Passive Heat Stimuli as a Systemic Training in Elite Endurance Athletes: A New Strategy to Promote Greater Metabolic Flexibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Statistical Analysis
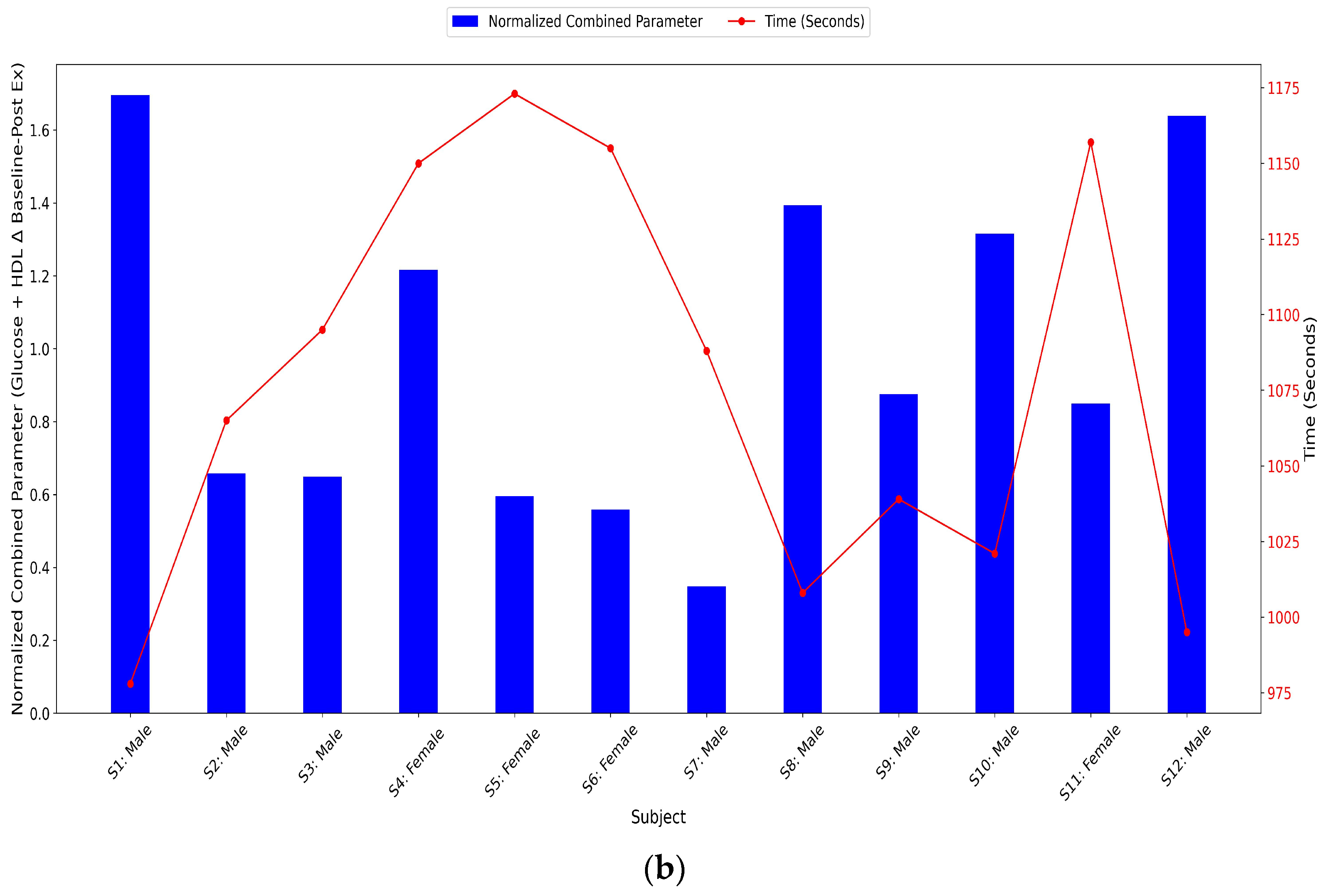
- Combined parameter (Δ Glucose + Δ HDL): Sum of normalized glucose Δ and normalized HDL Δ, where Δ represents the change from baseline to post-exercise.
