, A. The generation of centripetal force when walking in a circle: Insight from the distribution of ground reaction forces recorded by plantar insoles. J. Neuroeng. Rehabil. 2015, 12, 4. [Google Scholar] [CrossRef]
- Putti, A.B.; Arnold, G.P.; Cochrane, L.A.; Abboud, R.J. Normal pressure values and repeatability of the Emed ST4 system. Gait Posture 2008, 27, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.N.; Liang, W.D.; Zhou, F.H.; Li, H.T.; Zhang, L.X.; Zhang, Z.Q.; Li, J.J. Comparison of walking quality variables between incomplete spinal cord injury patients and healthy subjects by using a footscan plantar pressure system. Neural. Regen. Res. 2019, 14, 354–360. [Google Scholar] [CrossRef]
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Wojciechowski, E.; Mudge, A.; Burns, J. Spatiotemporal and plantar pressure patterns of 1000 healthy individuals aged 3–101 years. Gait Posture 2017, 58, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Thometz, J.G.; Tassone, C.; Barker, B.; Lyon, R. Dynamic plantar pressure measurement for the normal subject: Free-mapping model for the analysis of pediatric foot deformities. J. Pediatr. Orthop. 2005, 25, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Bosch, K.; Gerss, J.; Rosenbaum, D. Preliminary normative values for foot loading parameters of the developing child. Gait Posture 2007, 26, 238–247. [Google Scholar] [CrossRef] [PubMed]
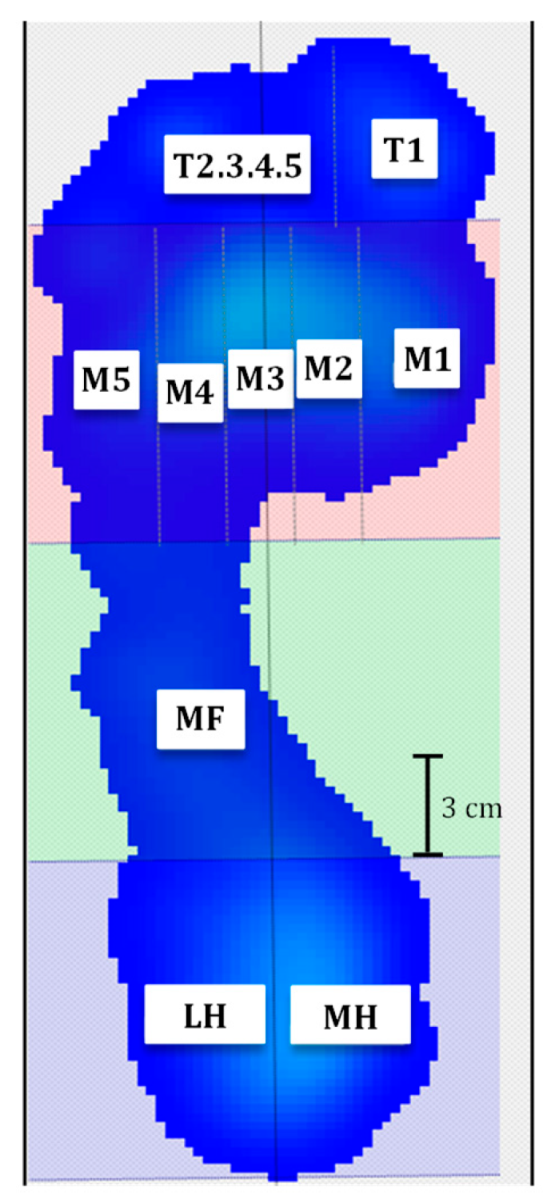
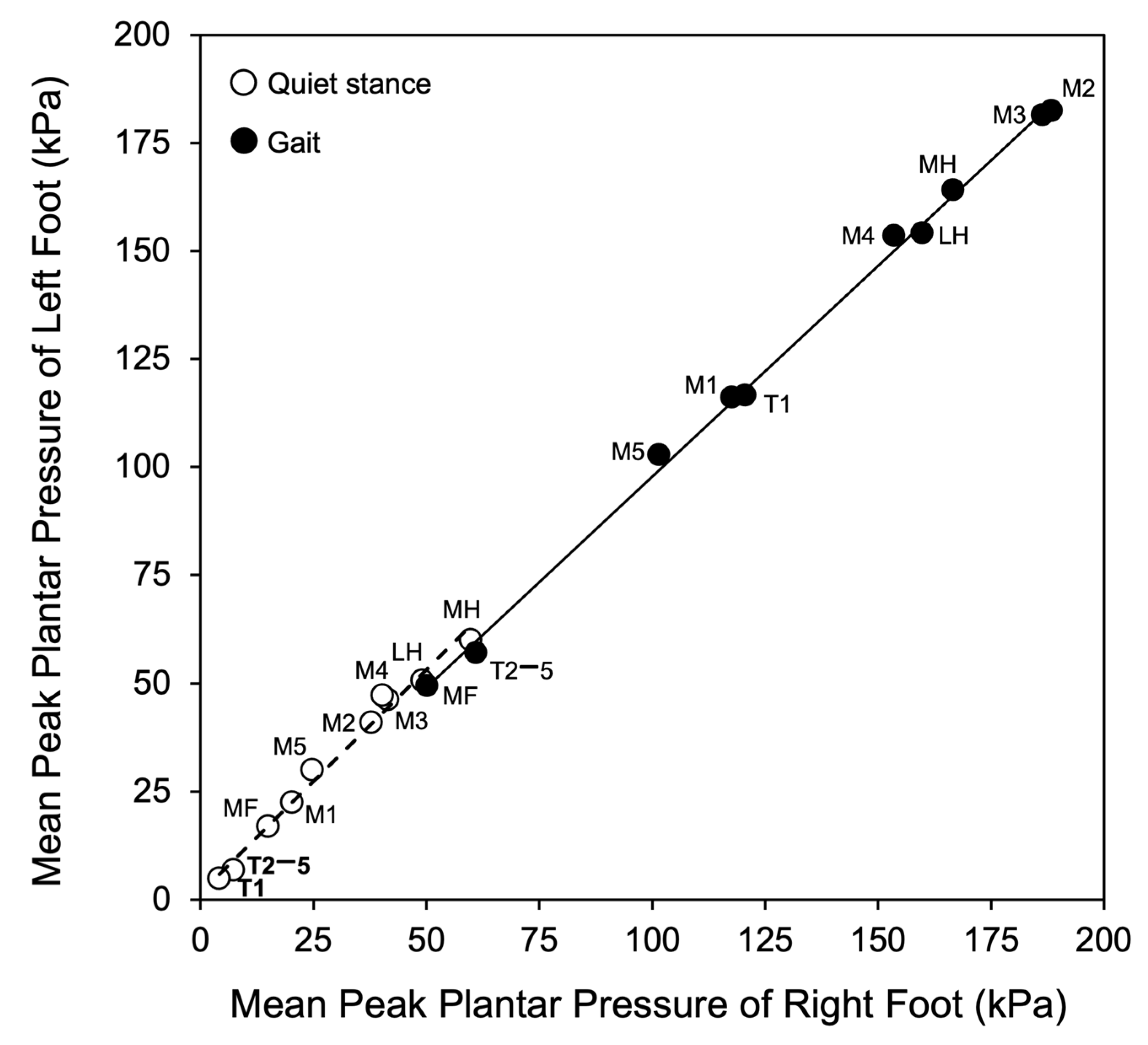
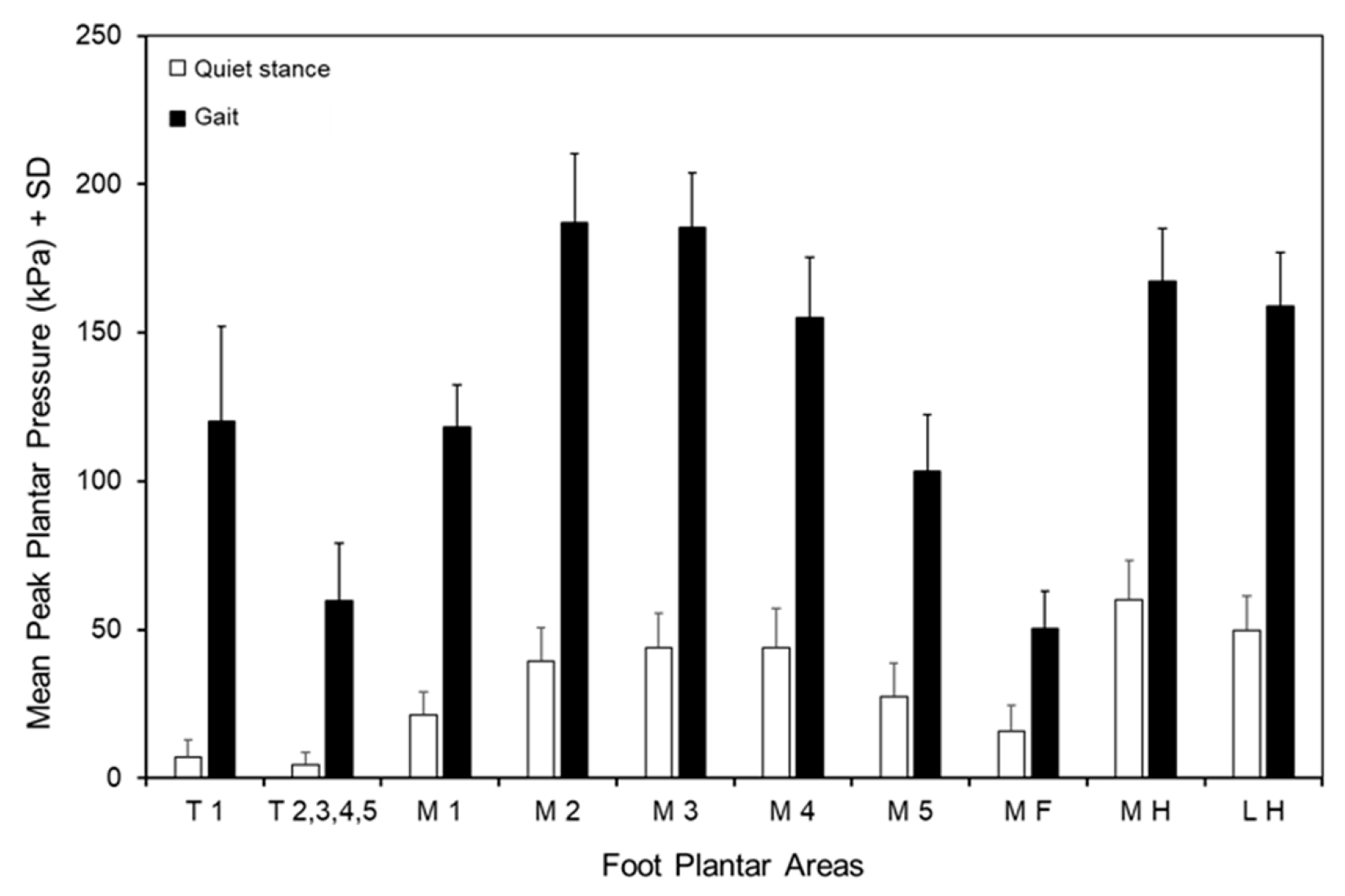
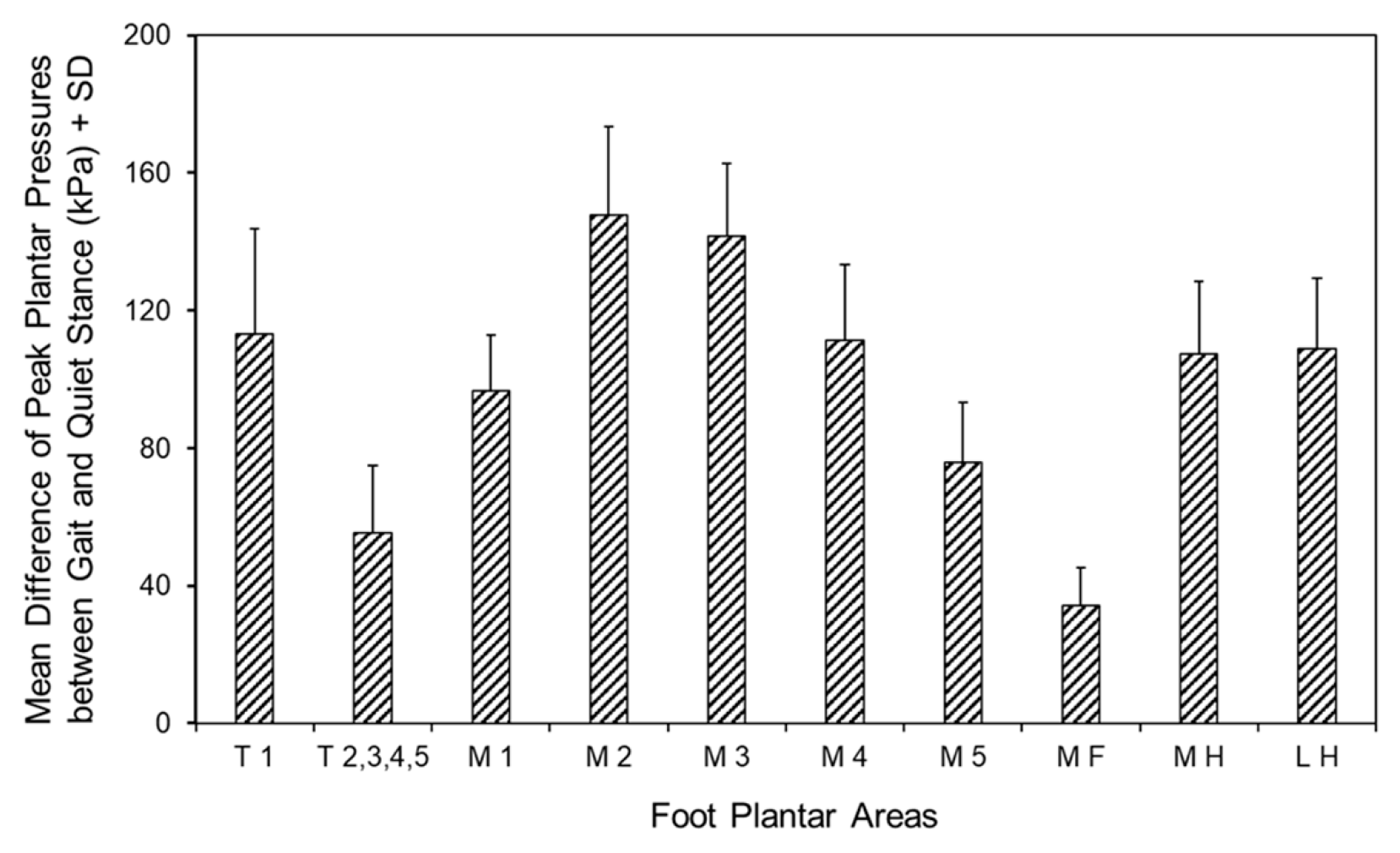

