Effects of Photomodulation Therapy for Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Data Sources
2.2. Data Extraction and Analysis
2.3. Statistical Analysis
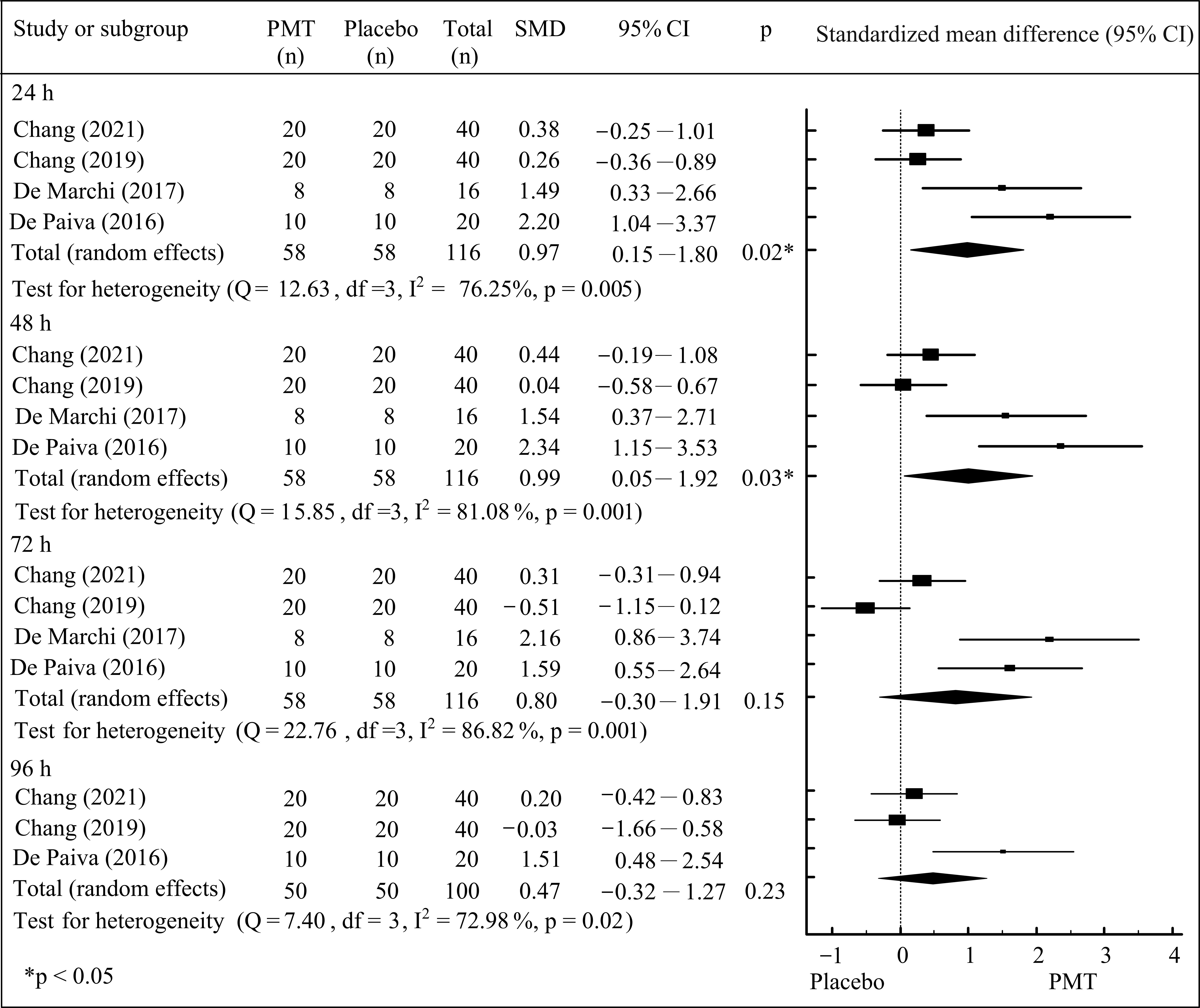
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glass, G.E. Photobiomodulation: The Clinical Applications of Low-Level Light Therapy. Aesthetic Surg. J. 2021, 41, 723–738. [Google Scholar] [CrossRef]
- Liu, H.; Cheema, U.; Player, D.J. Photobiomodulation therapy (PBMT) in skeletal muscle regeneration: A comprehensive review of mechanisms, clinical applications, and future directions. Photodiagnosis Photodyn. Ther. 2025, 53, 104634. [Google Scholar] [CrossRef]
- Lawrence, J.; Sorra, K. Photobiomodulation as Medicine: Low-level laser therapy (LLLT) for acute tissue injury or sport performance recovery. J. Funct. Morphol. Kinesiol. 2024, 9, 181. [Google Scholar] [CrossRef]
- Rola, P.; Wlodarczk, S.; Lesiak, M.; Doroszko, A.; Wlodarczk, A. Changes in cell biology under the influence of low-level laser therapy. Photonics 2022, 9, 502. [Google Scholar] [CrossRef]
- Cheung, K.; Hume, P.; Maxwell, L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef]
- Harrison, D.C.; Doma, K.; Rush, C.; Connor, J.D. Acute effects of exercise-induced muscle damage on sprint and change of direction performance: A systematic review and meta-analysis. Biol. Sport 2024, 41, 153–168. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Hubal, M.J. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 2014, 49, 155–170. [Google Scholar] [CrossRef]
- Heiss, R.; Lutter, C.; Freiwald, J.; Hoppe, M.W.; Grim, C.; Poettgen, K.; Forst, R.; Bloch, W.; Hüttel, M.; Hotfiel, T. Advances in delayed-onset muscle soreness (DOMS)—Part II: Treatment and prevention. Sportverletz. Sportschaden 2019, 33, 21–29. [Google Scholar] [CrossRef]
- Nahon, R.L.; Silva Lopes, J.S.; Monteiro de Magalhães Neto, A. Physical therapy interventions for the treatment of delayed onset muscle soreness (DOMS): Systematic review and meta-analysis. Phys. Ther. Sport 2021, 52, 1–12. [Google Scholar] [CrossRef]
- Dupuy, O.; Douzi, W.; Theurot, D.; Bosquet, L.; Dugué, B. An evidence-based approach for choosing post-exercise recovery techniques to reduce markers of muscle damage, soreness, fatigue, and inflammation: A systematic review with meta-analysis. Front. Physiol. 2018, 9, 403. [Google Scholar] [CrossRef]
- Vanin, A.A.; Verhagen, E.; Barboza, S.D.; Costa, L.O.P.; Leal-Junior, E.C.P. Photobiomodulation therapy for the improvement of muscular performance and reduction of muscular fatigue associated with exercise in healthy people: A systematic review and meta-analysis. Lasers Med. Sci. 2018, 33, 181–214. [Google Scholar] [CrossRef]
- Bettleyon, J.; Kaminski, T.W. Does low-level laser therapydecrease muscle-damaging mediators after performance in soccer athletes versus sham laser treatment? A critically appraised topic. J. Sport Rehabil. 2020, 29, 1210–1213. [Google Scholar] [CrossRef]
