Correlation of Physical Activity Level with Muscle Strength and Size During One Week of Knee Joint Immobilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Anthropometrics and Ultrasonography
2.4. Assessment of Isometric and Isokinetic Concentric Torque
2.5. Immobilization Procedures
2.6. Measurement of Compliance and Physical Activity
2.7. Nutrition Tracking
2.8. Statistical Analyses
3. Results
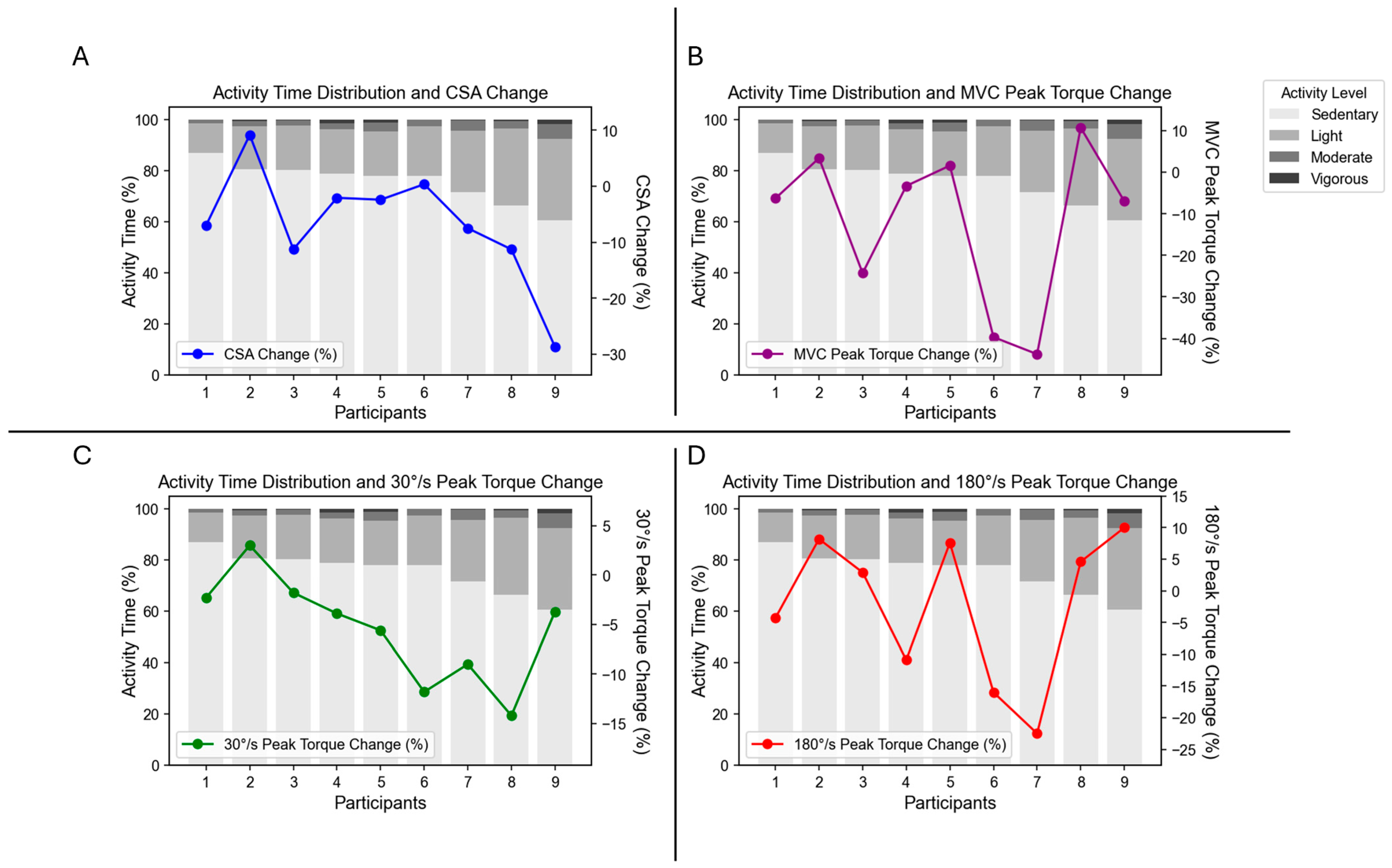
3.1. Compliance and Physical Activity
3.2. Muscle Strength and Size
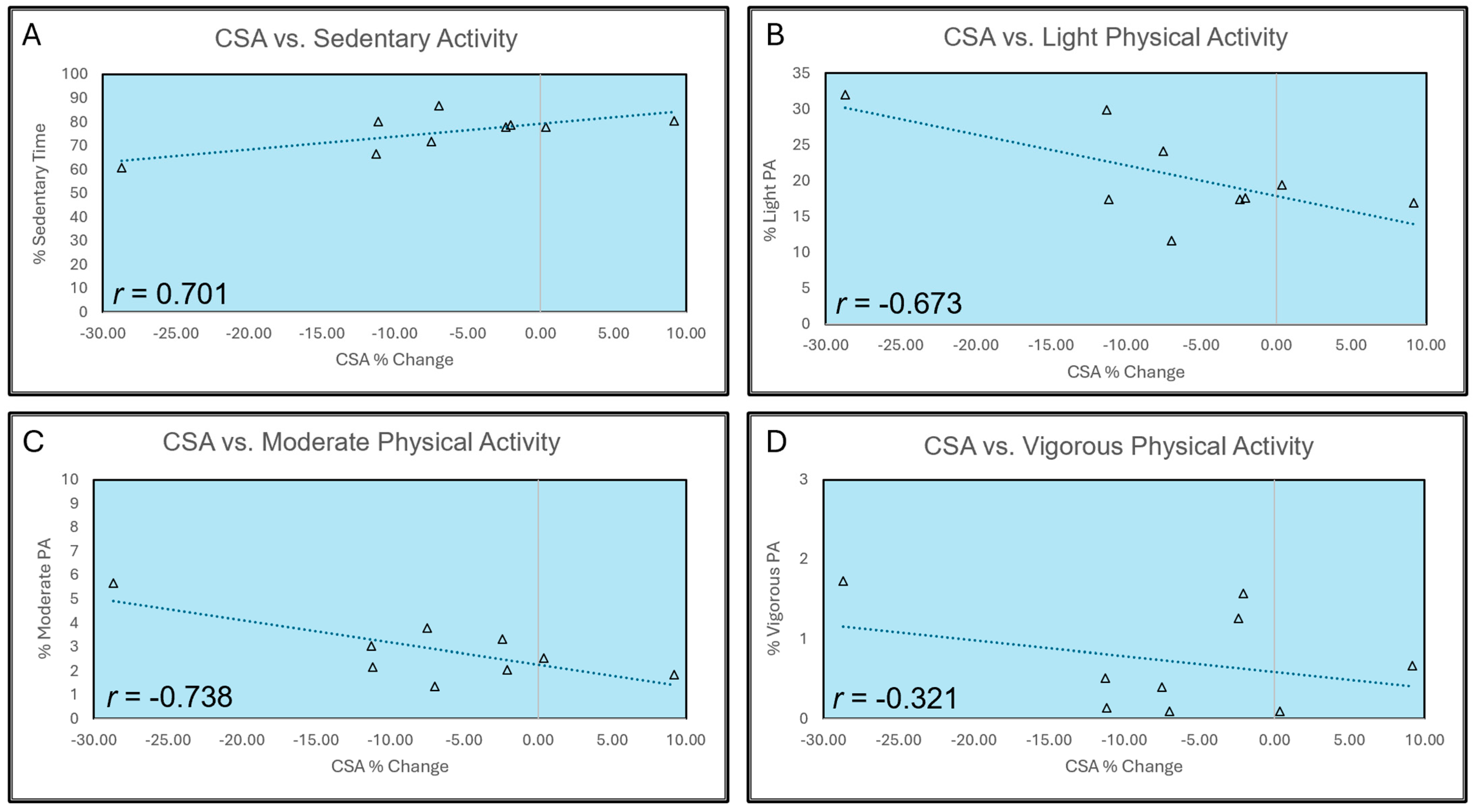
3.3. Correlation with Physical Activity Levels
3.4. Nutritional Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | Physical activity |
| MVC | Maximal voluntary contraction |
| CSA | Cross-sectional area |
| VL | Vastus lateralis |
| CPM | Counts per minute |
References
- Sommerfeldt, M.; Bouliane, M.; Otto, D.; Rowe, B.H.; Beaupre, L. The Use of Early Immobilization in the Management of Acute Soft-Tissue Injuries of the Knee: Results of a Survey of Emergency Physicians, Sports Medicine Physicians and Orthopedic Surgeons. Can. J. Surg. 2015, 58, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Varley-Campbell, J.; Fulford, J.; Taylor, B.; Mileva, K.N.; Bowtell, J.L. Effect of Immobilisation on Neuromuscular Function In Vivo in Humans: A Systematic Review. Sports Med. 2019, 49, 931–950. [Google Scholar] [CrossRef] [PubMed]
- Preobrazenski, N.; Seigel, J.; Halliday, S.; Janssen, I.; McGlory, C. Single-Leg Disuse Decreases Skeletal Muscle Strength, Size, and Power in Uninjured Adults: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 684–696. [Google Scholar] [CrossRef]
- Cerqueira, M.S.; Do Nascimento, J.D.S.; Maciel, D.G.; Barboza, J.A.M.; De Brito Vieira, W.H. Effects of Blood Flow Restriction without Additional Exercise on Strength Reductions and Muscular Atrophy Following Immobilization: A Systematic Review. J. Sport Health Sci. 2020, 9, 152–159. [Google Scholar] [CrossRef]
- Oates, B.R.; Glover, E.I.; West, D.W.; Fry, J.L.; Tarnopolsky, M.A.; Phillips, S.M. Low-Volume Resistance Exercise Attenuates the Decline in Strength and Muscle Mass Associated with Immobilization. Muscle Nerve 2010, 42, 539–546. [Google Scholar] [CrossRef]
- Tian, D.; Meng, J. Exercise for Prevention and Relief of Cardiovascular Disease: Prognoses, Mechanisms, and Approaches. Oxid. Med. Cell Longev. 2019, 2019, 3756750. [Google Scholar] [CrossRef]