| Foot Areas | Peak Pressure (kPa) During Quiet Stance | Peak Pressure (kPa) During Gait |
|---|---|---|
| T1 | 7.1 ± 5.8 | 118.6 ± 34.2 |
| T2–5 | 4.5 ± 4.0 | 59.0 ± 20.2 |
| M1 | 21.3 ± 7.7 | 116.8 ± 17.6 |
| M2 | 39.3 ± 11.3 | 185.4 ± 26.6 |
| M3 | 43.8 ± 11.8 | 183.9 ± 22.4 |
| M4 | 43.7 ± 13.2 | 153.6 ± 23.1 |
| M5 | 27.4 ± 11.3 | 102.2 ± 21.0 |
| MF | 15.9 ± 8.5 | 49.7 ± 13.5 |
| MH | 59.9 ± 13.5 | 165.3 ± 23.2 |
| LH | 49.8 ± 11.4 | 157.0 ± 23.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirando, M.; Pavese, C.; Pingue, V.; Sozzi, S.; Nardone, A. Can Plantar Pressure Distribution During Gait Be Estimated from Quiet Stance in Healthy Individuals? J. Funct. Morphol. Kinesiol. 2025, 10, 301. https://doi.org/10.3390/jfmk10030301
Mirando M, Pavese C, Pingue V, Sozzi S, Nardone A. Can Plantar Pressure Distribution During Gait Be Estimated from Quiet Stance in Healthy Individuals? Journal of Functional Morphology and Kinesiology. 2025; 10(3):301. https://doi.org/10.3390/jfmk10030301
Chicago/Turabian StyleMirando, Marta, Chiara Pavese, Valeria Pingue, Stefania Sozzi, and Antonio Nardone. 2025. "Can Plantar Pressure Distribution During Gait Be Estimated from Quiet Stance in Healthy Individuals?" Journal of Functional Morphology and Kinesiology 10, no. 3: 301. https://doi.org/10.3390/jfmk10030301
APA StyleMirando, M., Pavese, C., Pingue, V., Sozzi, S., & Nardone, A. (2025). Can Plantar Pressure Distribution During Gait Be Estimated from Quiet Stance in Healthy Individuals? Journal of Functional Morphology and Kinesiology, 10(3), 301. https://doi.org/10.3390/jfmk10030301