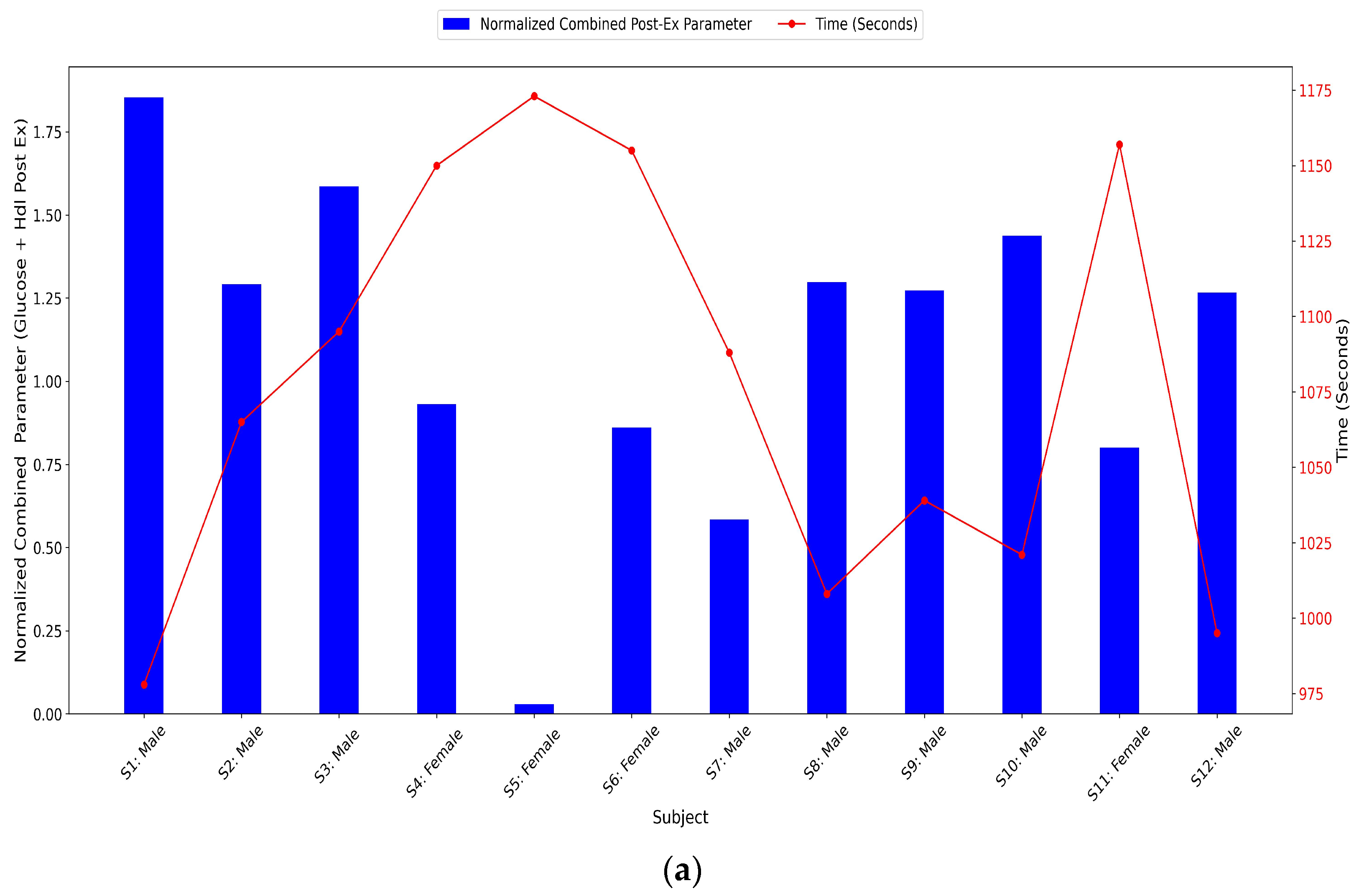
- Combined parameter (post-exercise glucose + post-exercise HDL): Sum of normalized post-exercise glucose and normalized post-exercise HDL to assess absolute metabolic values post-exercise rather than relative changes.