- Cashin, A.G.; McAuley, J.H. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J. Physiother. 2020, 66, 59. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D. Meta-analysis. Potentials and promise. BMJ 1997, 315, 1371–1374. [Google Scholar] [CrossRef]
- Ma, F.; Li, Y.; Chen, Q.; Mei, Y.; Hu, G.; Yang, Y.; Xu, C.; Zheng, S.; Jiang, J.; Xu, X.; et al. Effects of photobiomodulation and low-intensity stretching on delayed-onset muscle soreness: A randomized control trial. Photobiomodul. Photomed. Laser Surg. 2022, 40, 810–817. [Google Scholar] [CrossRef]
- D’Amico, A.; Silva, K.; Rubero, A.; Dion, S.; Gillis, J.; Gallo, J. The influence of phototherapy on recovery from exercise-induced muscle damage. Int. J. Sports Phys. Ther. 2022, 17, 658–668. [Google Scholar] [CrossRef]
- Azuma, R.H.E.; Merlo, J.K.; Jacinto, J.L.; Borim, J.M.; da Silva, R.A.; Pacagnelli, F.L.; Nunes, J.P.; Ribeiro, A.S.; Aguiar, A.F. Photobiomodulation therapy at 808 nm does not improve biceps brachii performance to exhaustion and delayed-onset muscle soreness in young adult women: A randomized, controlled, crossover trial. Front. Physiol. 2021, 12, 664582. [Google Scholar] [CrossRef]
- Chang, W.D.; Lin, H.Y.; Chang, N.J.; Wu, J.H. Effects of 830 nm light-emitting diode therapy on delayed-onset muscle soreness. Evid. Based Complement. Altern. Med. 2021, 2021, 6690572. [Google Scholar] [CrossRef]
- Chang, W.D.; Wu, J.H.; Chang, N.J.; Lee, C.L.; Chen, S. Effects of laser acupuncture on delayed onset muscle soreness of the biceps brachii muscle: A randomized controlled trial. Evid. Based Complement. Altern. Med. 2019, 2019, 6568976. [Google Scholar] [CrossRef]
- De Marchi, T.; Schmitt, V.M.; Machado, G.P.; de Sene, J.S.; de Col, C.D.; Tairova, O.; Salvador, M.; Leal-Junior, E.C. Does photobiomodulation therapy is better than cryotherapy in muscle recovery after a high-intensity exercise? A randomized, double-blind, placebo-controlled clinical trial. Lasers Med. Sci. 2017, 32, 429–437. [Google Scholar] [CrossRef]
- Fleckenstein, J.; Niederer, D.; Auerbach, K.; Bernhörster, M.; Hübscher, M.; Vogt, L.; Banzer, W. No Effect of acupuncture in the relief of delayed-onset muscle soreness: Results of a randomized controlled trial. Clin. J. Sport Med. 2016, 26, 471–477. [Google Scholar] [CrossRef]
- De Paiva, P.R.; Tomazoni, S.S.; Johnson, D.S.; Vanin, A.A.; Albuquerque-Pontes, G.M.; Machado, C.D.; Casalechi, H.L.; de Carvalho, P.T.; Leal-Junior, E.C. Photobiomodulation therapy (PBMT) and/or cryotherapy in skeletal muscle restitution, what is better? A randomized, double-blinded, placebo-controlled clinical trial. Lasers Med. Sci. 2016, 31, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Felismino, A.S.; Costa, E.C.; Aoki, M.S.; Ferraresi, C.; de Araújo Moura Lemos, T.M.; de Brito Vieira, W.H. Effect of low-level laser therapy (808 nm) on markers of muscle damage: A randomized double-blind placebo-controlled trial. Lasers Med. Sci. 2014, 29, 933–938. [Google Scholar] [CrossRef]
- Borges, L.S.; Cerqueira, M.S.; dos Santos Rocha, J.A.; Conrado, L.A.; Machado, M.; Pereira, R.; Pinto Neto, O. Light-emitting diode phototherapy improves muscle recovery after a damaging exercise. Lasers Med. Sci. 2014, 29, 1139–1144. [Google Scholar] [CrossRef]
