Manufacturing of a Granular Fertilizer Based on Organic Slurry and Hardening Agent
Abstract
:1. Introduction
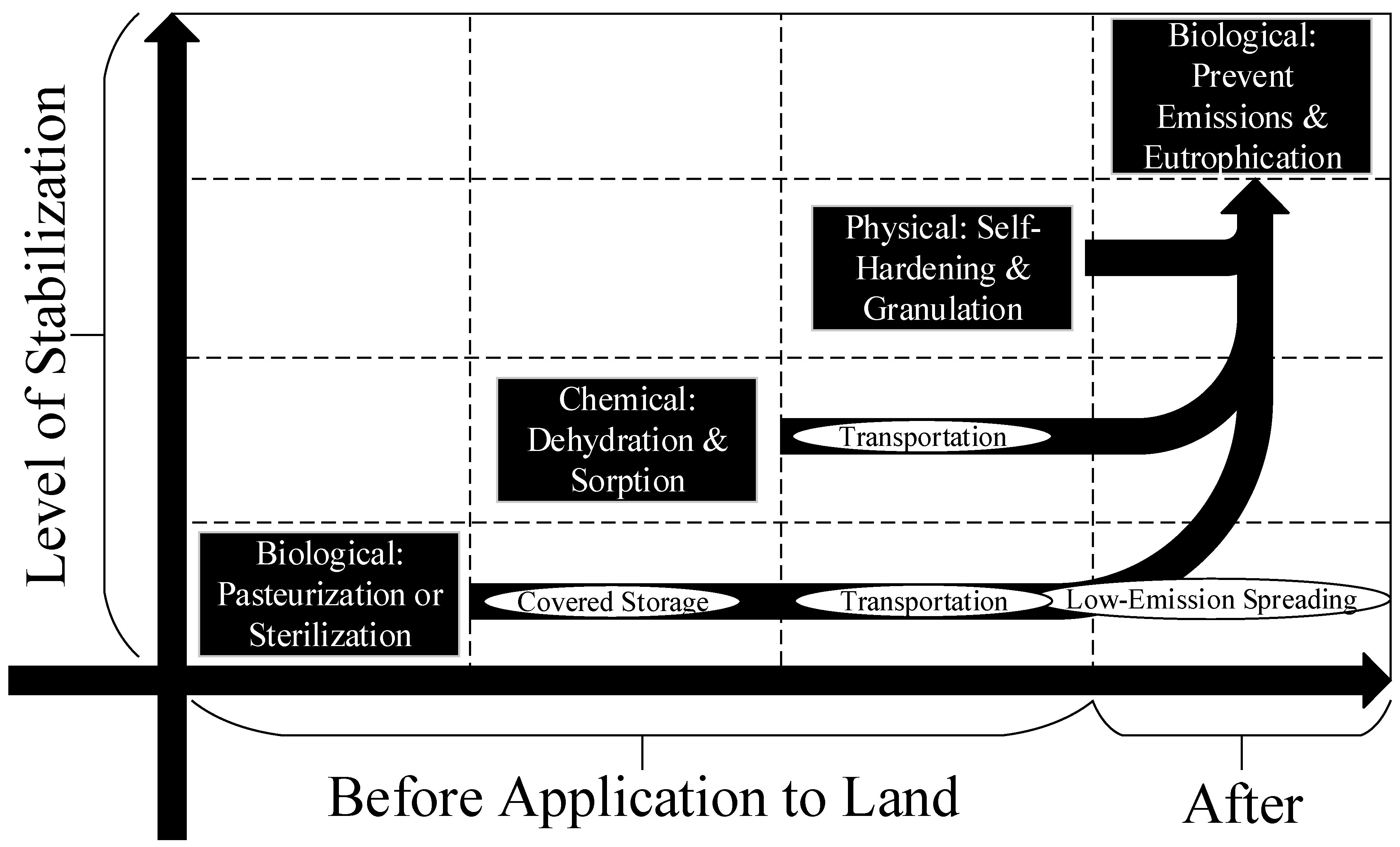
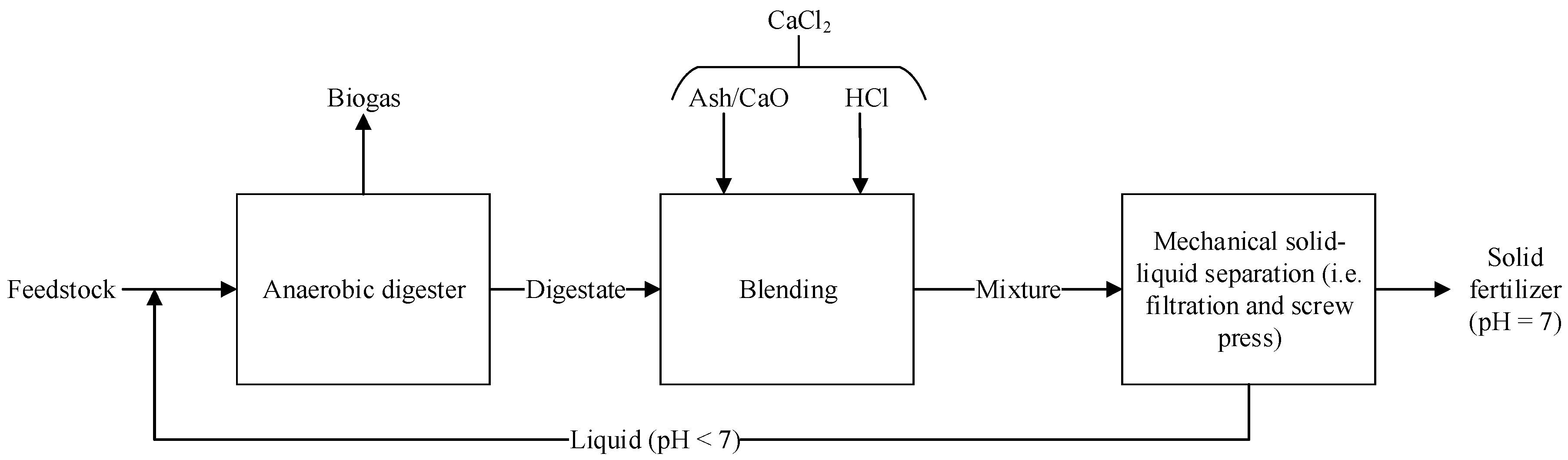
2. Materials and Methods: Level of Stability of the OS
2.1. Biological Stabilization before Land Application
2.2. Chemical Stabilization
2.3. Physical Stabilization
2.4. Biological Stabilization after Land Application