3. Results
3.1. Physiological Responses to Sauna and Exercise
3.2. Individual Physiological Responses to Heat Exposure and Running
3.3. Individual Physiological Values
3.3.1. Core Temperature
3.3.2. Arterial Oxygen Saturation Levels (SaO2)
3.3.3. Muscle Oxygen Saturation Levels (SmO2)
3.3.4. Hemoglobin Levels (Hb)
3.3.5. Glucose Values
3.3.6. Total Cholesterol Levels
3.3.7. Triglyceride Levels
3.3.8. HDL Cholesterol Levels
3.3.9. LDL Cholesterol Levels
3.3.10. Heart Rate Levels (HR)
3.3.11. Glucose and HDL Normalized Combined Parameters Levels
4. Discussion
4.1. Potential of Passive Heat Stimuli
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurz, A. Physiology of Thermoregulation. Best Pract. Res. Clin. Anaesthesiol. 2008, 22, 627–644. [Google Scholar] [CrossRef]
- Baker, L.B. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Wirth, A.; Knechtle, P.; Rosemann, T.; Senn, O. Do Ultra-Runners in a 24-h Run Really Dehydrate? Ir. J. Med. Sci. 2011, 180, 129–134. [Google Scholar] [CrossRef][Green Version]
- Ely, M.R.; Cheuvront, S.N.; Roberts, W.O.; Montain, S.J. Impact of Weather on Marathon-Running Performance. Med. Sci. Sports Exerc. 2007, 39, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.D.; Cramer, M.N.; Chapman, P.G.; Caillaud, C.; Thompson, M.W. Cardiovascular Strain Impairs Prolonged Self-Paced Exercise in the Heat. Exp. Physiol. 2011, 96, 134–144. [Google Scholar] [CrossRef]
- Bruck, K.; Olschewski, H. Body Temperature Related Factors Diminishing the Drive to Exercise. Can. J. Physiol. Pharmacol. 1987, 65, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Snow, R.J.; Stathis, C.G.; Hargreaves, M.; Carey, M.F. Effect of Heat Stress on Muscle Energy Metabolism during Exercise. J. Appl. Physiol. 1994, 77, 1274–1280. [Google Scholar] [CrossRef]
- Racinais, S.; Alonso, J.M.; Coutts, A.J.; Flouris, A.D.; Girard, O.; González-Alonso, J.; Hausswirth, C.; Jay, O.; Lee, J.K.W.; Mitchell, N.; et al. Consensus Recommendations on Training and Competing in the Heat. Scand. J. Med. Sci. Sports 2015, 25, 6–19. [Google Scholar] [CrossRef]
- Maresh, C.M.; Whittlesey, M.J.; Armstrong, L.E.; Yamamoto, L.M.; Judelson, D.A.; Fish, K.E.; Casa, D.J.; Kavouras, S.A.; Castracane, V.D. Effect of Hydration State on Testosterone and Cortisol Responses to Training-Intensity Exercise in Collegiate Runners. Int. J. Sports Med. 2006, 27, 765–770. [Google Scholar] [CrossRef]
- Mantzios, K.; Ioannou, L.G.; Panagiotaki, Z.; Ziaka, S.; Périard, J.D.; Racinais, S.; Nybo, L.; Flouris, A.D. Effects of Weather Parameters on Endurance Running Performance: Discipline-Specific Analysis of 1258 Races. Med. Sci Sports Exerc. 2022, 54, 153–161. [Google Scholar] [CrossRef]
- Bennett, S.; Tiollier, E.; Owens, D.J.; Brocherie, F.; Louis, J.B. Implications of Heat Stress-Induced Metabolic Alterations for Endurance Training. Int. J. Sports Med. 2024, 45, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Maunder, E.; Plews, D.J.; Wallis, G.A.; Brick, M.J.; Leigh, W.B.; Chang, W.-L.; Watkins, C.M.; Kilding, A.E. Temperate Performance and Metabolic Adaptations Following Endurance Training Performed under Environmental Heat Stress. Physiol. Rep. 2021, 9, e14849. [Google Scholar] [CrossRef]