- Marshall, N.E.; Keller, R.A.; Dines, J.; Bush-Joseph, C.; Limpisvasti, O. Current Practice: Postoperative and Return to Play Trends after ACL Reconstruction by Fellowship-Trained Sports Surgeons. Musculoskelet. Surg. 2019, 103, 55–61. [Google Scholar] [CrossRef]
- Saint-Maurice, P.F.; Troiano, R.P.; Bassett, D.R., Jr.; Graubard, B.I.; Carlson, S.A.; Shiroma, E.J.; Fulton, J.E.; Matthews, C.E. Association of Daily Step Count and Step Intensity With Mortality Among US Adults. JAMA 2020, 323, 1151–1160. [Google Scholar] [CrossRef]
- Arentson-Lantz, E.; Galvan, E.; Wacher, A.; Fry, C.S.; Paddon-Jones, D. 2000 Steps/Day Does Not Fully Protect Skeletal Muscle Health in Older Adults during Bed Rest. J. Aging Phys. Act. 2019, 27, 191–197. [Google Scholar] [CrossRef]
- MacLennan, R.J.; Sahebi, M.; Becker, N.; Davis, E.; Garcia, J.M.; Stock, M.S. Declines in Skeletal Muscle Quality vs. Size Following Two Weeks of Knee Joint Immobilization. PeerJ 2020, 8, e8224. [Google Scholar] [CrossRef]
- MacLennan, R.J.; Ogilvie, D.; McDorman, J.; Vargas, E.; Grusky, A.R.; Kim, Y.; Garcia, J.M.; Stock, M.S. The Time Course of Neuromuscular Impairment during Short-Term Disuse in Young Women. Physiol. Rep. 2021, 9, e14677. [Google Scholar] [CrossRef] [PubMed]
- Harmon, K.K.; Girts, R.M.; Pagan, J.I.; Rodriguez, G.; Stock, M.S. The Acute Effects of Action Observation on Muscle Strength/Weakness and Corticospinal Excitability in Older Adults. Exp. Brain Res. 2022, 240, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.B.; Kanaley, J.A.; Ploutz-Snyder, L.L. Neuromuscular Function Following Muscular Unloading and Blood Flow Restricted Exercise. Eur. J. Appl. Physiol. 2014, 114, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.B.; Clark, B.C.; Ploutz-Snyder, L.L. Accelerometry as a Measure of Subject Compliance in Unilateral Lower Limb Suspension. Aviat. Space Environ. Med. 2006, 77, 953–956. [Google Scholar]
- Bodine, S.C. Disuse-Induced Muscle Wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2200–2208. [Google Scholar] [CrossRef]
- Marusic, U.; Narici, M.; Simunic, B.; Pisot, R.; Ritzmann, R. Nonuniform Loss of Muscle Strength and Atrophy during Bed Rest: A Systematic Review. J. Appl. Physiol. 2021, 131, 194–206. [Google Scholar] [CrossRef]
- Sirago, G.; Pellegrino, M.A.; Bottinelli, R.; Franchi, M.V.; Narici, M.V. Loss of Neuromuscular Junction Integrity and Muscle Atrophy in Skeletal Muscle Disuse. Ageing Res. Rev. 2023, 83, 101810. [Google Scholar] [CrossRef]
