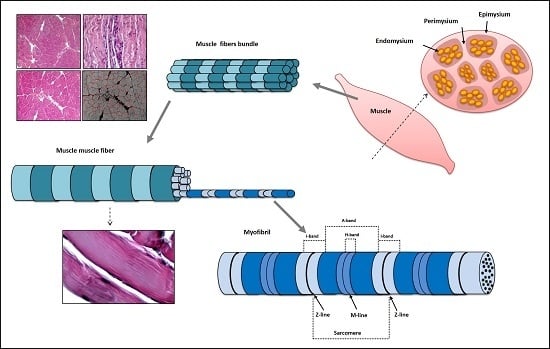
Morphological and Functional Aspects of Human Skeletal Muscle
Abstract
:1. Introduction
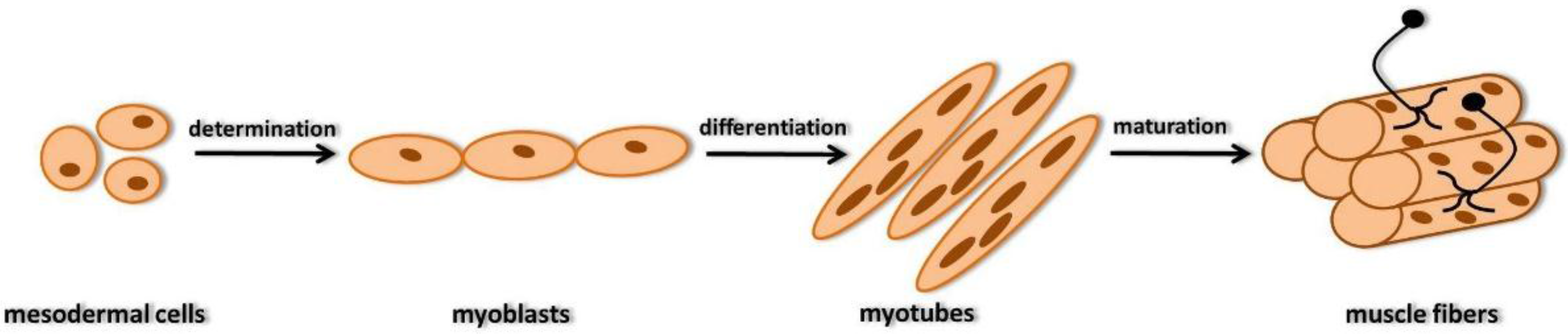
2. Development of Skeletal Muscle Fibers
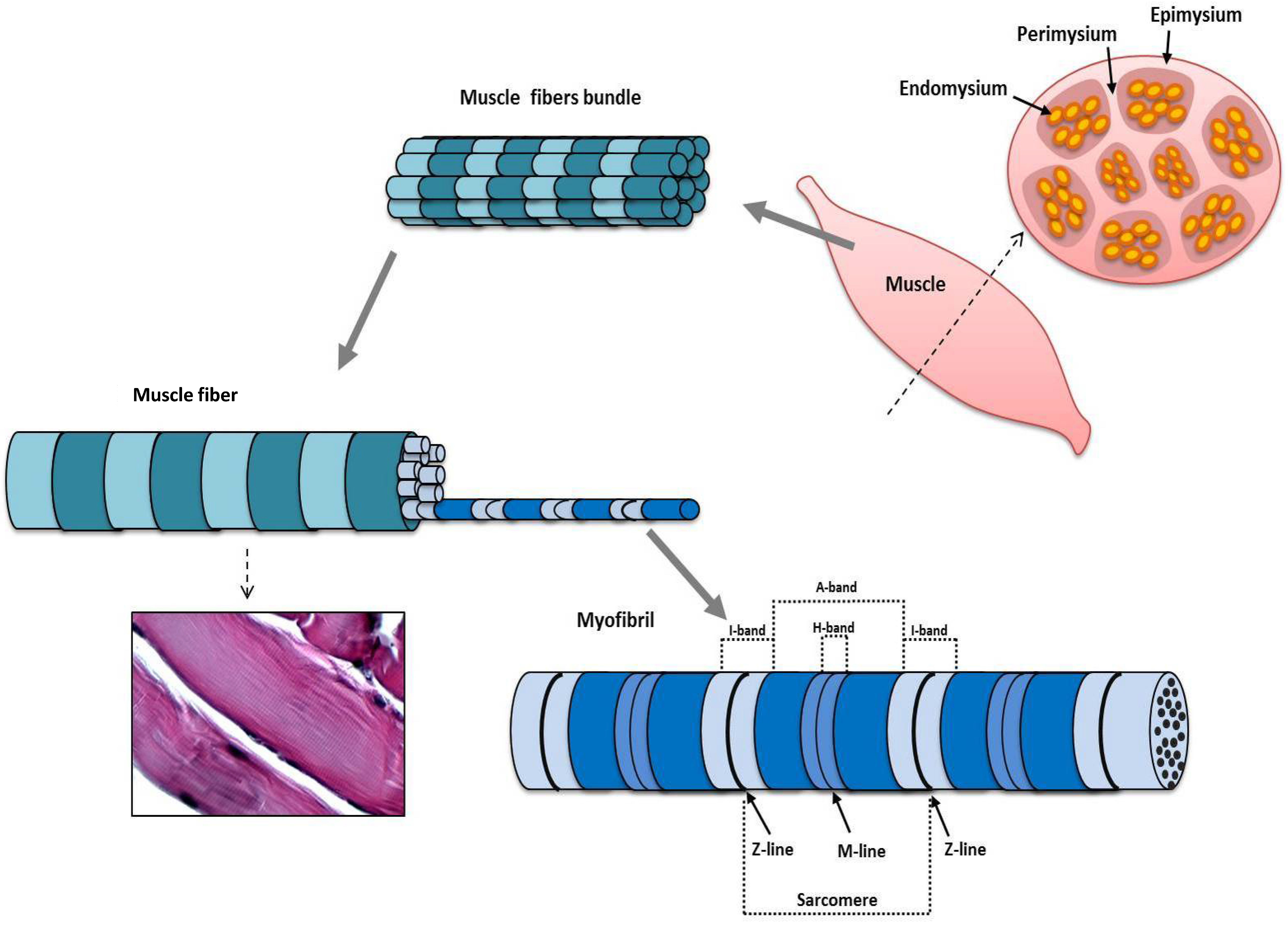
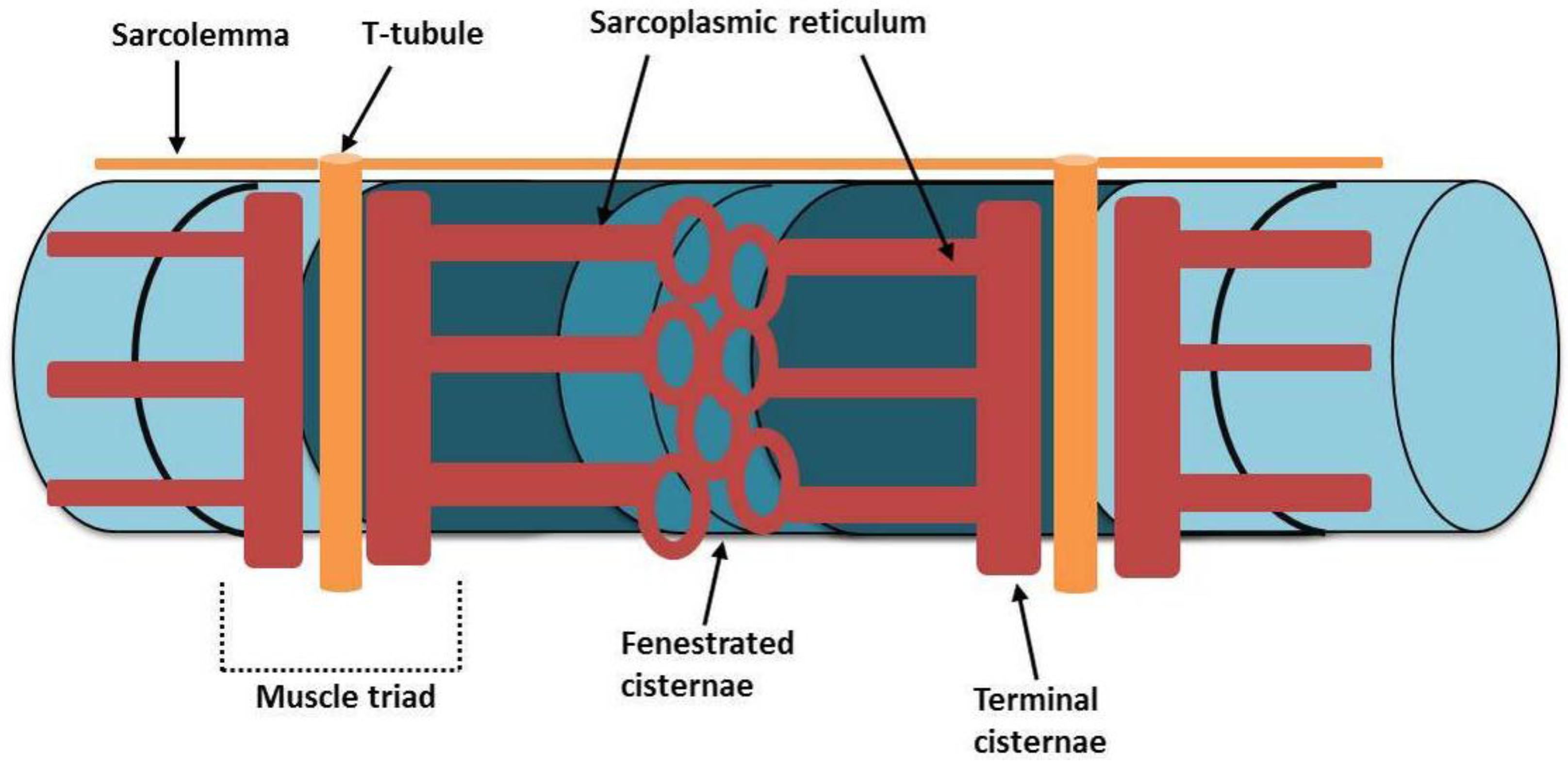
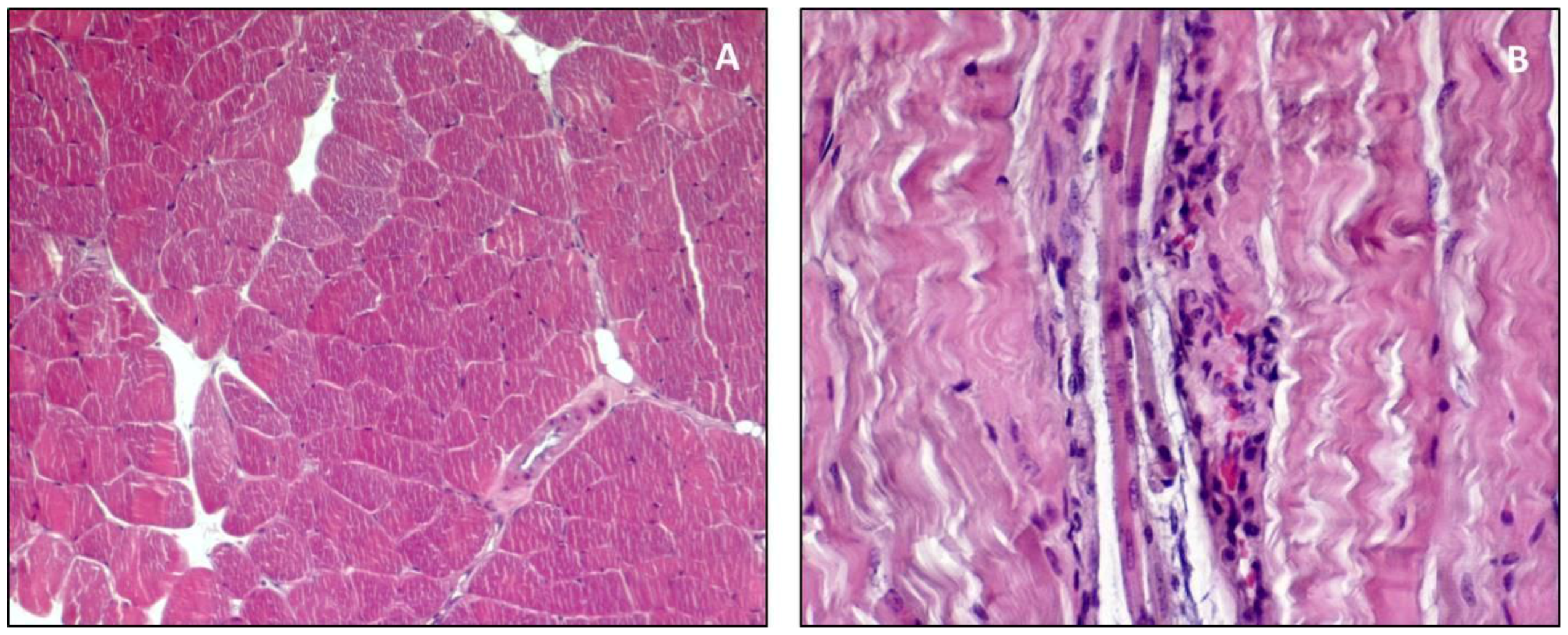
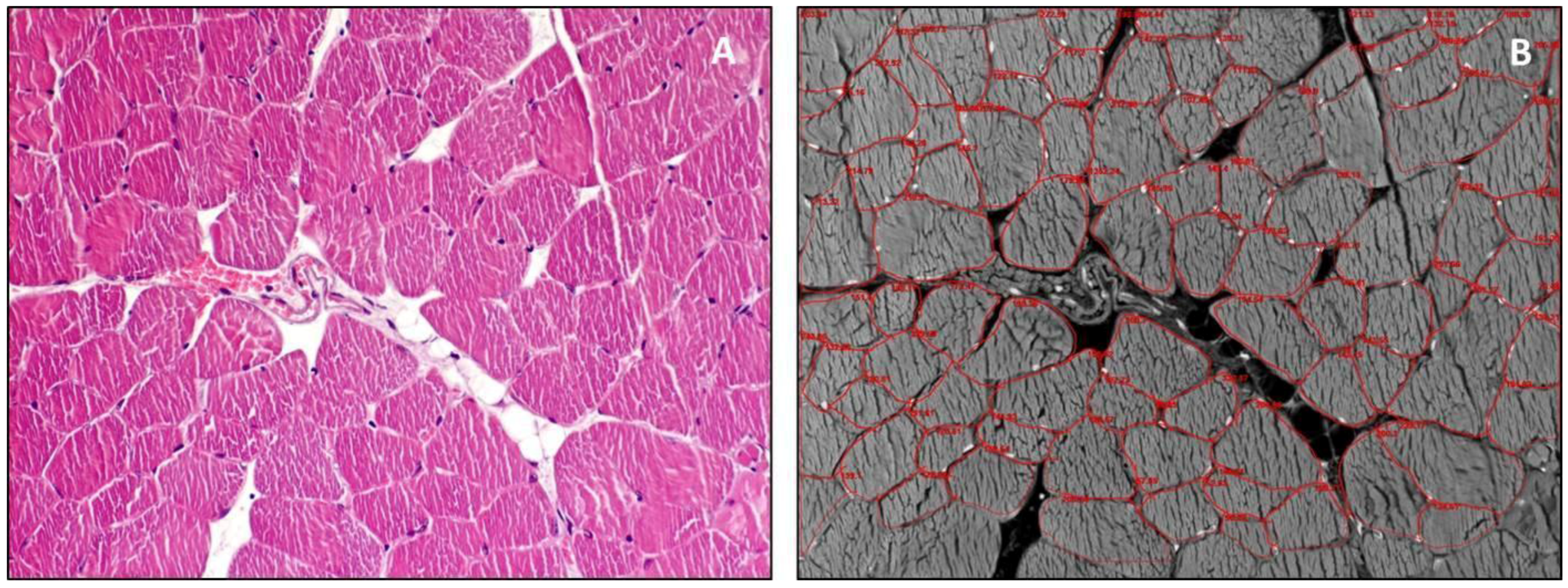
3. Structure and Function
4. Guidelines for Histology
5. Exercise
6. Sarcopenia
7. Ageing
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AchR | Acetylcholine Receptor |
| AVP | Vasopressin |
| ESPEN-SIG | European Society for Clinical Nutrition and Metabolism Special Interest Groups |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| F-actin | Actin Filaments |
| FGF-8 | Fibroblast Growth Factor 8 |
| H&E | Hematoxylin & Eosin |
| IGF-I | Insulin-like Growth Factor-I |
| IL-6 | Interleukin 6 |
| IWGS | International Working Group on Sarcopenia |
| MET | Mesenchymal-Epithelial Transition |
| PAS | Periodic Acid-Schiff staining |
| RA | Retinoic Acid |
| ROS | Reactive Oxygen Species |
| TNF-α | Tumor Necrosis Factor-α |
| T-tubule | Transverse Tubule |
References
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Aulehla, A.; Pourquiè, O. Signaling gradients during paraxial mesoderm development. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Imbesi, R.; D’Agata, V.; Musumeci, G.; Castogiovanni, P. Skeletal muscle: From development to function. Clin. Ter. 2014, 165, 47–56. [Google Scholar] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Coleman, R.; Szychlinska, M.A.; Salvatorelli, L.; Parenti, R.; Magro, G.; Imbesi, R. Somitogenesis: From somite to skeletal muscle. Acta Histochem. 2015, 117, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Palmeirim, I.; Rodrigues, S.; Dale, J.K.; Maroto, M. Development on time. Adv. Exp. Med. Biol. 2008, 641, 62–71. [Google Scholar] [PubMed]