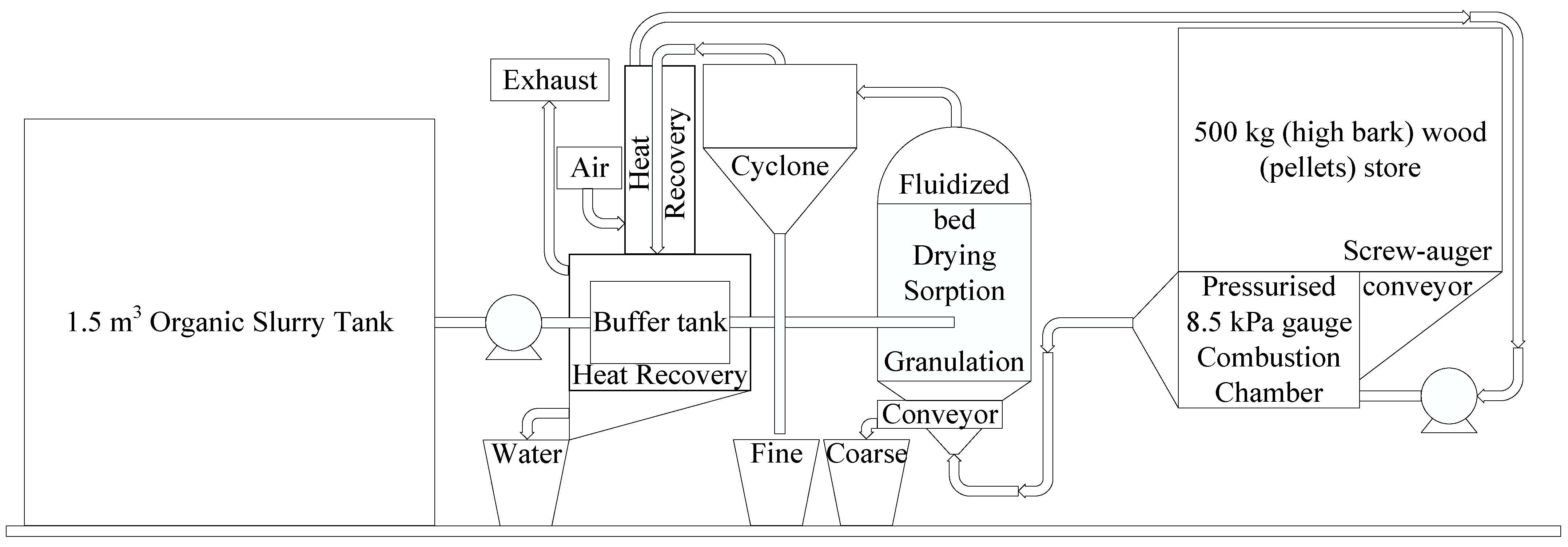
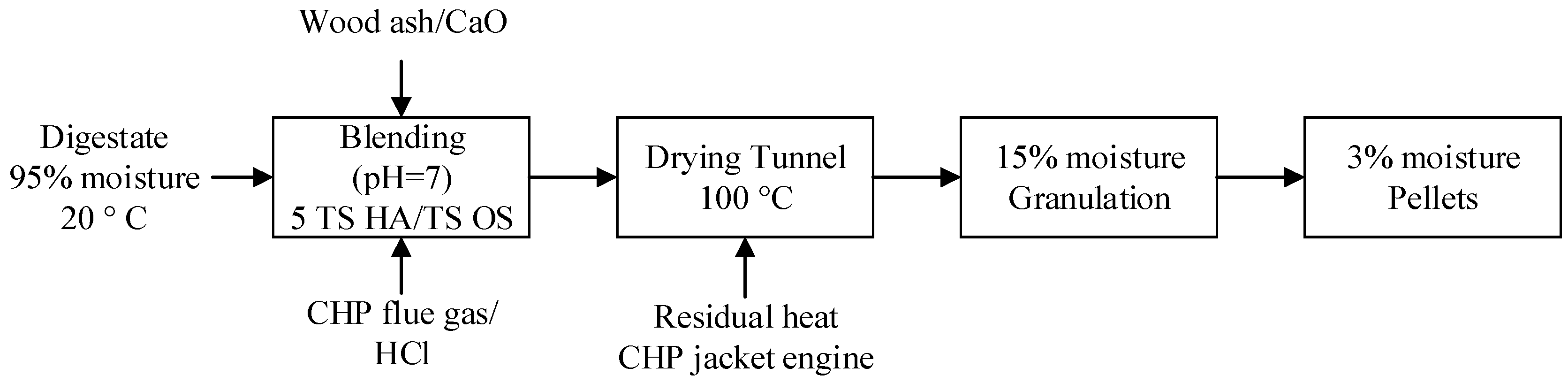
3. Results: Process Design
3.1. Dimensions of the Equipment
3.1.1. Power of the Fan
3.1.2. Dimensions of the Fluidized Bed Drier
3.1.3. Dimensions of the Cyclone
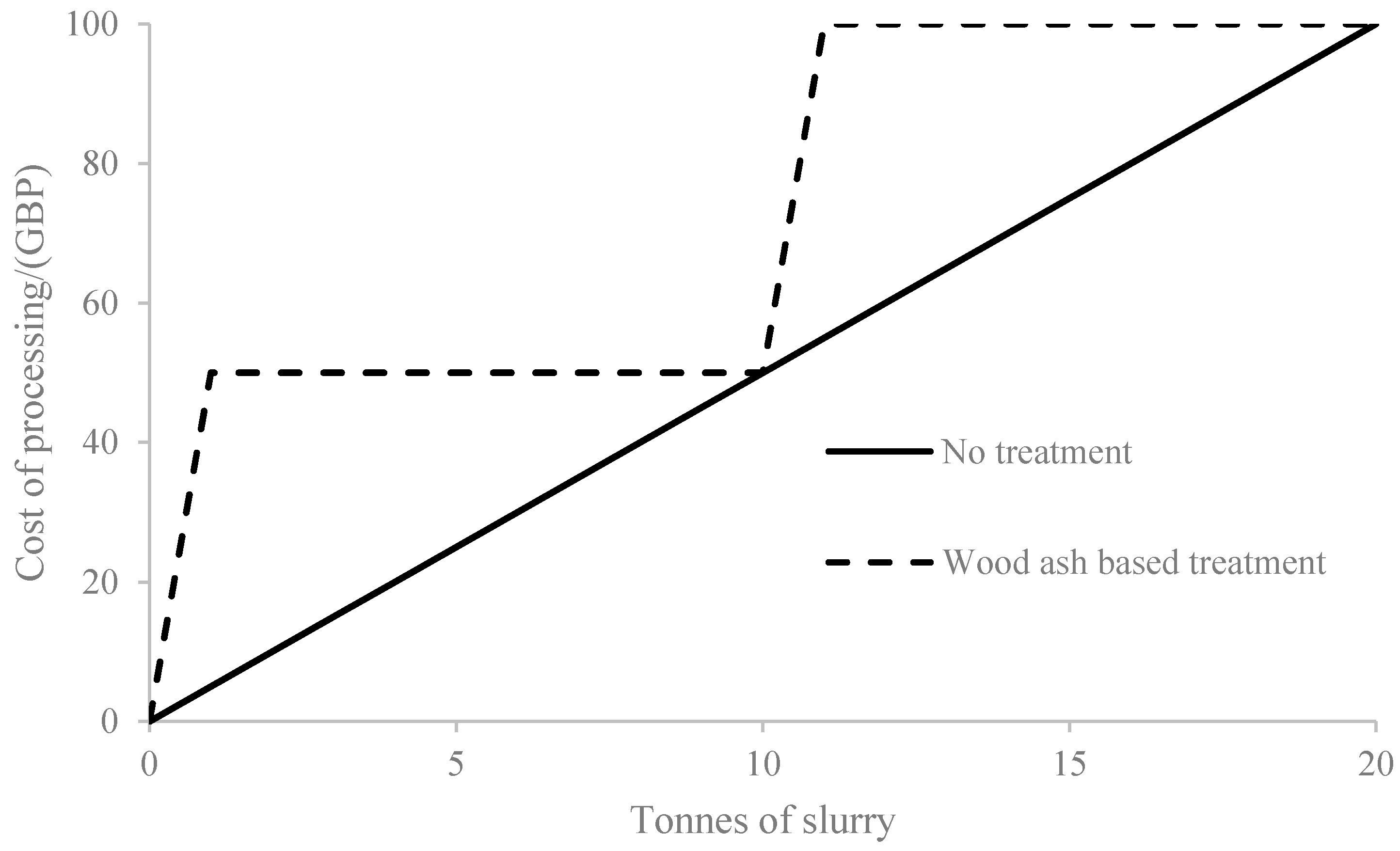
3.2. Economic Balance
- The longer the distance of transportation, the more economically viable the proposed process (Figure 4).