- Girts, R.M.; Harmon, K.K.; Rodriguez, G.; Beausejour, J.P.; Pagan, J.I.; Carr, J.; Garcia, J.M.; Stout, J.R.; Fukuda, D.H.; Stock, M.S. Sex Differences in Muscle Quality Recovery Following One Week of Knee Joint Immobilization and Subsequent Retraining. Appl. Physiol. Nutr. Metab. 2024, 49, 805–817. [Google Scholar] [CrossRef]
- Hardy, E.J.O.; Inns, T.B.; Hatt, J.; Doleman, B.; Bass, J.J.; Atherton, P.J.; Lund, J.N.; Phillips, B.E. The Time Course of Disuse Muscle Atrophy of the Lower Limb in Health and Disease. J. Cachexia Sarcopenia Muscle 2022, 13, 2616–2629. [Google Scholar] [CrossRef]
- Deschenes, M.R.; McCoy, R.W.; Davis, J.M.; McGinn, M.C.; Eason, M.K. The Efficacy of Prehabilitative Conditioning: Ameliorating Unloading-Induced Declines in the Muscle Function of Humans. Am. J. Phys. Med. Rehabil. 2009, 88, 136–144. [Google Scholar] [CrossRef]
- Deschenes, M.R.; McCoy, R.W.; Mangis, K.A. Chronic Resistance Training Does Not Ameliorate Unloading-Induced Decrements in Neuromuscular Function. Am. J. Phys. Med. Rehabil. 2017, 96, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.M.; Godwin, J.S.; Plotkin, D.L.; McIntosh, M.C.; Mattingly, M.L.; Agostinelli, P.J.; Mueller, B.J.; Anglin, D.A.; Kontos, N.J.; Berry, A.C.; et al. Effects of Leg Immobilization and Recovery Resistance Training on Skeletal Muscle-Molecular Markers in Previously Resistance-Trained versus Untrained Adults. J. Appl. Physiol. 2025, 138, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, E.; Stout, J.R.; Beyer, K.S.; Church, D.D.; Varanoske, A.N.; Fukuda, D.H.; Hoffman, J.R. Effects of Supine Rest Duration on Ultrasound Measures of the Vastus Lateralis. Clin. Physiol. Funct. Imaging 2018, 38, 155–157. [Google Scholar] [CrossRef]
- Girts, R.M.; Harmon, K.K.; Pagan, J.I.; Alberto, A.; Hernandez, M.G.; Stock, M.S. The Influence of Ultrasound Image Depth and Gain on Skeletal Muscle Echo Intensity. Appl. Physiol. Nutr. Metab. 2022, 47, 839–846. [Google Scholar] [CrossRef]
- Carr, J.C.; Gerstner, G.R.; Voskuil, C.C.; Harden, J.E.; Dunnick, D.; Badillo, K.M.; Pagan, J.I.; Harmon, K.K.; Girts, R.M.; Beausejour, J.P.; et al. The Influence of Sonographer Experience on Skeletal Muscle Image Acquisition and Analysis. J. Funct. Morphol. Kinesiol. 2021, 6, 91. [Google Scholar] [CrossRef]
- Deschenes, M.R.; Holdren, A.N.; McCoy, R.W. Adaptations to Short-Term Muscle Unloading in Young and Aged Men. Med. Sci. Sports Exerc. 2008, 40, 856–863. [Google Scholar] [CrossRef]