- Roy, P.; Bandyopadhyay, A. Spatio-temporally restricted expression of cell adhesion molecules during chicken embryonic development. PLoS ONE 2014, 9, e96837. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Muerdter, C.P.; Knickerbocker, A.D.; Walsh, R.M.; Zepeda-Rivera, M.A.; Depner, K.H.; Sangesland, M.; Cisneros, T.B.; Kim, J.Y.; Sanchez-Vazquez, P.; et al. Cdc42 GTPase and Rac1 GTPase act downstream of p120 catenin and require GTP exchange during gastrulation of zebrafish mesoderm. Dev. Dyn. 2012, 241, 1545–1561. [Google Scholar] [CrossRef] [PubMed]
- Dockter, J.L. Sclerotome induction and differentiation. Curr. Top. Dev. Biol. 2000, 48, 77–127. [Google Scholar] [PubMed]
- Mitchell, K.J.; Pannerec, A.; Cadot, B.; Parlakian, A.; Besson, V.; Gomes, E.R.; Marazzi, G.; Sassoon, D.A. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 2010, 12, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Gerhart, J.; Elder, J.; Neely, C.; Schure, J.; Kvist, T.; Knudsen, K.; George-Weinstein, M. MyoD-positive epiblast cells regulate skeletal muscle differentiation in the embryo. J. Cell Biol. 2006, 175, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Avola, R.; Imbesi, R.; Castrogiovanni, P. Serotonin/growth hormone/insulin-like growth factors axis on pre- and post-natal development: A contemporary review. OA Anat. 2013, 1, 12. [Google Scholar] [CrossRef]
- Yasa, I.C.; Gunduz, N.; Kilinc, M.; Guler, M.O.; Tekinay, A.B. Basal Lamina Mimetic Nanofibrous Peptide Networks for Skeletal Myogenesis. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Demonbreun, A.R.; Biersmith, B.H.; McNally, E.M. Membrane fusion in muscle development and repair. Semin. Cell Dev. Biol. 2015, 45, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Posey, A.D., Jr.; Demonbreun, A.; McNally, E.M. Ferlin proteins in myoblast fusion and muscle growth. Curr. Top. Dev. Biol. 2011, 96, 203–230. [Google Scholar] [PubMed]
- Abmayr, S.M.; Zhuang, S.; Geisbrecht, E.R. Myoblast fusion in Drosophila. Methods Mol. Biol. 2008, 475, 75–97. [Google Scholar] [PubMed]