- When applying the untreated digestate, it is necessary to take into account the cost of the emissions of GHGs, which is established at a rate of GBP 27/tonne CO2 equivalent [73,74]. Hence, the proposed fluidized drying and wood ash stabilization process (Figure 4) can be justified if it lowers emissions.
4. Discussion: Development of a Business Plan for the Commercialization of the Technology
4.1. The Opportunity
4.2. Primary Market Research
4.3. Competitors
4.4. Barriers
4.5. Value Proposition and Customer Creation
5. Future Research Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| CATNAP | cheapest technology narrowly avoiding prosecution |
| GHG | greenhouse gas |
| HA | hardening agent |
| HTC | hydrothermal carbonization |
| NH3 | ammonia |
| NH4+-N | ammoniacal nitrogen |
| OS | organic slurry |
| OWAS | organic waste adsorption stabilization |
| PAS 110 | publicly available specification of anaerobic digestate in the UK |
| TS | total solids |
| VFA | volatile fatty acids |
| CaCl2 | calcium chloride |
| CO2 | carbon dioxide |
| CUE | carbon use efficiency |
| STRUBIAS | STRUvite, BIochar and Ash |
| Teagasc | agriculture and food development authority of Ireland |
| pH | potential of hydrogen |
| Al2(SO4)3 | aluminium sulphate |
| FeCl2 | iron chloride |
| CHP | combined heat and power plant |
References
- Hou, Y.; Velthof, G.L.; Case, S.D.C.; Oelofse, M.; Grignani, C.; Balsari, P.; Zavattaro, L.; Gioelli, F.; Bernal, M.P.; Fangueiro, D.; et al. Stakeholder perceptions of manure treatment technologies in Denmark, Italy, the Netherlands and Spain. J. Clean. Prod. 2018, 172, 1620–1630. [Google Scholar] [CrossRef]
- Blumenthal, K. Generation and Treatment of Municipal Waste. Available online: https://op.europa.eu/en/publication-detail/-/publication/bd8a43dc-8076-4134-987d-c3081c8311e8 (accessed on 14 December 2021).
- European Commission. Commission Staff Working Document: Accompanying the Communication from the Commission on Future Steps in Bio-Waste Management in the European Union. Available online: https://op.europa.eu/en/publication-detail/-/publication/95477ff0-0730-4758-9867-379c89d3bb2f/language-en (accessed on 30 January 2022).
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Mu, Z.X.; He, C.S.; Jiang, J.K.; Zhang, J.; Yang, H.Y.; Mu, Y. A modified two-point titration method for the determination of volatile fatty acids in anaerobic systems. Chemosphere 2018, 204, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, F.; Buratti, C. Biogas production from different substrates in an experimental Continuously Stirred Tank Reactor anaerobic digester. Bioresour. Technol. 2009, 100, 5783–5789. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, J.J.; Lefcourt, A.M.; Van Kessel, J.A.S.; Wilkerson, V. Managing Ammonia Emissions from Dairy Cows by Amending Slurry with Alum or Zeolite or by Diet Modification. Sci. World J. 2001, 1, 860–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemi, H.; Kianmehr, M.H.; Borghaee, A.M. Effect of Pellet Processing of Fertilizer on Slow-Release Nitrogen in Soil. Asian J. Plant Sci. 2010, 9, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.R.; Watabe, M.; Stewart, T.A.; Millar, B.C.; Moore, J.E. Pelleted organo-mineral fertilisers from composted pig slurry solids, animal wastes and spent mushroom compost for amenity grasslands. Waste Manag. 2007, 27, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Zafari, A.; Hosein Kianmehr, M. Effect of Temperature, Pressure and Moisture Content on Durability of Cattle Manure Pellet in Open-end Die Method. J. Agric. Sci. 2012, 4, 203–208. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.; Aggidis, G.; Aiouache, F. Alkaline Wood Ash, Turbulence, and Traps with Excess of Sulfuric Acid Do Not Strip Completely the Ammonia off an Agro-waste Digestate. Edelweiss Chem. Sci. J. 2021, 4, 19–24. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Impact of sulphuric, hydrochloric, nitric, and lactic acids in the preparation of a blend of agro-industrial digestate and wood ash to produce a novel fertiliser. J. Environ. Chem. Eng. 2021, 9, 105021. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Effects of Wood Ash-Based Alkaline Treatment on Nitrogen, Carbon, and Phosphorus Availability in Food Waste and Agro-Industrial Waste Digestates. Waste Biomass Valorization 2020, 12, 3355–3370. [Google Scholar] [CrossRef]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Kinetic study of the stabilization of an agro-industrial digestate by adding wood fly ash. Chem. Eng. J. Adv. 2021, 7, 100127. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to sulfuric acid for slurry acidification: Impact on slurry composition and ammonia emissions during storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- Regueiro, I.; Coutinho, J.; Gioelli, F.; Balsari, P.; Dinuccio, E.; Fangueiro, D. Acidification of raw and co-digested pig slurries with alum before mechanical separation reduces gaseous emission during storage of solid and liquid fractions. Agric. Ecosyst. Environ. 2016, 227, 42–51. [Google Scholar] [CrossRef]
- Podmirseg, S.M.; Seewald, M.S.A.; Knapp, B.A.; Bouzid, O.; Biderre-Petit, C.; Peyret, P.; Insam, H. Wood ash amendment to biogas reactors as an alternative to landfilling? A preliminary study on changes in process chemistry and biology. Waste Manag. Res. 2013, 31, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.; Pelkonen, M.; Lagerkvist, A. Co-digestion of sewage sludge and wood fly ash. Environ. Technol. 2020, 1–7. [Google Scholar] [CrossRef]
- Richards, S.; Marshall, R.; Lag-Brotons, A.J.; Semple, K.T.; Stutter, M. Phosphorus solubility changes following additions of bioenergy wastes to an agricultural soil: Implications for crop availability and environmental mobility. Geoderma 2021, 401, 115150. [Google Scholar] [CrossRef]
- Silva, A.; Fangueiro, D. Application of dairy manure amended with mineral fertilizer on stubble-covered soil: Effects on ammonia emissions. Biol. Life Sci. Forum 2021, 3, 19. [Google Scholar] [CrossRef]
- Mahfouz, S.a.; Shamf-Eldin, M.a. Effect of mineral vs. biofertilizer on growth, yield, and essential oil content of fennel (Foeniculum vulgare Mill.). Int. Agrophysics 2007, 21, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Insam, H.; Franke-Whittle, I.H.; Knapp, B.A.; Plank, R. Use of wood ash and anaerobic sludge for grassland fertilization: Effects on plants and microbes. Die Bodenkult. 2009, 60, 39–51. Available online: https://diebodenkultur.boku.ac.at/volltexte/band-60/heft-2/insam.pdf (accessed on 30 January 2022).