- Leal Junior, E.C.; de Godoi, V.; Mancalossi, J.L.; Rossi, R.P.; De Marchi, T.; Parente, M.; Grosselli, D.; Generosi, R.A.; Basso, M.; Frigo, L.; et al. Comparison between cold water immersion therapy (CWIT) and light emitting diode therapy (LEDT) in short-term skeletal muscle recovery after high-intensity exercise in athletes—Preliminary results. Lasers Med. Sci. 2011, 26, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Douris, P.; Southard, V.; Ferrigi, R.; Grauer, J.; Katz, D.; Nascimento, C.; Podbielski, P. Effect of phototherapy on delayed onset muscle soreness. Photomed. Laser Surg. 2006, 24, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.A.; Barron, J.; Walsh, D.M.; Baxter, G.D. Lack of effect of combined low intensity laser therapy/phototherapy (CLILT) on delayed onset muscle soreness in humans. Lasers Surg. Med. 1999, 24, 223–230. [Google Scholar] [CrossRef]
- Craig, J.A.; Barlas, P.; Baxter, G.D.; Walsh, D.M.; Allen, J.M. Delayed-onset muscle soreness: Lack of effect of combined phototherapy/low-intensity laser therapy at low pulse repetition rates. J. Clin. Laser Med. Surg. 1996, 14, 375–380. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef]
- Da Silva, T.G.; Ribeiro, R.S.; Mencalha, A.L.; de Souza Fonseca, A. Photobiomodulation at molecular, cellular, and systemic levels. Lasers Med. Sci. 2023, 38, 136. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.R.; Goya, A.G.; Urias, Ú.; Jannig, P.R.; Bacurau, A.V.; Mello, W.G.; Faleiros, P.L.; Oliveira, S.H.; Garcia, V.G.; Ervolino, E.; et al. Strength training prior to muscle injury potentiates low-level laser therapy (LLLT)-induced muscle regeneration. Lasers Med. Sci. 2017, 32, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Souza, N.H.C.; Mesquita-Ferrari, R.A.; Rodrigues, M.F.S.D.; da Silva, D.F.T.; Ribeiro, B.G.; Alves, A.N.; Garcia, M.P.; Nunes, F.D.; da Silva Junior, E.M.; França, C.M.; et al. Photobiomodulation and different macrophages phenotypes during muscle tissue repair. J. Cell. Mol. Med. 2018, 22, 4922–4934. [Google Scholar] [CrossRef] [PubMed]
- Deonese, B.; Nimphius, S. Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2016; Volume 12, pp. 522–548. [Google Scholar]

| Author (Years) | Study Design | DOMS Muscle | Group (Sample Size, n) | Quality |
|---|---|---|---|---|
| Ma (2022) [15] | Parallel | Quadriceps | PMT (n = 12) Control (n = 15) | 6 |
| D’Amico (2022) [16] | Parallel | Quadriceps, hamstring and calf | PMT (n = 16) Placebo (n = 17) | 7 |
| Azuma (2021) [17] | Crossover | Biceps brachii | PMT (n = 15) Placebo (n = 15) | 7 |
| Chang (2021) [18] | Parallel | Quadriceps | PMT (n = 20) Placebo (n = 20) | 6 |
| Chang (2019) [19] | Parallel | Biceps brachii | PMT (n = 20) Placebo (n = 20) | 5 |
| De Marchi (2017) [20] | Parallel | Biceps brachii | PMT (n = 8) Placebo (n = 8) | 9 |
| Fleckenstein(2016) [21] | Parallel | Biceps brachii | PMT (n = 12) Placebo (n = 12) Control (n = 12) | 7 |
| De Paiva (2016) [22] | Parallel | Quadriceps | PMT (n = 10) Placebo (n = 10) | 9 |