- Bouscaren, N.; Millet, G.Y.; Racinais, S. Heat Stress Challenges in Marathon vs. Ultra-Endurance Running. Front. Sports Act. Living 2019, 1, 59. [Google Scholar] [CrossRef]
- Pugh, L.G.; Corbett, J.L.; Johnson, R.H. Rectal Temperatures, Weight Losses, and Sweat Rates in Marathon Running. J. Appl. Physiol. 1967, 23, 347–352. [Google Scholar] [CrossRef]
- Wyndham, C.H.; Strydom, N.B. The Danger of an Inadequate Water Intake during Marathon Running. S. Afr. Med. J. 1969, 43, 893–896. [Google Scholar]
- Maron, M.B.; Wagner, J.A.; Horvath, S.M. Thermoregulatory Responses during Competitive Marathon Running. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 42, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.W.; Nio, A.Q.X.; Lim, C.L.; Teo, E.Y.N.; Byrne, C. Thermoregulation, Pacing and Fluid Balance during Mass Participation Distance Running in a Warm and Humid Environment. Eur. J. Appl. Physiol. 2010, 109, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Herms, J.; Jornet, K. Physiological Data of Kilian Jornet During the Victory of UTMB 2022: An Exceptional Report of Maximal Metabolical Limits. Sports Med. 2024, 54, 3211–3214. [Google Scholar] [CrossRef]
- Périard, J.D.; Racinais, S.; Sawka, M.N. Adaptations and Mechanisms of Human Heat Acclimation: Applications for Competitive Athletes and Sports. Scand. J. Med. Sci. Sports 2015, 25, 20–38. [Google Scholar] [CrossRef]
- Gibson, O.R.; James, C.A.; Mee, J.A.; Willmott, A.G.B.; Turner, G.; Hayes, M.; Maxwell, N.S. Heat Alleviation Strategies for Athletic Performance: A Review and Practitioner Guidelines. Temperature 2020, 7, 3–36. [Google Scholar] [CrossRef]
- Murray, K.O.; Clanton, T.L.; Horowitz, M. Epigenetic Responses to Heat: From Adaptation to Maladaptation. Exp. Physiol. 2022, 107, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Halliday, A.; D’Auria, S.; Buchheit, M.; Leicht, A.S. Effect of Sauna-Based Heat Acclimation on Plasma Volume and Heart Rate Variability. Eur. J. Appl. Physiol. 2015, 115, 785–794. [Google Scholar] [CrossRef]
- Sánchez-Pérez, E.A.; Lozano-Nuevo, J.J.; Huerta-Ramírez, S.; Cerda-Téllez, F.; Mendoza-Portillo, E.; Sánchez-Pérez, E.A.; Lozano-Nuevo, J.J.; Huerta-Ramírez, S.; Cerda-Téllez, F.; Mendoza-Portillo, E. Validación de cinco pulsioxímetros. Med. Interna México 2017, 33, 723–729. [Google Scholar] [CrossRef]
- Jaén-Carrillo, D.; Roche-Seruendo, L.E.; Cartón-Llorente, A.; García-Pinillos, F. Agreement between Muscle Oxygen Saturation from Two Commercially Available Systems in Endurance Running: Moxy Monitor versus Humon Hex. Proc. Inst. Mech. Eng. Part P J. Sports Eng. Technol. 2022, 236, 231–237. [Google Scholar] [CrossRef]
- Ranjan, V.; Rana, R.; Khillan, K.; Chauhan, K. A Comparative Quality Evaluation of Point-of-Care Methodology for Testing Hemoglobin in Blood Donors by Two Different Technologies. Curr. Med. Res. Pract. 2020, 10, 90–92. [Google Scholar] [CrossRef]
- Tanner, R.K.; Fuller, K.L.; Ross, M.L.R. Evaluation of Three Portable Blood Lactate Analysers: Lactate Pro, Lactate Scout and Lactate Plus. Eur. J. Appl. Physiol. 2010, 109, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Brengelmann, G.L.; Savage, M.V. Temperature Regulation in the Neutral Zone. Ann. N. Y. Acad. Sci. 1997, 813, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hanninen, O. The Sauna-Stimulating and Relaxing. Physiology 1986, 1, 179–181. [Google Scholar] [CrossRef]