- Walsh, F.S.; Celeste, A.J. Myostatin: A modulator of skeletal-muscle stem cells. Biochem. Soc. Trans. 2005, 33, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.F.; Celeste, A.J. Myostatin, a negative regulator of muscle mass: Implications for muscle degenerative diseases. Curr. Opin. Pharmacol. 2005, 5, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Parenti, R.; Szychlinska, M.A.; Imbesi, R. Pregnancy, embryo-fetal development and nutrition: Physiology around fetal programming. J. Histol. Histopathol. 2015, 2. [Google Scholar] [CrossRef]
- Fortin, M.; Videman, T.; Gibbons, L.E.; Battiè, M.C. Paraspinal muscle morphology and composition: A 15-yr longitudinal magnetic resonance imaging study. Med. Sci. Sports Exerc. 2014, 46, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Gransee, H.M.; Mantilla, C.B.; Sieck, G.C. Systems biology of skeletal muscle: Fiber type as an organizing principle WIREs. Syst. Biol. Med. 2012, 4, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.D. Functional muscle ischemia in Duchenne and Becker muscular dystrophy. Front. Physiol. 2013, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ottenheijm, C.A.C.; Granzier, H. Lifting the nebula: Novel insights into skeletal muscle contractility. Physiology 2010, 25, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Monroy, J.A.; Powers, K.L.; Gilomre, L.A.; Uyeno, T.A.; Lindstedt, S.L.; Nishikawa, K.C. What is the role of titin in active muscle? Exerc. Sports Sci. Rev. 2012, 40, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lamboley, C.R.; Murphy, R.M.; McKenna, M.J.; Lamb, G.D. Sarcoplasmic reticulum Ca2+ uptake and leak properties, and SERCA isoform expression, in type I and type II fibres of human skeletal muscle. J. Physiol. 2014, 592, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, I.D.; Launikonis, B.S. Three-dimensional reconstruction and analysis of the tubular system of vertebrate skeletal muscle. J. Cell Sci. 2013, 126, 4048–4058. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.; Larsen, S.; Dohlmann, T.L.; Qvortrup, K.; Helge, J.W.; Dela, F.; Prats, C. Three-dimensional reconstruction of the human skeletal muscle mitochondrial network as a tool to assess mitochondrial content and structural organization. Acta Physiol. 