- Fernández-Delgado Juárez, M.; Waldhuber, S.; Knapp, A.; Partl, C.; Gómez-Brandón, M.; Insam, H. Wood ash effects on chemical and microbiological properties of digestate- and manure-amended soils. Biol. Fertil. Soils 2013, 49, 575–585. [Google Scholar] [CrossRef]
- Bougnom, B.P.; Niederkofler, C.; Knapp, B.A.; Stimpfl, E.; Insam, H. Residues from renewable energy production: Their value for fertilizing pastures. Biomass Bioenergy 2012, 39, 290–295. [Google Scholar] [CrossRef]
- de França, A.A.; von Tucher, S.; Schmidhalter, U. Effects of combined application of acidified biogas slurry and chemical fertilizer on crop production and N soil fertility. Eur. J. Agron. 2021, 123. [Google Scholar] [CrossRef]
- Essel, B.; Abaidoo, R.C.; Opoku, A.; Ewusi-Mensah, N. Economically Optimal Rate for Nutrient Application to Maize in the Semi-deciduous Forest Zone of Ghana. J. Soil Sci. Plant Nutr. 2020, 20, 1703–1713. [Google Scholar] [CrossRef]
- European Commission. The Nitrates Directive. Available online: https://ec.europa.eu/environment/water/water-nitrates/index_en.html (accessed on 24 January 2022).
- UK AHDB. Nutrient Management Guide (RB209). Available online: https://ahdb.org.uk/knowledge-library/rb209-section-2-organic-materials (accessed on 28 January 2022).
- UK AHDB. Nutrient Management Guide (RB209). Available online: https://ahdb.org.uk/knowledge-library/rb209-section-4-arable-crops (accessed on 28 January 2022).
- Moure Abelenda, A.; Aiouache, F. Wood Ash Based Treatment of Anaerobic Digestate: State-of-the-Art and Possibilities. Processes 2022, 10, 147. [Google Scholar] [CrossRef]
- Forbes, M.S.; Raison, R.J.; Skjemstad, J.O. Formation, transformation and transport of black carbon (charcoal) in terrestrial and aquatic ecosystems. Sci. Total Environ. 2006, 370, 190–206. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef]
- Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Exploring technological alternatives of nutrient recovery from digestate as a secondary resource. Renew. Sustain. Energy Rev. 2020, 134, 110379. [Google Scholar] [CrossRef]
- Hoffmann, J.; Rudra, S.; Toor, S.S.; Holm-Nielsen, J.B.; Rosendahl, L.A. Conceptual design of an integrated hydrothermal liquefaction and biogas plant for sustainable bioenergy production. Bioresour. Technol. 2013, 129, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Latham, K.G.; Kozyatnyk, I.; Figueira, J.; Carlborg, M.; Rosenbaum, E. Self-generation of low ash carbon microspheres from the hydrothermal supernatant of anaerobic digestate: Formation insights and supercapacitor performance. Chem. Eng. J. Adv. 2021, 6, 100097. [Google Scholar] [CrossRef]
- Yun, S.; Fang, W.; Du, T.; Hu, X.; Huang, X.; Li, X.; Zhang, C.; Lund, P.D. Use of bio-based carbon materials for improving biogas yield and digestate stability. Energy 2018, 164, 898–909. [Google Scholar] [CrossRef]
- Shi, W.; Healy, M.G.; Ashekuzzaman, S.M.; Daly, K.; Leahy, J.J.; Fenton, O. Dairy processing sludge and co-products: A review of present and future re-use pathways in agriculture. J. Clean. Prod. 2021, 314, 128035. [Google Scholar] [CrossRef]
- WRAP. BSI PAS 110:2014 Specification for Whole Digestate, Separated Liquor and Separated Fibre Derived from the Anaerobic Digestion of Source-Segregated Biodegradable Materials. Available online: https://wrap.org.uk/resources/guide/bsi-pas-110-producing-quality-anaerobic-digestate (accessed on 7 January 2022).
- Banks, C.J.; Haeven, S.; Zhang, Y.; Sapp, M. Review of the application of the Residual Biogas Potential Test. Available online: http://www.organics-recycling.org.uk/uploads/article2652/PAS110%20digestate%20stability%20review.pdf (accessed on 14 December 2021).
- Dijkstra, P.; Salpas, E.; Fairbanks, D.; Miller, E.B.; Hagerty, S.B.; Jan, K.; Groenigen, V.; Hungate, B.A.; Marks, J.C.; Koch, G.W.; et al. High carbon use efficiency in soil microbial communities is related to balanced growth, not storage compound synthesis. Soil Biol. Biochem. 2015, 89, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Saveyn, H.; Eder, P. End-of-Waste Criteria for Biodegradable Waste Subjected to Biological Treatment (Compost & Digestate): Technical Proposals. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC87124 (accessed on 14 December 2021).
- UK Government. Quality Protocol: Compost—End of Waste Criteria for the Production and Use of Quality Compost from Source-Segregated Biodegradable Waste. Available online: https://www.gov.uk/government/publications/quality-protocol-for-the-production-and-use-of-compost-from-waste (accessed on 14 December 2021).
- UK WRAP. PAS 100:2011 Specification for Composted Materials. Available online: http://www.organics-recycling.org.uk/page.php?article=3483#:~:text=PAS%20100%3A2018%20requires%20producers%20to%20set%20up%20a,also%20relates%20to%20the%20new%20%C3%82%C2%91compost%20quality%C3%82%C2%92%20clause (accessed on 15 December 2021).
- European Parliament. Regulation (EU) 2019/1009 Fertilizer Products. Off. J. Eur. Union. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32019R1009 (accessed on 30 January 2022).