- Zapara, M.A.; Dudnik, E.N.; Samartseva, V.G.; Kryzhanovskaya, S.Y.; Susta, D.; Glazachev, O.S. Passive Whole-Body Hyperthermia Increases Aerobic Capacity and Cardio-Respiratory Efficiency in Amateur Athletes. Health 2020, 12, 14–26. [Google Scholar] [CrossRef][Green Version]
- Schenaarts, L.; Hendriks, F.K.; Fuchs, C.J.; Sluijsmans, W.E.; Snijders, T.; van Loon, L.J. A Single Sauna Session Does Not Improve Postprandial Blood Glucose Handling in Individuals with Type 2 Diabetes Mellitus: A Cross-Over, Randomized, Controlled Trial. Exp. Clin. Endocrinol. Diabetes 2024, 11, 622–630. [Google Scholar] [CrossRef]
- Sakurai, R.; Fujiwara, Y.; Saito, K.; Fukaya, T.; Kim, M.-J.; Yasunaga, M.; Kim, H.; Ogawa, K.; Tanaka, C.; Tsunoda, N.; et al. Effects of a Comprehensive Intervention Program, Including Hot Bathing, on Overweight Adults: A Randomized Controlled Trial. Geriatr. Gerontol. Int. 2013, 13, 638–645. [Google Scholar] [CrossRef]
- Vangelova, K.; Deyanov, C.; Ivanova, M. Dyslipidemia in Industrial Workers in Hot Environments. Cent. Eur. J. Public Health 2006, 14, 15–17. [Google Scholar] [CrossRef]
- Leppäluoto, J.; Tuominen, M.; Väänänen, A.; Karpakka, J.; Vuori, J. Some Cardiovascular and Metabolic Effects of Repeated Sauna Bathing. Acta Physiol. Scand. 1986, 128, 77–81. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Klein, S. Lipid Metabolism during Endurance Exercise123. Am. J. Clin. Nutr. 2000, 72, 558S–563S. [Google Scholar] [CrossRef]
- Sgouraki, E.; Tsopanakis, A.; Tsopanakis, C. Acute Exercise: Response of HDL-C, LDL-C Lipoproteins and HDL-C Subfractions Levels in Selected Sport Disciplines. J. Sports Med. Phys. Fit. 2001, 41, 386–391. [Google Scholar] [PubMed]
- Sirtori, C.R.; Ruscica, M.; Calabresi, L.; Chiesa, G.; Giovannoni, R.; Badimon, J.J. HDL Therapy Today: From Atherosclerosis, to Stent Compatibility to Heart Failure. Ann. Med. 2019, 51, 345–359. [Google Scholar] [CrossRef]
- Jose, A.D.; Stitt, F.; Collison, D. The Effects of Exercise and Changes in Body Temperature on the Intrinsic Heart Rate in Man. Am. Heart J. 1970, 79, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.J.; Proppe, D.W. Mechanisms Producing Tachycardia in Conscious Baboons during Environmental Heat Stress. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 56, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Balcerek, B.; Steinach, M.; Lichti, J.; Maggioni, M.A.; Becker, P.N.; Labes, R.; Gunga, H.-C.; Persson, P.B.; Fähling, M. A Broad Diversity in Oxygen Affinity to Haemoglobin. Sci. Rep. 2020, 10, 16920. [Google Scholar] [CrossRef]
- Zinchuk, V.V.; Zhad’ko, D.D. Sauna effect on blood oxygen transport function and proxidant/antioxidant balance in youths. Fiziol Cheloveka 2012, 38, 112–119. [Google Scholar]
- Patrick, R.P.; Johnson, T.L. Sauna Use as a Lifestyle Practice to Extend Healthspan. Exp. Gerontol. 2021, 154, 111509. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, M.; Littmann, A.E.; Chang, S.-H.; Wester, L.A.; Knipper, J.S.; Shields, R.K. Heat Stress and Cardiovascular, Hormonal, and Heat Shock Proteins in Humans. J. Athl. Train. 2012, 47, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ihsan, M.; Deldicque, L.; Molphy, J.; Britto, F.; Cherif, A.; Racinais, S. Skeletal Muscle Signaling Following Whole-Body and Localized Heat Exposure in Humans. Front. Physiol. 2020, 11, 839. [Google Scholar] [CrossRef]