2015, 213, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mackey, A.L.; Srikuea, R.; Esser, K.A.; Yang, L. Automated image segmentation of haematoxylin and eosin stained skeletal muscle cross-sections. J. Microsc. 2013, 252, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.R.; Barton, E.R. SMASH—Semi-automatic muscle analysis using segmentation of histology: A MATLAB application. Skelet. Muscle 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Briguet, A.; Courdier-Fruh, I.; Foster, M.; Meier, T.; Magyar, J.P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 2004, 14, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Flisinski, M.; Brymora, A.; Elminowska-Wenda, G.; Bogucka, J.; Walasik, K.; Stefanska, A.; Strozecki, P.; Manitius, J. Morphometric analysis of muscle fibre types in rat locomotor and postural skeletal muscles in different stages of chronic kidney disease. J. Physiol. Pharmacol. 2014, 65, 567–576. [Google Scholar] [PubMed]
- Lamon, S.; Wallace, M.A.; Russell, A.P. The STARS signaling pathway: A key regulator of skeletal muscle function. Pflugers Arch. 2014, 466, 1659–1671. [Google Scholar] [CrossRef] [PubMed]
- Seene, T.; Kaasik, P.; Alev, K. Muscle protein turnover in endurance training: A review. Int. J. Sports Med. 2011, 32, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lira, V.A.; Greene, N.P. Exercise training-induced regulation of mitochondrial quality. Exerc. Sport Sci. Rev. 2012, 40, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Imbesi, R.; Szychlinska, M.A.; Castrogiovanni, P. Apoptosis and Skeletal Muscle in Aging. Open J. Apoptosis 2015, 4, 41–46. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Menshikova, E.V.; Ritov, V.B.; Fairfull, L.; Ferrell, R.E.; Kelley, D.E.; Goodpaster, B.H. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 6, 1534–1540. [Google Scholar] [CrossRef]