- Szymula, A.; Wlazło, Ł.; Sasáková, N.; Wnuk, W.; Nowakowicz-Dębek, B. The Use of Natural Sorbents to Reduce Ammonia Emissions from Cattle Faeces. Agronomy 2021, 11, 2543. [Google Scholar] [CrossRef]
- Ashekuzzaman, S.M.; Fenton, O.; Meers, E.; Forrestal, P.J. Differing Phosphorus Crop Availability of Aluminium and Calcium Precipitated Dairy Processing Sludge Potential Recycled Alternatives to Mineral Phosphorus Fertiliser. Agronomy 2021, 11, 427. [Google Scholar] [CrossRef]
- Yagi, S.; Fukushi, K. Removal of phosphate from solution by adsorption and precipitation of calcium phosphate onto monohydrocalcite. J. Colloid Interface Sci. 2012, 384, 128–136. [Google Scholar] [CrossRef]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Randall, D.G.; Krähenbühl, M.; Köpping, I.; Larsen, T.A.; Udert, K.M. A novel approach for stabilizing fresh urine by calcium hydroxide addition. Water Res. 2016, 95, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Simha, P.; Friedrich, C.; Randall, D.G.; Vinnerås, B. Alkaline Dehydration of Human Urine Collected in Source-Separated Sanitation Systems Using Magnesium Oxide. Front. Environ. Sci. 2021, 8, 619901. [Google Scholar] [CrossRef]
- Simha, P.; Lalander, C.; Nordin, A.; Vinnerås, B. Alkaline dehydration of source-separated fresh human urine: Preliminary insights into using different dehydration temperature and media. Sci. Total Environ. 2020, 733, 139313. [Google Scholar] [CrossRef] [PubMed]
- Simha, P.; Senecal, J.; Nordin, A.; Lalander, C.; Vinnerås, B. Alkaline dehydration of anion–exchanged human urine: Volume reduction, nutrient recovery and process optimisation. Water Res. 2018, 142, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.R.; Tilley, E.; Udert, K.M. Wood ash as a magnesium source for phosphorus recovery from source-separated urine. Sci. Total Environ. 2012, 419, 68–75. [Google Scholar] [CrossRef]
- James, A.K.; Thring, R.W.; Helle, S.; Ghuman, H.S. Ash management review-applications of biomass bottom ash. Energies 2012, 5, 3856–3873. [Google Scholar] [CrossRef]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef]
- Miranda, C.; Soares, A.S.; Coelho, A.C.; Trindade, H.; Teixeira, C.A. Environmental implications of stored cattle slurry treatment with sulphuric acid and biochar: A life cycle assessment approach. Environ. Res. 2021, 194, 110640. [Google Scholar] [CrossRef]
- Kavanagh, I.; Burchill, W.; Healy, M.G.; Fenton, O.; Krol, D.J.; Lanigan, G.J. Mitigation of ammonia and greenhouse gas emissions from stored cattle slurry using acidifiers and chemical amendments. J. Clean. Prod. 2019, 237, 117822. [Google Scholar] [CrossRef]
- Dinuccio, E.; Berg, W.; Balsari, P. Gaseous emissions from the storage of untreated slurries and the fractions obtained after mechanical separation. Atmos. Environ. 2008, 42, 2448–2459. [Google Scholar] [CrossRef] [Green Version]
- Dinuccio, E.; Gioelli, F.; Balsari, P.; Dorno, N. Ammonia losses from the storage and application of raw and chemo-mechanically separated slurry. Agric. Ecosyst. Environ. 2012, 153, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Dinuccio, E.; Balsari, P.; Berg, W. GHG emissions during the storage of rough pig slurry and the fractions obtained by mechanical separation. Aust. J. Exp. Agric. 2008, 48, 93–95. [Google Scholar] [CrossRef]
- Kavanagh, I.; Fenton, O.; Healy, M.G.; Burchill, W.; Lanigan, G.J.; Krol, D.J. Mitigating ammonia and greenhouse gas emissions from stored cattle slurry using agricultural waste, commercially available products and a chemical acidifier. J. Clean. Prod. 2021, 294, 126251. [Google Scholar] [CrossRef]
- Klages, S.; Heidecke, C.; Osterburg, B.; Bailey, J.; Calciu, I.; Casey, C.; Dalgaard, T.; Frick, H.; Glavan, M.; D’Haene, K.; et al. Nitrogen Surplus—A Unified Indicator for Water Pollution in Europe? Water 2020, 12, 1197. [Google Scholar] [CrossRef] [Green Version]
- Huygens, D.; Orveillon, G.; Lugato, E.; Tavazzi, S.; Comero, S.; Jones, A.; Gawlik, B.; Saveyn, H. Technical Proposals for the Safe Use of Processed Manure above the Threshold Established for Nitrate Vulnerable Zones by the Nitrates Directive (91/676/EEC). Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC121636 (accessed on 24 January 2022).
- UK Government. Nitrate Vulnerable Zones. Available online: https://www.gov.uk/government/collections/nitrate-vulnerable-zones (accessed on 24 January 2022).
- UK Government. Clean Air Strategy 2019. Available online: https://www.gov.uk/government/publications/clean-air-strategy-2019 (accessed on 24 January 2022).
- UK DEFRA. The Path to Sustainable Farming: An Agricultural Transition Plan 2021 to 2024. Available online: https://www.gov.uk/government/publications/agricultural-transition-plan-2021-to-2024 (accessed on 30 December 2021).
- UK DEFRA. Farming is Changing. Available online: https://www.gov.uk/government/publications/future-farming-changes-to-farming-in-england (accessed on 30 December 2021).
- UK DEFRA. Overview: How Farming is Changing. Available online: https://defrafarming.blog.gov.uk/2021/06/23/how-farming-is-changing/ (accessed on 24 January 2022).
- Buckley, C.; Krol, D.; Lanigan, G.; Donnellan, T.; Spink, J.; Hanrahan, K.; Boland, A.; Forrestal, P.; Humphreys, J.; Murphy, P.; et al. An Analysis of the Cost of the Abatement of Ammonia Emissions in Irish Agriculture to 2030. Available online: https://www.teagasc.ie/media/website/publications/2020/NH3-Ammonia-MACC.pdf (accessed on 31 December 2021).
- Lanigan, G.J.; Donnellan, T.; Lanigan, G.J.; Hanrahan, K.; Paul, C.; Shalloo, L.; Krol, D.; Forrestal, P.; Farrelly, N.; O’brien, D.; et al. An Analysis of Abatement Potential of Greenhouse Gas Emissions in Irish Agriculture 2021-2030 Prepared by the Teagasc Greenhouse Gas Working Group Authors. Available online: https://www.teagasc.ie/media/website/publications/2018/An-Analysis-of-Abatement-Potential-of-Greenhouse-Gas-Emissions-in-Irish-Agriculture-2021-2030.pdf (accessed on 31 December 2021).
- UK DEFRA. Code of Good Agricultural Practice (COGAP) for Reducing Ammonia Emissions. Available online: https://www.gov.uk/government/publications/code-of-good-agricultural-practice-for-reducing-ammonia-emissions (accessed on 30 December 2021).
- European Commission. BAT Conclusions for the Intensive Rearing of Poultry or Pigs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017D0302&from=ES (accessed on 28 January 2022).