- Hoekstra, S.P.; Bishop, N.C.; Leicht, C.A. Elevating Body Termperature to Reduce Low-Grade Inflammation: A Welcome Strategy for Those Unable to Exercise. Exerc. Immunol. Rev. 2020, 26, 42–55. [Google Scholar]
- Zaccardi, F.; Laukkanen, T.; Willeit, P.; Kunutsor, S.K.; Kauhanen, J.; Laukkanen, J.A. Sauna Bathing and Incident Hypertension: A Prospective Cohort Study. Am. J. Hypertens. 2017, 30, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Barroso-Aranda, J.; Contreras, F. Regular Thermal Therapy May Promote Insulin Sensitivity While Boosting Expression of Endothelial Nitric Oxide Synthase—Effects Comparable to Those of Exercise Training. Med. Hypotheses 2009, 73, 103–105. [Google Scholar] [CrossRef]
- Sobajima, M.; Nozawa, T.; Ihori, H.; Shida, T.; Ohori, T.; Suzuki, T.; Matsuki, A.; Yasumura, S.; Inoue, H. Repeated Sauna Therapy Improves Myocardial Perfusion in Patients with Chronically Occluded Coronary Artery-Related Ischemia. Int. J. Cardiol. 2013, 167, 237–243. [Google Scholar] [CrossRef]
- Bouchama, A.; Aziz, M.A.; Mahri, S.A.; Gabere, M.N.; Dlamy, M.A.; Mohammad, S.; Abbad, M.A.; Hussein, M. A Model of Exposure to Extreme Environmental Heat Uncovers the Human Transcriptome to Heat Stress. Sci. Rep. 2017, 7, 9429. [Google Scholar] [CrossRef]


| Variable | Baseline | Sauna | Post-Sauna | Post-Exercise |
|---|---|---|---|---|
| Age | ||||
| Height (cm) | ||||
| Weight (kg) | ** | |||
| BMI | 21.01 | 20.84 | ||
| Temperature (°C) | * | * | ||
| SaO2 (%) | ** | ** | ||
| SmO2 (%) | 58.9 ± 16.8 ** | NS | ||
| Hb (g/dL) | NS | 9 * | ||
| Glucose (mg/dL) | 6.8 ** | 11.8 ** | ||
| TC (mg/dL) | NS | NS | ||
| TG (mg/dL) | * | NS | ||
| HDL Chol (mg/dL) | * | * | ||
| LDL Chol (mg/dL) | 68.8 | NS | 36.3 * ± 8.9 ** | |
| HR (beats/min) | ** | ** | ||
| BL (mmol/L) | ** |
| Variable | T (°C) | SaO2 (%) | SmO2 (%) | Hb (g/dL) | GL (mg/dL) | TC (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | HR (bpm) | BL (mmol/L) | WL (g) | Time (s) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | B | 36.4 | 98 | 63.1 | 15.1 | 77 | 250 | 110 | 77 | 56 | 52 | 0.7 | 300 | 978 |
| S | 39.7 | 90 | 66.8 | 15.8 | 96 | 268 | 119 | 99 | 50 | 130 | 1.9 | |||
| E | 39.1 | 89 | 44 | 14.4 | 136 | 233 | 131 | 184 | 46 | 181 | 14.1 | |||
| S2 | B | 36.8 | 99 | 60.2 | 15 | 80 | 260 | 99 | 101 | 88 | 44 | 1.1 | 600 | 1065 |
| S | 39.8 | 88 | 65.7 | 15.4 | 106 | 233 | 80 | 109 | 86 | 112 | 2.1 | |||
| E | 39.7 | 86 | 52 | 13.6 | 126 | 258 | 118 | 176 | 52 | 177 | 12.7 | |||
| S3 | B | 36.6 | 100 | 66.8 | 14.2 | 92 | 240 | 80 | 99 | 106 | 40 | 1.6 | 590 | 1095 |
| S | 38.9 | 91 | 72.3 | 14.4 | 109 | 218 | 114 | 106 | 101 | 121 | 1.9 | |||
| E | 39.8 | 90 | 44 | 13.1 | 136 | 242 | 107 | 176 | 36 | 173 | 13.3 | |||
| S4 | B | 36.2 | 99 | 62.1 | 12.9 | 81 | 220 | 77 | 60 | 68 | 46 | 1.1 | 700 | 1150 |
| S | 39.4 | 88 | 70.2 | 11.7 | 111 | 203 | 60 | 102 | 58 | 102 | 1.7 | |||