- Phillips, S.M. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014, 44, S71–S77. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.; Reggiani, C. The role of satellite cells in muscle hypertrophy. J. Muscle Res. Cell Motil. 2014, 35, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Nader, G.A.; von Walden, F.; Liu, C.; Lindvall, J.; Gutmann, L.; Pistilli, E.E.; Gordon, P.M. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J. Appl. Physiol. 2014, 116, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Stathokostas, L.; Roland, K.P.; Jakobi, J.M.; Patterson, C.; Vandervoort, A.A.; Jones, G.R. The effectiveness of exercise interventions for the management of frailty: A systematic review. J. Aging Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kwak, H.B.; Lawler, J.M. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid. Redox Signal. 2006, 8, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Tarnopolsky, M.A.; Beckman, K.; Felkey, K.; Hubbard, A. Resistance exercise reverses aging in human skeletal muscle. PLoS ONE 2007, 2, e465. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Imbesi, R. Oxidative stress and skeletal muscle in exercise. Ital. J. Anat. Embryol. 2012, 117, 107–117. [Google Scholar] [PubMed]
- Brioche, T.; Pagano, A.F.; Py, G.; Chopard, A. Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention. Mol. Asp. Med. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J. Strategies for reducing oxidative damage in ageing skeletal muscle. Adv. Drug Deliv. Rev. 2009, 61, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Viña, J.; Ji, L.L. Interplay of oxidants and antioxidants during exercise: Implications for muscle health. Phys. Sportsmed. 2009, 37, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Szychlinska, M.A.; Mobasheri, A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: Molecular markers of senescent chondrocytes. Histol. Histopathol. 2015, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Imbesi, R. Muscle in exercise. Role of different fatty acids in diets. Ital. J. Anat. Embryol. 