- Wróbel, M.; Jewiarz, M.; Mudryk, K.; Fraczek, J.; Dziedzic, K. Conceptual design of the mobile granulation line for production fertilizers from digestates and ash mixtures. MATEC Web Conf. 2018, 168, 04003. [Google Scholar] [CrossRef] [Green Version]
- Al-Mallahi, J.; Sürmeli, R.Ö.; Çalli, B. Recovery of phosphorus from liquid digestate using waste magnesite dust. J. Clean. Prod. 2020, 272. [Google Scholar] [CrossRef]
- Mudryk, K.; Frączek, J.; Wróbel, M.; Jewiarz, M.; Dziedzic, K. Agglomeration of Ash-Based Fertilizer Mixtures from Biomass Combustion and Digestate. In Renewable Energy Sources: Engineering, Technology, Innovation; Krzysztof, M., Sebastian, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 823–834. [Google Scholar] [CrossRef]
- Pesonen, J.; Kuokkanen, V.; Kuokkanen, T.; Illikainen, M. Co-granulation of bio-ash with sewage sludge and lime for fertilizer use. J. Environ. Chem. Eng. 2016, 4, 4817–4821. [Google Scholar] [CrossRef]
- Steenari, B.M.; Lindqvist, O. Stabilisation of biofuel ashes for recycling to forest soil. Biomass Bioenergy 1997, 13, 39–50. [Google Scholar] [CrossRef]
- Pampuro, N.; Bagagiolo, G.; Priarone, P.C.; Cavallo, E. Effects of pelletizing pressure and the addition of woody bulking agents on the physical and mechanical properties of pellets made from composted pig solid fraction. Powder Technol. 2017, 311, 112–119. [Google Scholar] [CrossRef]
- Brennan, R.B.; Healy, M.G.; Fenton, O.; Lanigan, G.J. The effect of chemical amendments used for phosphorus abatement on greenhouse gas and ammonia emissions from dairy cattle slurry: Synergies and pollution swapping. PLoS ONE 2015, 10, e0111965. [Google Scholar] [CrossRef] [Green Version]
- Stevens, C.J.; Quinton, J.N. Policy implications of pollution swapping. Phys. Chem. Earth 2009, 34, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Manzoni, S.; Moorhead, D.L.; Richter, A. Carbon use efficiency of microbial communities: Stoichiometry, methodology and modelling. Ecol. Lett. 2013, 16, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Bastami, M.S.B.; Jones, D.L.; Chadwick, D.R. Reduction of Methane Emission during Slurry Storage by the Addition of Effective Microorganisms and Excessive Carbon Source from Brewing Sugar. J. Environ. Qual. 2016, 45, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Briceño, C.I.; Pozarlik, A.K.; Bramer, E.A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Brem, G. Hydrothermal carbonization of wet biomass from nitrogen and phosphorus approach: A review. Renew. Energy 2021, 171, 401–415. [Google Scholar] [CrossRef]
- Magdziarz, A.; Mlonka-Mędrala, A.; Sieradzka, M.; Aragon-Briceño, C.; Pożarlik, A.; Bramer, E.A.; Brem, G.; Niedzwiecki, Ł.; Pawlak-Kruczek, H. Multiphase analysis of hydrochars obtained by anaerobic digestion of municipal solid waste organic fraction. Renew. Energy 2021, 175, 108–118. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Niedzwiecki, L.; Sieradzka, M.; Mlonka-Mędrala, A.; Baranowski, M.; Serafin-Tkaczuk, M.; Magdziarz, A. Hydrothermal carbonization of agricultural and municipal solid waste digestates—Structure and energetic properties of the solid products. Fuel 2020, 275. [Google Scholar] [CrossRef]
- Fujioka, M.; Ito, R. Development of Separation Process of Soluble Nutrients from Synthetic Dairy Slurry by Modified Solvay Process. Sanit. Value Chain 2020, 4, 17–26. [Google Scholar] [CrossRef]
- Drapanauskaite, D.; Handler, R.M.; Fox, N.; Baltrusaitis, J. Transformation of Liquid Digestate from the Solid-Separated Biogas Digestion Reactor Effluent into a Solid NH4HCO3 Fertilizer: Sustainable Process Engineering and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 580–588. [Google Scholar] [CrossRef]
- UK WRAP. Conversion of Struvite via Gas Infusion Acidification. Available online: https://wrap.org.uk/sites/files/wrap/CwmHarry-DIAD2feasibilitystudy.pdf (accessed on 1 June 2018).
- Jewiarz, M.; Wróbel, M.; Fraczek, J.; Mudryk, K.; Dziedzic, K. Digestate, ash and Trichoderm based fertilizer-production line concept design. MATEC Web Conf. 2018, 168, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mor, S.; Chhoden, K.; Ravindra, K. Application of agro-waste rice husk ash for the removal of phosphate from the wastewater. J. Clean. Prod. 2016, 129, 673–680. [Google Scholar] [CrossRef]
- Balcas Energy Ltd. Wood Pellets Specifications. Available online: https://balcasenergy.com/online-shop/bulk-wood-pellets/ (accessed on 28 January 2022).
- Nara Machinery Ltd. Drying Unit for Slurry Material. Available online: https://www.nara-m.co.jp/english/product/dryer/msd.html (accessed on 31 December 2021).
- Chhabra, R.; Basavaraj, M.G. Fluidisation. In Coulson and Richardson’s Chemical Engineering, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 445–455. [Google Scholar] [CrossRef]
- Air Control Industries Ltd. ACI Product Selector. Available online: https://fans-uk.aircontrolindustries.com/ (accessed on 31 December 2021).
- Berg, W.; Salamat, R.; Scaar, H.; Mellmann, J. Investigation of nitrogen loss during laboratory scale fixed-bed drying of digestate. Waste Manag. 2021, 129, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Weigand, H.; Bertau, M.; Hübner, W.; Bohndick, F.; Bruckert, A. RecoPhos: Full-scale fertilizer production from sewage sludge ash. Waste Manag. 2013, 33, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Grass, H.; Lory, M.; Krämer, T.; Thali, M.; Bartsch, C. Lethal carbon monoxide poisoning in wood pellet storerooms-two cases and a review of the literature. Ann. Occup. Hyg. 2012, 56, 755–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, P. Biomass Handling. In Biomass Gasification, Pyrolysis and Torrefaction; Academic Press: Cambridge, MA, USA, 2018; pp. 445–478. [Google Scholar] [CrossRef]
- Balcas Energy Ltd. Wood Pellets’ Plant in Asphalt Production. Available online: https://www.youtube.com/watch?v=D4E3COSsyVk (accessed on 29 January 2022).
- Moilanen, M.; Saarsalmi, A.; Kukkola, M.; Issakainen, J. Effects of stabilized wood ash on nutrient status and growth of Scots pine—Comparison between uplands and peatlands. For. Ecol. Manag. 2013, 295, 136–144. [Google Scholar] [CrossRef]
- Gemco Energy. Flat Die Feed Pellet Mill Technical Data. Available online: http://www.gemco-energy.com/Flat-die-pellet-mill/feed-pellet-mill/ (accessed on 28 January 2022).
- Voshell, S.; Mäkelä, M.; Dahl, O. A review of biomass ash properties towards treatment and recycling. Renew. Sustain. Energy Rev. 2018, 96, 479–486. [Google Scholar] [CrossRef]
- Merino-Martín, L.; Stokes, A.; Gweon, H.S.; Moragues-Saitua, L.; Staunton, S.; Plassard, C.; Oliver, A.; Le Bissonnais, Y.; Griffiths, R.I. Interacting effects of land use type, microbes and plant traits on soil aggregate stability. Soil Biol. Biochem. 2021, 154, 108072. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Thermodynamic and Experimental Investigation of Solar-Driven Biomass Pyro-Gasification Using H2O, CO2, or ZnO Oxidants for Clean Syngas and Metallurgical Zn Production. Processes 2021, 9, 687. [Google Scholar] [CrossRef]
- Pipe Flow Calculations. Flue Gases Properties Table: Density, Specific Heat, Viscosity. Available online: https://www.pipeflowcalculations.com/tables/flue-gas.xhtml (accessed on 31 December 2021).
