Ultrasonic Synthesis of Nanochitosan and Its Size Effects on Turbidity Removal and Dealkalization in Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Chitosan Nanoparticles
2.2.2. Characterization of Chitosan Nanoparticles
2.2.3. Preparation of Wastewater Samples
2.2.4. Residual Turbidity Removal and Dealkalization Efficiency Studies
3. Results and Discussion
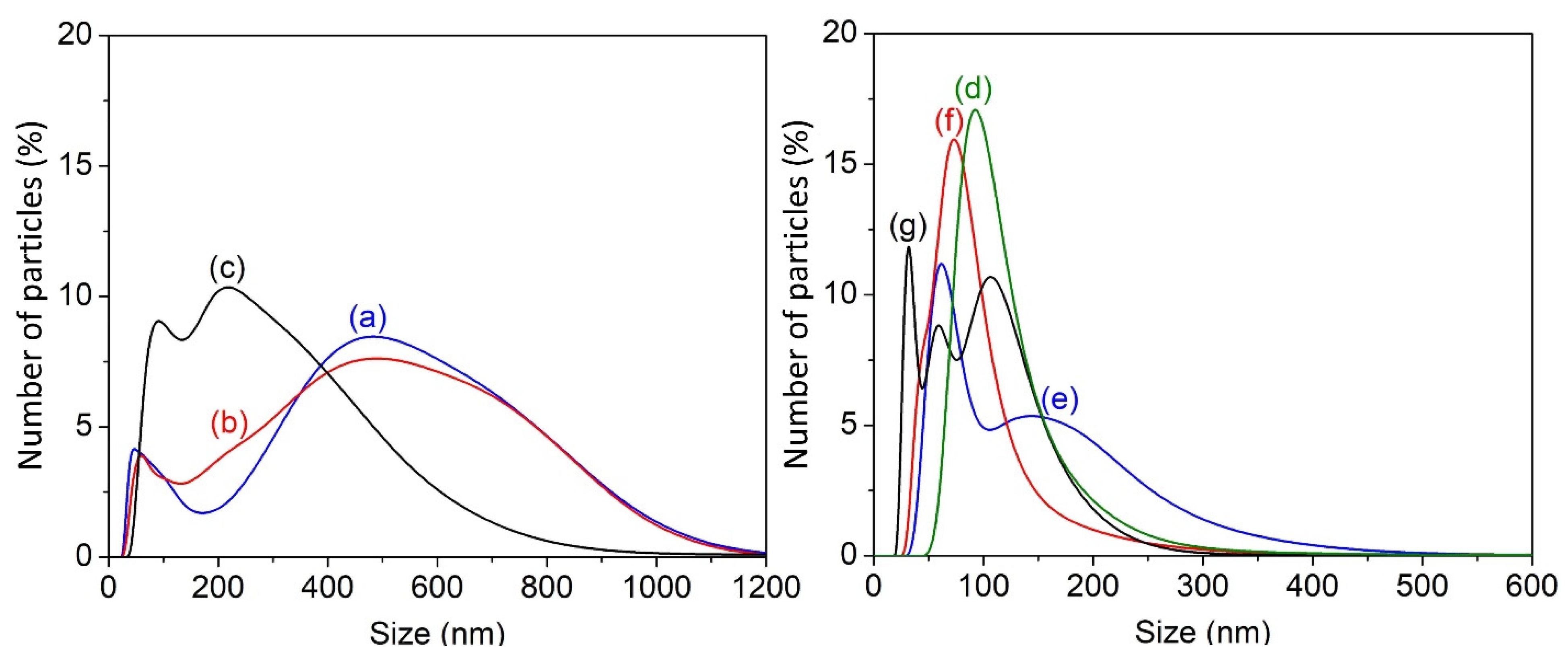
3.1. Particle Size Analysis of Chitosan Nanoparticles