- Deschenes, M.R.; McCoy, R.W.; Holdren, A.N.; Eason, M.K. Gender Influences Neuromuscular Adaptations to Muscle Unloading. Eur. J. Appl. Physiol. 2009, 105, 889–897. [Google Scholar] [CrossRef]
- Troiano, R.P. Large-Scale Applications of Accelerometers: New Frontiers and New Questions. Med. Sci. Sports Exerc. 2007, 39, 1501. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; McDowell, M. Physical Activity in the United States Measured by Accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Clark, B.C.; Manini, T.M. What Is Dynapenia? Nutrition 2012, 28, 495–503. [Google Scholar] [CrossRef]
- Papa, E.V.; Dong, X.; Hassan, M. Skeletal Muscle Function Deficits in the Elderly: Current Perspectives on Resistance Training. J. Nat. Sci. 2017, 3, e272. [Google Scholar] [PubMed]
- Dirks, M.L.; Backx, E.M.P.; Wall, B.T.; Verdijk, L.B.; van Loon, L. May Bed Rest Cause Greater Muscle Loss than Limb Immobilization? Acta Physiol. 2016, 218, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Carroll, T.J.; Herbert, R.D.; Munn, J.; Lee, M.; Gandevia, S.C. Contralateral Effects of Unilateral Strength Training: Evidence and Possible Mechanisms. J. Appl. Physiol. 2006, 101, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.M.; Lamon, S. The Cross-Education Phenomenon: Brain and Beyond. Front. Physiol. 2017, 8, 297. [Google Scholar] [CrossRef]
- Lee, M.; Carroll, T.J. Cross Education: Possible Mechanisms for the Contralateral Effects of Unilateral Resistance Training. Sports Med. 2007, 37, 1–14. [Google Scholar] [CrossRef]
- Voskuil, C.C.; Andrushko, J.W.; Huddleston, B.S.; Farthing, J.P.; Carr, J.C. Exercise Prescription and Strategies to Promote the Cross-Education of Strength: A Scoping Review. Appl. Physiol. Nutr. Metab. 2023, 48, 569–582. [Google Scholar] [CrossRef]
- Andrushko, J.W.; Lanovaz, J.L.; Björkman, K.M.; Kontulainen, S.A.; Farthing, J.P. Unilateral Strength Training Leads to Muscle-Specific Sparing Effects during Opposite Homologous Limb Immobilization. J. Appl. Physiol. 2018, 124, 866–876. [Google Scholar] [CrossRef]
- Farthing, J.P.; Krentz, J.R.; Magnus, C.R.A. Strength Training the Free Limb Attenuates Strength Loss during Unilateral Immobilization. J. Appl. Physiol. 2009, 106, 830–836. [Google Scholar] [CrossRef]
- Magnus, C.R.A.; Barss, T.S.; Lanovaz, J.L.; Farthing, J.P. Effects of Cross-Education on the Muscle after a Period of Unilateral Limb Immobilization Using a Shoulder Sling and Swathe. J. Appl. Physiol. 2010, 109, 1887–1894. [Google Scholar] [CrossRef]