| E | 38.9 | 89 | 52 | 11.2 | 116 | 207 | 99 | 174 | 41 | 170 | 10.3 | |||
| S5 | B | 36.4 | 99 | 62.5 | 11.3 | 85 | 113 | 139 | 54 | 79 | 63 | 1.2 | 500 | 1173 |
| S | 39.6 | 89 | 76 | 16.1 | 98 | 127 | 115 | 59 | 143 | 100 | 1.1 | |||
| E | 39.3 | 80 | 52 | 13.4 | 108 | 198 | 88 | 154 | 39 | 175 | 11.6 | |||
| S6 | B | 37.3 | 100 | 65.2 | 16.4 | 88 | 175 | 84 | 80 | 78 | 51 | 1.3 | 400 | 1155 |
| S | 38.6 | 97 | 71 | 16 | 88 | 194 | 78 | 92 | 86 | 97 | 1 | |||
| E | 39.7 | 95 | 43 | 13 | 117 | 231 | 112 | 171 | 36 | 195 | 12.5 | |||
| S7 | B | 36.5 | 98 | 35.7 | 15.7 | 90 | 166 | 99 | 77 | 64 | 51 | 1.5 | 500 | 1088 |
| S | 39.7 | 92 | 37.8 | 14.6 | 92 | 141 | 75 | 78 | 62 | 116 | 1.7 | |||
| E | 39.3 | 90 | 39 | 14.5 | 111 | 104 | 183 | 168 | 42 | 176 | 10.8 | |||
| S8 | B | 37.1 | 99 | 35.6 | 15.1 | 83 | 156 | 116 | 66 | 54 | 45 | 1.2 | 400 | 1008 |
| S | 39.8 | 97 | 36.9 | 13.7 | 100 | 200 | 86 | 60 | 123 | 118 | 2.4 | |||
| E | 39.6 | 91 | 52 | 13.8 | 133 | 199 | 75 | 170 | 20 | 173 | 9.7 | |||
| S9 | B | 36.9 | 99 | 32.2 | 15.8 | 85 | 179 | 111 | 86 | 33 | 70 | 1.2 | 200 | 1039 |
| S | 39.4 | 94 | 36.1 | 13.5 | 98 | 150 | 70 | 90 | 60 | 82 | 1.7 | |||
| E | 39.8 | 90 | 28 | 14.1 | 131 | 100 | 135 | 171 | 23 | 176 | 7.7 | |||
| S10 | B | 36.6 | 98 | 38.2 | 15 | 90 | 211 | 77 | 62 | 82 | 52 | 1.5 | 400 | 1021 |
| S | 39.9 | 89 | 64.2 | 14.7 | 101 | 189 | 90 | 100 | 89 | 122 | 1.6 | |||
| E | 38.2 | 89 | 31 | 13.8 | 123 | 211 | 99 | 183 | 35 | 172 | 9.9 | |||
| S11 | B | 36.4 | 98 | 64.2 | 14.7 | 81 | 147 | 108 | 70 | 60 | 40 | 1.1 | 300 | 1157 |
| S | 39.1 | 91 | 72 | 15.2 | 99 | 212 | 103 | 107 | 105 | 119 | 1.9 | |||
| E | 39.3 | 90 | 52 | 12 | 107 | 178 | 102 | 178 | 30 | 175 | 13.4 | |||
| S12 | B | 36.2 | 99 | 60.1 | 13.7 | 85 | 166 | 127 | 54 | 58 | 55 | 1.2 | 300 | 995 |
| S | 38.9 | 90 | 37.8 | 14.3 | 93 | 174 | 80 | 93 | 81 | 127 | 2.1 | |||
| E | 39.6 | 88 | 32.1 | 14.3 | 141 | 199 | 100 | 162 | 36 | 171 | 12.2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinca-Morros, S.; Burtscher, M.; Benito-Lopez, F.; Álvarez-Herms, J. Passive Heat Stimuli as a Systemic Training in Elite Endurance Athletes: A New Strategy to Promote Greater Metabolic Flexibility. J. Funct. Morphol. Kinesiol. 2025, 10, 220. https://doi.org/10.3390/jfmk10020220
Cinca-Morros S, Burtscher M, Benito-Lopez F, Álvarez-Herms J. Passive Heat Stimuli as a Systemic Training in Elite Endurance Athletes: A New Strategy to Promote Greater Metabolic Flexibility. Journal of Functional Morphology and Kinesiology. 2025; 10(2):220. https://doi.org/10.3390/jfmk10020220
Chicago/Turabian StyleCinca-Morros, Sergi, Martin Burtscher, Fernando Benito-Lopez, and Jesús Álvarez-Herms. 2025. "Passive Heat Stimuli as a Systemic Training in Elite Endurance Athletes: A New Strategy to Promote Greater Metabolic Flexibility" Journal of Functional Morphology and Kinesiology 10, no. 2: 220. https://doi.org/10.3390/jfmk10020220
APA StyleCinca-Morros, S., Burtscher, M., Benito-Lopez, F., & Álvarez-Herms, J. (2025). Passive Heat Stimuli as a Systemic Training in Elite Endurance Athletes: A New Strategy to Promote Greater Metabolic Flexibility. Journal of Functional Morphology and Kinesiology, 10(2), 220. https://doi.org/10.3390/jfmk10020220