2006, 111, 199–214. [Google Scholar] [PubMed]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Imbesi, R.; Castrogiovanni, P. Effects of dietary extra-virgin olive oil on oxidative stress resulting from exhaustive exercise in rat skeletal muscle: A morphological study. Acta Histochem. 2014, 116, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ryall, J.G.; Schertzer, J.D.; Lynch, G.S. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology 2008, 9, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. Liver cirrhosis. Lancet 2014, 383, 1749–1761. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Cooper, C.; Rizzoli, R.; Kanis, J.A.; Appelboom, G.; Bautmans, I.; Bischoff-Ferrari, H.A.; Boers, M.; Brandi, M.L.; Bruyère, O.; et al. Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin. Exp. Res. 2016, 28, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical definition of sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.M.; Al-Jaouni, R.; Filippi, J.; Wiroth, J.B.; Zeanandin, G.; Arab, K.; Hébuterne, X. Sarcopenia is prevalent in patients with Crohn’s disease in clinical remission. Inflamm. Bowel Dis. 2008, 14, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Martyn, C.N.; Cooper, C.; Sayer, A.A. Grip strength, body composition, and mortality. Int. J. Epidemiol. 2007, 36, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, C.; di Rosa, M. Modulation of myotilin and fylamin C in various muscle diseases: A microarray analysis. J. Funct. Morphol. Kinesiol. 2016, 1, 90–101. [Google Scholar] [CrossRef]
- Trovato, F.M.; Aiello, F.C.; Larocca, L.; Taylor-Robinson, S.D. The role of physical activity and nutrition in the sarcopenia of cirrhosis. J. Funct. Morphol. Kinesiol. 2016, 1, 118–125. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: Overlap of clinical features. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.; Lim, J.Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hikida, R.S. Aging changes in satellite cells and their functions. Curr. Aging Sci. 2011, 4, 279–297. [Google Scholar] [CrossRef] [PubMed]