| Felismino(2014) [23] | Parallel | Biceps brachii | PMT (n = 11) Placebo (n = 11) | 6 |
| Borges (2014) [24] | Parallel | Biceps brachii | PMT (n = 8) Placebo (n = 9) | 5 |
| Leal Junior (2011) [25] | Crossover | Hamstring and calf | PMT (n = 6) Placebo (n = 6) | 5 |
| Douris (2006) [26] | Parallel | Biceps brachii | PMT (n = 9) Placebo (n = 9) Control (n = 9) | 4 |
| Craig (1999) [27] | Parallel | Biceps brachii | PMT (n = 12) Placebo (n = 12) Control (n = 12) | 4 |
| Craig (1996) [28] | Parallel | Biceps brachii | PMT (n = 12) Placebo (n = 12) Control (n = 12) | 4 |
| Author (Years) | Wavelength (Nm) | Frequency (Hz) | Output (mW) | PMT Intervention |
|---|---|---|---|---|
| Ma (2022) [15] | 810 ± 30 | Continuous | 400 | Number of points: 6 points of quadriceps, 2 points of hamstring and 2 points of calf muscle; time per point: 125 s; 1 section per day |
| D’Amico (2022) [16] | 650 | Continuous | 200 | Number of points: 6 points of quadriceps muscle; time per point: 30 s |
| Azuma (2021) [17] | 808 | Continuous | 100 | Number of points: 4 points of biceps muscle; time per point: 70 s |
| Chang (2021) [18] | 830 | 10 | 210 | Number of points: 6 points of quadriceps muscle; time per point 10 min |
| Chang (2019) [19] | 830 | 10 | 60 | Number of points: 2 points of biceps muscle; time per point 10 min |
| De Marchi (2017) [20] | 660/850 | Continuous | 10/30 | Number of points: 1 points of biceps muscle; time per point: 30 s |
| Fleckenstein (2016) [21] | NA | NA | NA | Number of points: 8 points of biceps muscle |
| De Paiva (2016) [22] | 905/875 | 1000/16 | 15/70 | Number of points: 6 points of quadriceps muscle; time per point 300 s |
| Felismino (2014) [23] | 808 | Continuous | 100 | Number of points: 4 points of biceps muscle; time per point: 10 s |
| Borges (2014) [24] | 630 | Continuous | 300 | Number of points: 4 points of biceps muscle; time per point: 30 s |
| Leal Junior (2011) [25] | 660/835 | Continuous | 10 | Number of points: 4 points of bilateral hamstring muscle and 1 point of calf muscle; time per point: 30 s |
| Douris (2006) [26] | 660/880 | NA | NA | Number of points: 2 points of biceps muscle; time per point: 80 s |
| Craig (1999) [27] | 660–950 | 73 | 534 | Number of points: NA; time per point: 4 min on biceps muscle |
| Craig (1996) [28] | 660–950 | 2.5, 5 or 20 | NA | Number of points: NA; time per point: 12 min on biceps muscle |
| Author (Years) | Assessments | Time Point | Outcomes |
|---|---|---|---|
| Ma (2022) [15] | VAS, PPT, muscle strength, single-leg forward jump | Before, 24 h, 48 h, 72 h and 96 h | No significant differences in all assessments within and between groups |
| D’Amico (2022) [16] | VAS, vertical jump, agility T-test | Before, immediately, 24 h, 48 h, 72 h and 96 h | A decrease of VAS on calf muscle between groups * No significant differences in vertical jump, and agility T-test |
| Azuma (2021) [17] | VAS, RPE | Before, immediately, 24 h, 48 h, and 72 h | An increase of RPE in PMT group * No significant differences in VAS and PRE between groups |