- Someshwar, A.V. Wood and Combination Wood-Fired Boiler Ash Characterization. J. Environ. Qual. 1996, 25, 962–972. [Google Scholar] [CrossRef]
- Pu, Y.; Li, L.; Wang, Q.; Shi, X.; Fu, L.; Zhang, G.; Luan, C.; Abomohra, A.E.-F. Accelerated carbonation treatment of recycled concrete aggregates using flue gas: A comparative study towards performance improvement. J. CO2 Util. 2021, 43. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y.; Serna-Guerrero, R. Flue gas treatment via CO2 adsorption. Chem. Eng. J. 2011, 171, 760–774. [Google Scholar] [CrossRef]
- Martínez, I.; Arias, B.; Grasa, G.S.; Abanades, J.C. CO2 capture in existing power plants using second generation Ca-Looping systems firing biomass in the calciner. J. Clean. Prod. 2018, 187, 638–649. [Google Scholar] [CrossRef]
- Crutchik, D.; Morales, N.; Vázquez-Padín, J.R.; Garrido, J.M. Enhancement of struvite pellets crystallization in a full-scale plant using an industrial grade magnesium product. Water Sci. Technol. 2017, 75, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Garrido Fernández, J.M.; Crutchik Pedemonte, D. Method and System for the Crystallisation of Struvite for Recovering Phosphates in Wastewater. Available online: https://patents.google.com/patent/EP3112320A1/en (accessed on 30 January 2022).
- Xu, Y.; Lu, B.; Luo, C.; Chen, J.; Zhang, Z.; Zhang, L. Sorption enhanced steam reforming of ethanol over Ni-based catalyst coupling with high-performance CaO pellets. Chem. Eng. J. 2021, 406, 126903. [Google Scholar] [CrossRef]
- Duroudier, J.-P. Separation Between a Fluid and a Divided Solid Through Centrifugal Force. In Liquid-Gas and Solid-Gas Separators; Elsevier: Amsterdam, The Netherlands, 2016; pp. 103–143. [Google Scholar]
- Victor, L. AD and Composting Industry Market Survey Report 2020. Available online: https://wrap.org.uk/resources/report/anaerobic-digestion-and-composting-latest-industry-survey-report-new-summaries (accessed on 14 December 2021).
- ADAS UK Ltd. Digestate distribution models. Available online: http://www.wrap.org.uk/sites/files/wrap/Digestatedistributionmodelsreport.pdf (accessed on 3 February 2017).
- Galician Environmental Agency. Technical Guidelines for Management of Farm Manures as Fertilizers. Available online: https://cmatv.xunta.gal/seccion-organizacion/c/CMAOT_SX_de_Calidade_e_Avaliacion_Ambiental?content=SX_Calidade_Avaliacion_Ambiental/Autorizacion_ambiental_integrada/seccion.html&std=ProxectosAgroforestais.html (accessed on 29 January 2022).
- Welsh Government. Evaluate Cost Effective Slurry Storage and Management Options. Available online: https://businesswales.gov.wales/farmingconnect/news-and-events/news/evaluate-cost-effective-slurry-storage-and-management-options (accessed on 31 December 2021).
- Wood, E.; James, K.; Barker, E. Comparison of the Environmental Impacts of Nitrogenous Materials. Available online: https://wrap.org.uk/sites/default/files/2021-01/Nitrogenous%20Materials%20Report%202020.pdf (accessed on 14 December 2021).
- US Department of Agriculture. Farm Business Planning Course. Available online: https://nesfp.org/farmer-training/farm-business-planning (accessed on 29 January 2022).
- Teagasc. Farm Business Planning. Available online: https://www.teagasc.ie/rural-economy/farm-management/financial-analysis/farm-business-planning/ (accessed on 29 January 2022).
- Global Research Alliance on Agricultural Greenhouse Gases. Webinar: Development of Bio-Based Fertilizers for a Circular Bioeconomy. Available online: https://globalresearchalliance.org/event/webinar-development-of-bio-based-fertilisers-for-a-circular-bioeconomy/ (accessed on 29 January 2022).
- ADAS UK Ltd. Farm Business Planning Webinar. Available online: https://www.facebook.com/events/464480348570377?ref=newsfeed (accessed on 29 January 2022).
- Norwood, F.B.; Peel, D. Supply Chain Mapping to Prepare for Future Pandemics. Appl. Econ. Perspect. Policy 2020, 43, 412–429. [Google Scholar] [CrossRef]
- Pereira, A.; Turnes, A.; Vence, X. Barriers to shifting to a servicized model of crop protection in smallholding viticulture. J. Clean. Prod. 2017, 149, 701–710. [Google Scholar] [CrossRef]
- Van der Stelt, B.; Temminghoff, E.J.M.; Van Vliet, P.C.J.; Van Riemsdijk, W.H. Volatilization of ammonia from manure as affected by manure additives, temperature and mixing. Bioresour. Technol. 2007, 98, 3449–3455. [Google Scholar] [CrossRef]
- Joseph Morton Ltd. Slurry Additives. Available online: https://www.josephmorton.co.uk/slurry-additives (accessed on 31 December 2021).
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Evaluation of strategies for the production of a stable blended fertilizer of anaerobic digestates and wood ashes. Nat.-Based Solut. 2021. Forthcoming. [Google Scholar]
- Gonzalez-Perez, A.; Hägg, K.; Duteil, F. Optimizing NOM Removal: Impact of Calcium Chloride. Sustainability 2021, 13, 6338. [Google Scholar] [CrossRef]
- UK Sentencing Council. Environmental Offences Definitive Guideline. Available online: https://www.sentencingcouncil.org.uk/publications/item/environmental-offences-definitive-guideline/ (accessed on 30 January 2022).
- Huotari, N.; Tillman-Sutela, E.; Moilanen, M.; Laiho, R. Recycling of ash—For the good of the environment? For. Ecol. Manag. 2015, 348, 226–240. [Google Scholar] [CrossRef]
- UK Government. Quality Protocol Poultry Litter Ash. End of Waste Criteria for the Production and use of Treated Ash from the Incineration of Poultry Litter, Feathers and Straw. Available online: https://www.gov.uk/government/publications/quality-protocol-poultry-litter-ash (accessed on 14 December 2021).
- Zheng, Y.; Ke, L.; Xia, D.; Zheng, Y.; Wang, Y.; Li, H.; Li, Q. Enhancement of digestates dewaterability by CTAB combined with CFA pretreatment. Sep. Purif. Technol. 2016, 163, 282–289. [Google Scholar] [CrossRef]
- UK Government. Quality Protocol of Anaerobic Digestate. End of Waste Criteria for the Production and Use of Quality Outputs from Anaerobic Digestion of Source-Segregated Biodegradable Waste. Available online: https://www.gov.uk/government/publications/quality-protocol-anaerobic-digestate (accessed on 14 December 2021).
- UK Environmental Agency. Converting Waste into Products: End of Waste Submission Form Guidance. Available online: https://www.gov.uk/government/collections/quality-protocols-end-of-waste-frameworks-for-waste-derived-products (accessed on 30 January 2022).
- UK Environmental Agency. A Waste Comparator Tool to Support End-of-waste Applications. Available online: https://www.gov.uk/government/publications/defining-product-comparators-to-use-when-applying-waste-derived-materials-to-land (accessed on 30 January 2022).
- European Commission. Directive 86/278/EEC Protection of the Environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Communities 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01986L0278-20180704 (accessed on 30 January 2022).