- McKay, B.R.; Ogborn, D.I.; Baker, J.M.; Toth, K.G.; Tarnopolsky, M.A.; Parise, G. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am. J. Physiol. Cell Physiol. 2013, 304, C717–C728. [Google Scholar] [CrossRef] [PubMed]
- Weisleder, N.; Brotto, M.; Komazaki, S.; Pan, Z.; Zhao, X.; Nosek, T.; Parness, J.; Takeshima, H.; Ma, J. Muscle aging is associated with compromised Ca2+ spark signaling and segregated intracellular Ca2+ release. J. Cell Biol. 2006, 174, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.S.; Toth, M.J. Myofilament protein alterations promote physical disability in aging and disease. Exerc. Sport Sci. Rev. 2013, 41, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Moen, R.J.; Klein, J.C.; Thomas, D.D. Electron paramagnetic resonance resolves effects of oxidative stress on muscle proteins. Exerc. Sport Sci. Rev. 2014, 42, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Broskey, N.T.; Greggio, C.; Boss, A.; Boutant, M.; Dwyer, A.; Schlueter, L.; Hans, D.; Gremion, G.; Kreis, R.; Boesch, C.; et al. Skeletal muscle mitochondria in the elderly: Effects of physical fitness and exercise training. J. Clin. Endocrinol. Metab. 2014, 99, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Tudorascu, I.; Sfredel, V.; Riza, A.L.; Dănciulescu Miulescu, R.; Ianoşi, S.L.; Dănoiu, S. Motor unit changes in normal aging: A brief review. Rom. J. Morphol. Embryol. 2014, 55, 1295–1301. [Google Scholar] [PubMed]
- Leeuwenburgh, C. Role of apoptosis in sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Brioche, T.; Lemoine-Morel, S. Oxidative stress, sarcopenia, antioxidant strategies and exercise: Molecular aspects. Curr. Pharm. Des. 2016, 22, 2664–2678. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; van Loon, L.J. Aging, exercise, and muscle protein metabolism. J. Appl. Physiol. 2009, 106, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Hofer, T.; Seo, A.Y.; Leeuwenburgh, C. Molecular mechanisms of life- and health-span extension: Role of calorie restriction and exercise intervention. Appl. Physiol. Nutr. Metab. 