- Pearce, A.J.; Hendy, A.; Bowen, W.A.; Kidgell, D.J. Corticospinal Adaptations and Strength Maintenance in the Immobilized Arm Following 3 Weeks Unilateral Strength Training. Scand. J. Med. Sci. Sports 2013, 23, 740–748. [Google Scholar] [CrossRef]
- Rozier, C.K.; Elder, J.D. Cross-Training Effects of Isokinetic Exercise on Skeletal Muscle. Int. J. Rehabil. Res. 1980, 3, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C. In Vivo Alterations in Skeletal Muscle Form and Function after Disuse Atrophy. Med. Sci. Sports Exerc. 2009, 41, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D. Cellular Mechanism of Immobilization-Induced Muscle Atrophy: A Mini Review. Sports Med. Health Sci. 2019, 1, 19–23. [Google Scholar] [CrossRef]
- Howard, E.E.; Pasiakos, S.M.; Fussell, M.A.; Rodriguez, N.R. Skeletal Muscle Disuse Atrophy and the Rehabilitative Role of Protein in Recovery from Musculoskeletal Injury. Adv. Nutr. 2020, 11, 989–1001. [Google Scholar] [CrossRef]
- Wall, B.T.; van Loon, L.J.C. Nutritional Strategies to Attenuate Muscle Disuse Atrophy. Nutr. Rev. 2013, 71, 195–208. [Google Scholar] [CrossRef]
- Kilroe, S.P.; Fulford, J.; Holwerda, A.M.; Jackman, S.R.; Lee, B.P.; Gijsen, A.P.; van Loon, L.J.C.; Wall, B.T. Short-Term Muscle Disuse Induces a Rapid and Sustained Decline in Daily Myofibrillar Protein Synthesis Rates. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E117–E130. [Google Scholar] [CrossRef]
- Glover, E.I.; Phillips, S.M.; Oates, B.R.; Tang, J.E.; Tarnopolsky, M.A.; Selby, A.; Smith, K.; Rennie, M.J. Immobilization Induces Anabolic Resistance in Human Myofibrillar Protein Synthesis with Low and High Dose Amino Acid Infusion. J. Physiol. 2008, 586, 6049–6061. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of Ubiquitin Ligases Required for Skeletal Muscle Atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Dirks, M.L.; Wall, B.T.; Snijders, T.; Ottenbros, C.L.P.; Verdijk, L.B.; van Loon, L.J.C. Neuromuscular Electrical Stimulation Prevents Muscle Disuse Atrophy during Leg Immobilization in Humans. Acta Physiol. 2014, 210, 628–641. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt Pathway Prevents Expression of Muscle Atrophy-Induced Ubiquitin Ligases by Inhibiting FOXO Transcription Factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Goodman, C.A.; Hornberger, T.A.; Ji, L.L. PGC-1α Overexpression by in Vivo Transfection Attenuates Mitochondrial Deterioration of Skeletal Muscle Caused by Immobilization. FASEB J. 2015, 29, 4092–4106. [Google Scholar] [CrossRef] [PubMed]