- Ondrasek, G.; Kranjčec, F.; Filipović, L.; Filipović, V.; Bubalo Kovačić, M.; Badovinac, I.J.; Peter, R.; Petravić, M.; Macan, J.; Rengel, Z. Biomass bottom ash & dolomite similarly ameliorate an acidic low-nutrient soil, improve phytonutrition and growth, but increase Cd accumulation in radish. Sci. Total Environ. 2021, 753, 141902. [Google Scholar] [CrossRef]
- Vogel, C.; Adam, C.; Peplinski, B.; Wellendorf, S. Chemical reactions during the preparation of P and NPK fertilizers from thermochemically treated sewage sludge ashes. Soil Sci. Plant Nutr. 2010, 56, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Pawlak-Kruczek, H.; Wnukowski, M.; Niedzwiecki, L.; Kowal, M.; Krochmalny, K. Gasification of Torrefied Sewage Sludge With the Addition of Calcium Carbonate. J. Energy Resour. Technol. 2020, 142, 070910. [Google Scholar] [CrossRef]
- Anton, C.; Micu, A.E.; Rusu, E. Introducing the Living Lab Approach in the Coastal Area of Constanta (Romania) by Using Design Thinking. Inventions 2022, 7, 19. [Google Scholar] [CrossRef]
- Moure Abelenda, A. Prototype with CaCl2 as Dessicant. Available online: https://www.youtube.com/watch?v=6bM9fVIaOow (accessed on 31 December 2021).
- Brienza, C.; Sigurnjak, I.; Meier, T.; Michels, E.; Adani, F.; Schoumans, O.; Vaneeckhaute, C.; Meers, E. Techno-economic assessment at full scale of a biogas refinery plant receiving nitrogen rich feedstock and producing renewable energy and biobased fertilisers. J. Clean. Prod. 2021, 308, 127408. [Google Scholar] [CrossRef] [PubMed]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- IGAPE. Guide for Elaboration of a Business Plan and Finantial Study. Available online: http://www.igape.es/es/crear-unha-empresa/crear-unha-empresa/plan-de-negocio/plan-de-empresa (accessed on 31 December 2021).
- Hanak, D.P.; Erans, M.; Nabavi, S.A.; Jeremias, M.; Romeo, L.M.; Manovic, V. Technical and economic feasibility evaluation of calcium looping with no CO2 recirculation. Chem. Eng. J. 2018, 335, 763–773. [Google Scholar] [CrossRef]


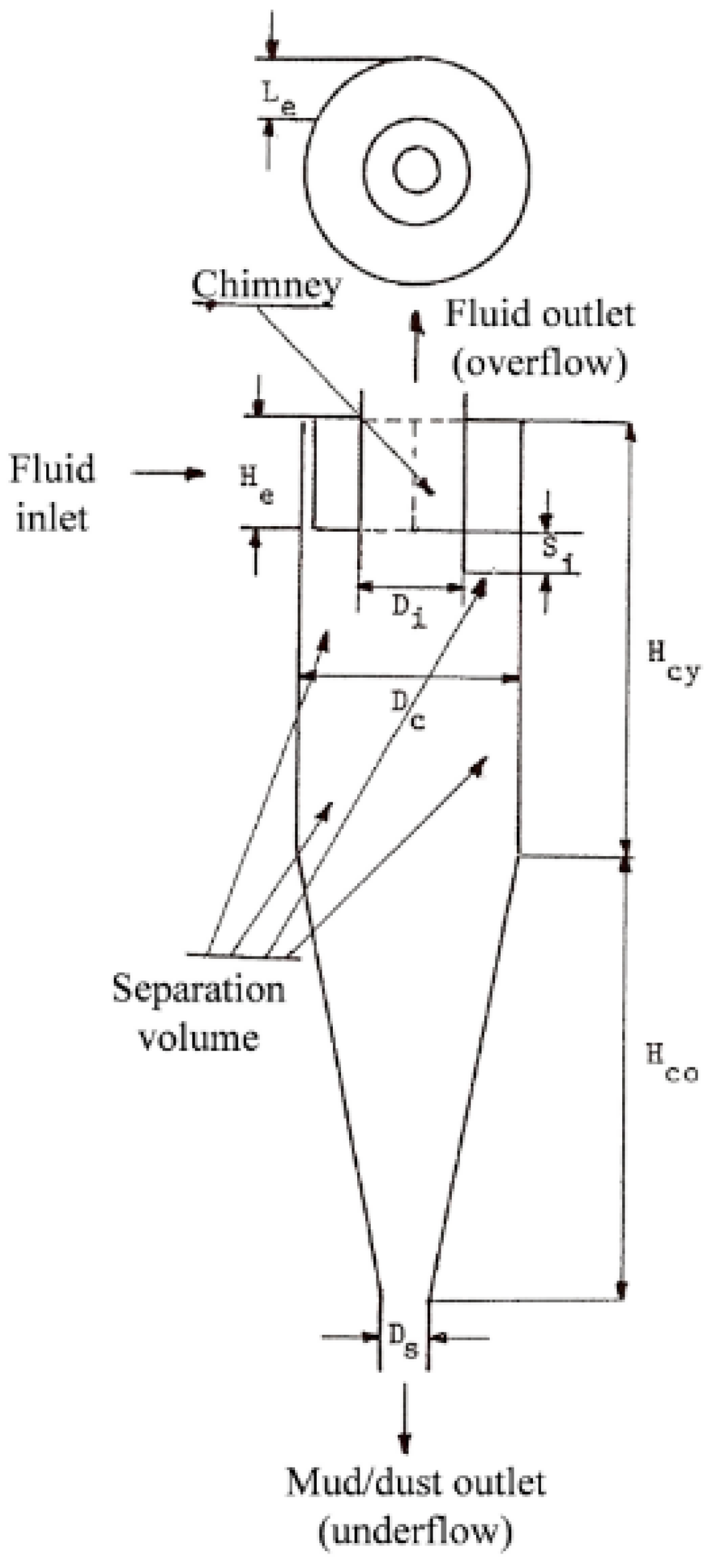

| Dimension | cm |
|---|---|
| Dc | 21 |
| Le | 5 |
| He | 11 |
| Di | 11 |
| Si | 3 |
| Hcy | 43 |
| Hco | 43 |
| Ds | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moure Abelenda, A.; Amaechi, C.V. Manufacturing of a Granular Fertilizer Based on Organic Slurry and Hardening Agent. Inventions 2022, 7, 26. https://doi.org/10.3390/inventions7010026
Moure Abelenda A, Amaechi CV. Manufacturing of a Granular Fertilizer Based on Organic Slurry and Hardening Agent. Inventions. 2022; 7(1):26. https://doi.org/10.3390/inventions7010026
Chicago/Turabian StyleMoure Abelenda, Alejandro, and Chiemela Victor Amaechi. 2022. "Manufacturing of a Granular Fertilizer Based on Organic Slurry and Hardening Agent" Inventions 7, no. 1: 26. https://doi.org/10.3390/inventions7010026
APA StyleMoure Abelenda, A., & Amaechi, C. V. (2022). Manufacturing of a Granular Fertilizer Based on Organic Slurry and Hardening Agent. Inventions, 7(1), 26. https://doi.org/10.3390/inventions7010026