| Chang (2021) [18] | VAS, PPT, limb circumference, ROM, muscle strength | Before, immediately, 24 h, 48 h, 72 h, and 96 h | Significant improvements on PPT and ROM between groups * No significant differences in limb circumference, muscle strength between groups between groups. |
| Chang (2019) [19] | VAS, PPT, force sense, limb circumference, muscle strength | Before, immediately, 24 h, 48 h, 72 h, and 96 h | Significant changes on VAS, PPT, limb circumference, muscle strength in PMT group* Only a significant difference in limb circumference between groups * |
| De Marchi (2017) [20] | VAS, muscle strength, CK, TBARS, DNPH | Before, immediately, 60 min, and 24 h, 48 h and 72 h | Significant differences in CK, TBARS, and DNPH between groups * |
| Fleckenstein (2016) [21] | VAS, PPT, muscle strength | Before, 24, 48 and 72 h | No significant differences in VAS, PPT, muscle strength among the groups |
| De Paiva (2016) [22] | VAS, muscle strength, CK | Before, immediately, 1 h, 24 h, 48 h, 72 h and 96 h | Significant differences in VAS, muscle strength and CK between the groups * |
| Felismino (2014) [23] | RPE, muscle strength, CK | Before, immediately, 24 h, 48 h, and 72 h | A significant difference in CK between groups * |
| Borges (2014) [24] | VAS, ROM, muscle strength | Before, 24 h, 48 h, 72 h and 96 h | Significant differences in VAS, ROM, muscle strength between groups * |
| Leal Junior (2011) [25] | CK, BL, C-reactive protein | Before and immediately | Significant decreases in CK and BL in PMT group*, but no significant differences in all variables between the groups |
| Douris (2006) [26] | VAS, McGill pain questionnaire, limb circumference, ROM | Before, 24 h, 48 h, 72 h and 96 h | Significant differences in VAS, McGill pain questionnaire between groups * |
| Craig (1999) [27] | VAS, PPT, ROM | Before and 1–11 days | No significant differences in VAS, PPT and ROM among the groups |
| Craig (1996) [28] | VAS, McGill pain questionnaire, PPT, ROM, | Before, 24 h, 48 h, and 72 h | No significant differences in VAS, McGill pain questionnaire, PPT and ROM among the groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsou, Y.-A.; Chang, N.-J.; Chang, W.-D. Effects of Photomodulation Therapy for Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis. J. Funct. Morphol. Kinesiol. 2025, 10, 277. https://doi.org/10.3390/jfmk10030277
Tsou Y-A, Chang N-J, Chang W-D. Effects of Photomodulation Therapy for Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis. Journal of Functional Morphology and Kinesiology. 2025; 10(3):277. https://doi.org/10.3390/jfmk10030277
Chicago/Turabian StyleTsou, Yung-An, Nai-Jen Chang, and Wen-Dien Chang. 2025. "Effects of Photomodulation Therapy for Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis" Journal of Functional Morphology and Kinesiology 10, no. 3: 277. https://doi.org/10.3390/jfmk10030277
APA StyleTsou, Y.-A., Chang, N.-J., & Chang, W.-D. (2025). Effects of Photomodulation Therapy for Delayed Onset Muscle Soreness: A Systematic Review and Meta-Analysis. Journal of Functional Morphology and Kinesiology, 10(3), 277. https://doi.org/10.3390/jfmk10030277