- Guttridge, D.C.; Mayo, M.W.; Madrid, L.V.; Wang, C.-Y.; Baldwin, A.S., Jr. NF-ΚB-Induced Loss of MyoD Messenger RNA: Possible Role in Muscle Decay and Cachexia. Science 2000, 289, 2363–2366. [Google Scholar] [CrossRef]
- Sv, F.; Rp, P. Energy Cost of Ambulation with Crutches. Arch. Phys. Med. Rehabil. 1981, 62, 250–256. [Google Scholar]
- Smith-Ryan, A.E.; Hirsch, K.R.; Saylor, H.E.; Gould, L.M.; Blue, M.N.M. Nutritional Considerations and Strategies to Facilitate Injury Recovery and Rehabilitation. J. Athl. Train. 2020, 55, 918–930. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef]
- Wall, B.T.; Snijders, T.; Senden, J.M.G.; Ottenbros, C.L.P.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J.C. Disuse Impairs the Muscle Protein Synthetic Response to Protein Ingestion in Healthy Men. J. Clin. Endocrinol. Metab. 2013, 98, 4872–4881. [Google Scholar] [CrossRef]
- Kilroe, S.P.; Fulford, J.; Jackman, S.; Holwerda, A.; Gijsen, A.; van Loon, L.; Wall, B.T. Dietary Protein Intake Does Not Modulate Daily Myofibrillar Protein Synthesis Rates or Loss of Muscle Mass and Function during Short-Term Immobilization in Young Men: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2021, 113, 548–561. [Google Scholar] [CrossRef]
- Phillips, S.M.; McGlory, C. CrossTalk Proposal: The Dominant Mechanism Causing Disuse Muscle Atrophy Is Decreased Protein Synthesis. J. Physiol. 2014, 592, 5341–5343. [Google Scholar] [CrossRef]
- Tipton, K.D. Nutritional Support for Exercise-Induced Injuries. Sports Med. 2015, 45, 93–104. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M.; Hoffman, R.L.; Russ, D.W. Restoration of Voluntary Muscle Strength after 3 Weeks of Cast Immobilization Is Suppressed in Women Compared with Men. Arch. Phys. Med. Rehabil. 2009, 90, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R.; McCoy, R.W.; Mangis, K.A. Factors Relating to Gender Specificity of Unloading-Induced Declines in Strength. Muscle Nerve 2012, 46, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. Inflammation and Its Role in Regeneration and Repair. Circ. Res. 2019, 124, 1166–1168. [Google Scholar] [CrossRef]
- Nunes, E.A.; Stokes, T.; McKendry, J.; Currier, B.S.; Phillips, S.M. Disuse-Induced Skeletal Muscle Atrophy in Disease and Nondisease States in Humans: Mechanisms, Prevention, and Recovery Strategies. Am. J. Physiol. Cell Physiol. 2022, 322, C1068–C1084. [Google Scholar] [CrossRef]