2007, 32, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.; Leeuwenburgh, C. Muscle fiber specific apoptosis and TNF-α signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005, 19, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of skeletal muscle: A 12-year longitudinal study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [PubMed]
- Yamada, M.; Moriguch, Y.; Mitani, T.; Aoyama, T.; Arai, H. Age-dependent changes in skeletal muscle mass and visceral fat area in Japanese adults from 40 to 79 years-of-age. Geriatr. Gerontol. Int. 2014, 14, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.F.; Pasha, E.; Doros, G.; Clark, D.J.; Patten, C.; Phillips, E.M.; Frontera, W.R.; Fielding, R.A. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: Influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur. J. Appl. Physiol. 2014, 114, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Frontera, W.R.; Wood, M.; Evans, W.J.; Dallal, G.E.; Roubenoff, R.; Fiatarone Singh, M.A. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B209–B217. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trovato, F.M.; Imbesi, R.; Conway, N.; Castrogiovanni, P. Morphological and Functional Aspects of Human Skeletal Muscle. J. Funct. Morphol. Kinesiol. 2016, 1, 289-302. https://doi.org/10.3390/jfmk1030289
Trovato FM, Imbesi R, Conway N, Castrogiovanni P. Morphological and Functional Aspects of Human Skeletal Muscle. Journal of Functional Morphology and Kinesiology. 2016; 1(3):289-302. https://doi.org/10.3390/jfmk1030289
Chicago/Turabian StyleTrovato, Francesca Maria, Rosa Imbesi, Nerys Conway, and Paola Castrogiovanni. 2016. "Morphological and Functional Aspects of Human Skeletal Muscle" Journal of Functional Morphology and Kinesiology 1, no. 3: 289-302. https://doi.org/10.3390/jfmk1030289
APA StyleTrovato, F. M., Imbesi, R., Conway, N., & Castrogiovanni, P. (2016). Morphological and Functional Aspects of Human Skeletal Muscle. Journal of Functional Morphology and Kinesiology, 1(3), 289-302. https://doi.org/10.3390/jfmk1030289