| PA Level | Percent of Time |
|---|---|
| Sedentary | 75.68 ± 8.04% |
| Light PA | 20.73 ± 6.63% |
| Moderate PA | 2.87 ± 1.30% |
| Vigorous PA | 0.72 ± 0.64% |
| PRE | POST | p-Value (Effect Size) | |
|---|---|---|---|
| MVC peak torque (Nm) | 183.58 ± 60.19 | 160.23 ± 56.34 | 0.039 (0.820) |
| Concentric peak torque @ 30°/s (Nm) | 149.80 ± 36.40 | 142.34 ± 35.31 | 0.022 (0.947) |
| Concentric peak torque @ 180°/s (Nm) | 106.23 ± 35.90 | 104.06 ± 32.55 | 0.587 (0.189) |
| VL CSA (cm2) | 25.68 ±7.90 | 24.57 ± 8.36 | 0.062 (0.724) |
| Pearson r (p) | Sedentary | Light | Moderate | Vigorous |
|---|---|---|---|---|
| MVC peak torque | 0.286 (0.456) | −0.373 (0.323) | −0.146 (0.709) | 0.574 (0.106) |
| Concentric peak torque @ 30°/s | 0.440 (0.236) | −0.497 (0.173) | −0.275 (0.474) | 0.197 (0.611) |
| Concentric peak torque @ 180°/s | −0.197 (0.611) | 0.170 (0.661) | 0.174 (0.654) | 0.359 (0.343) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmon, K.K.; Pourhatami, Z.; Malinowski, D.; Girts, R.M.; Beausejour, J.P.; Wydra, J.S.; Carr, J.C.; Garcia, J.; Stock, M.S. Correlation of Physical Activity Level with Muscle Strength and Size During One Week of Knee Joint Immobilization. J. Funct. Morphol. Kinesiol. 2025, 10, 192. https://doi.org/10.3390/jfmk10020192
Harmon KK, Pourhatami Z, Malinowski D, Girts RM, Beausejour JP, Wydra JS, Carr JC, Garcia J, Stock MS. Correlation of Physical Activity Level with Muscle Strength and Size During One Week of Knee Joint Immobilization. Journal of Functional Morphology and Kinesiology. 2025; 10(2):192. https://doi.org/10.3390/jfmk10020192
Chicago/Turabian StyleHarmon, Kylie K., Zahra Pourhatami, Dylan Malinowski, Ryan M. Girts, Jonathan P. Beausejour, Jeremy S. Wydra, Joshua C. Carr, Jeanette Garcia, and Matt S. Stock. 2025. "Correlation of Physical Activity Level with Muscle Strength and Size During One Week of Knee Joint Immobilization" Journal of Functional Morphology and Kinesiology 10, no. 2: 192. https://doi.org/10.3390/jfmk10020192
APA StyleHarmon, K. K., Pourhatami, Z., Malinowski, D., Girts, R. M., Beausejour, J. P., Wydra, J. S., Carr, J. C., Garcia, J., & Stock, M. S. (2025). Correlation of Physical Activity Level with Muscle Strength and Size During One Week of Knee Joint Immobilization. Journal of Functional Morphology and Kinesiology, 10(2), 192. https://doi.org/10.3390/jfmk10020192