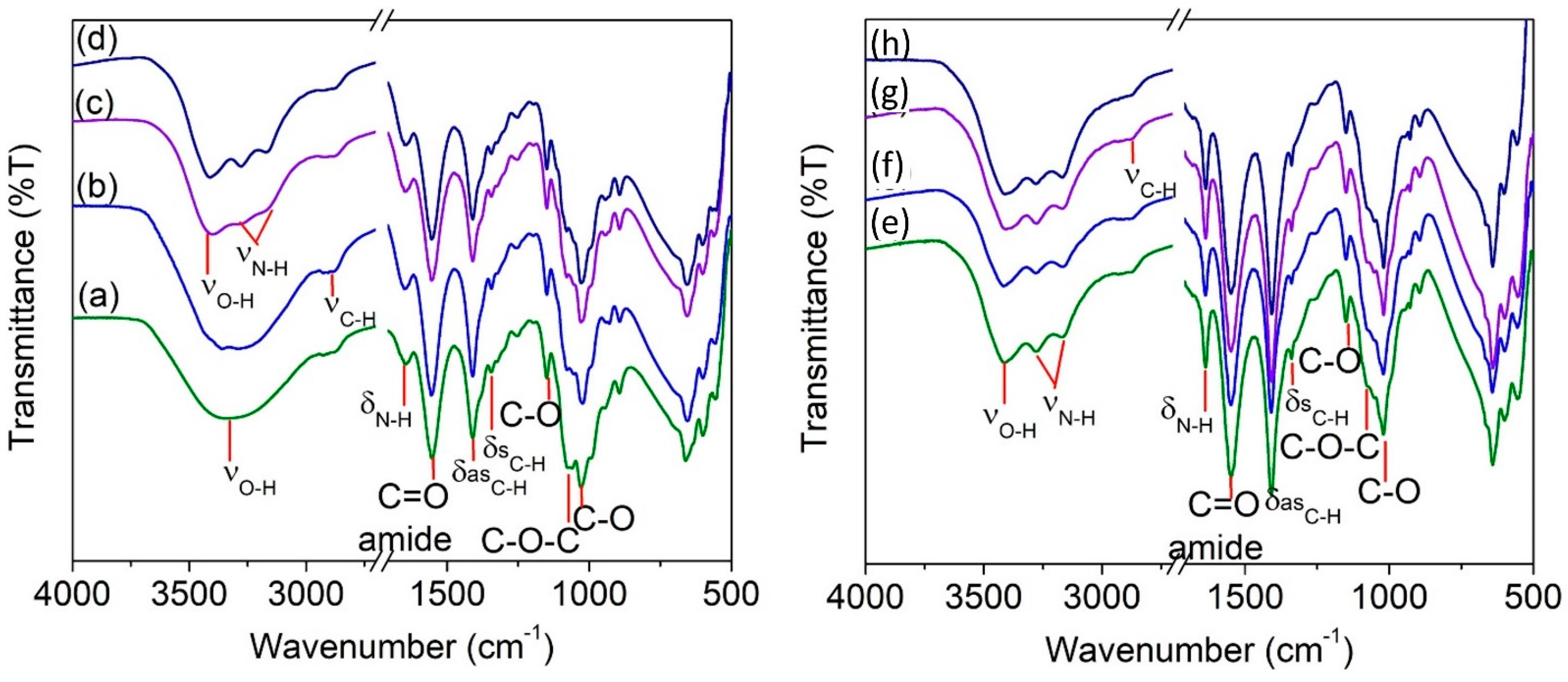
3.2. FTIR Spectroscopy Analysis
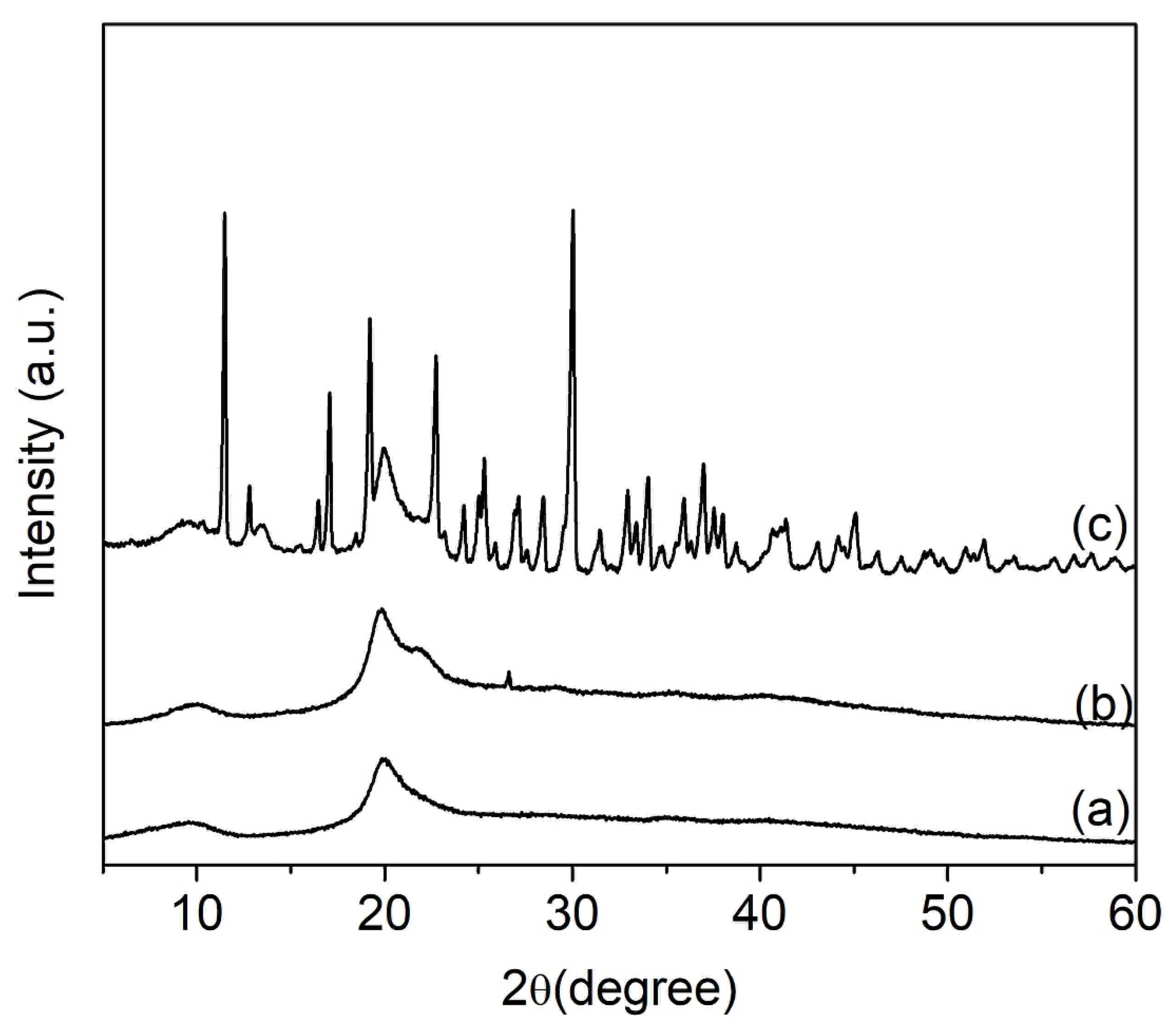
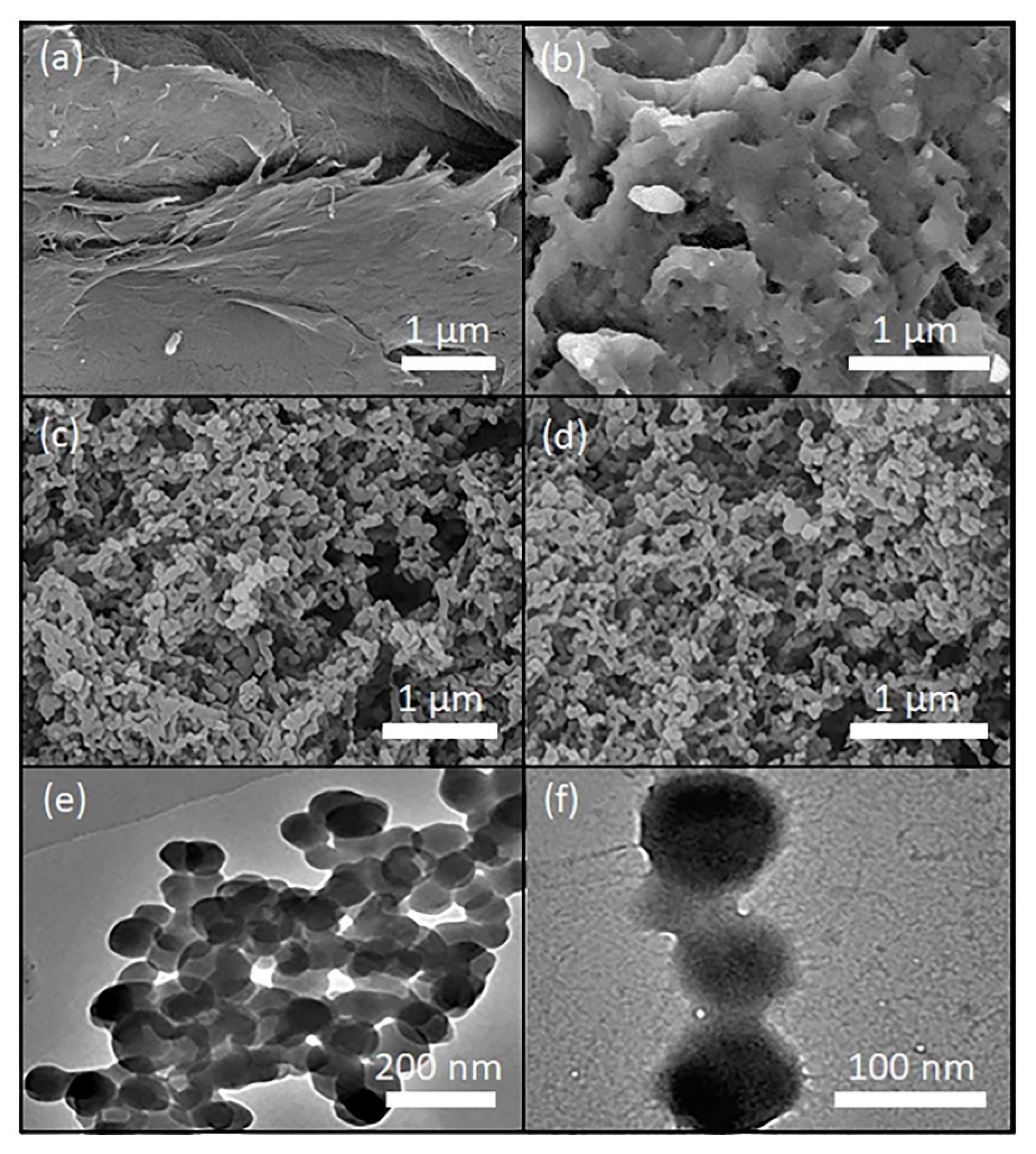
3.3. Morphological and Crystallinity Analyses
3.4. Coagulation Performance in Wastewater Treatment
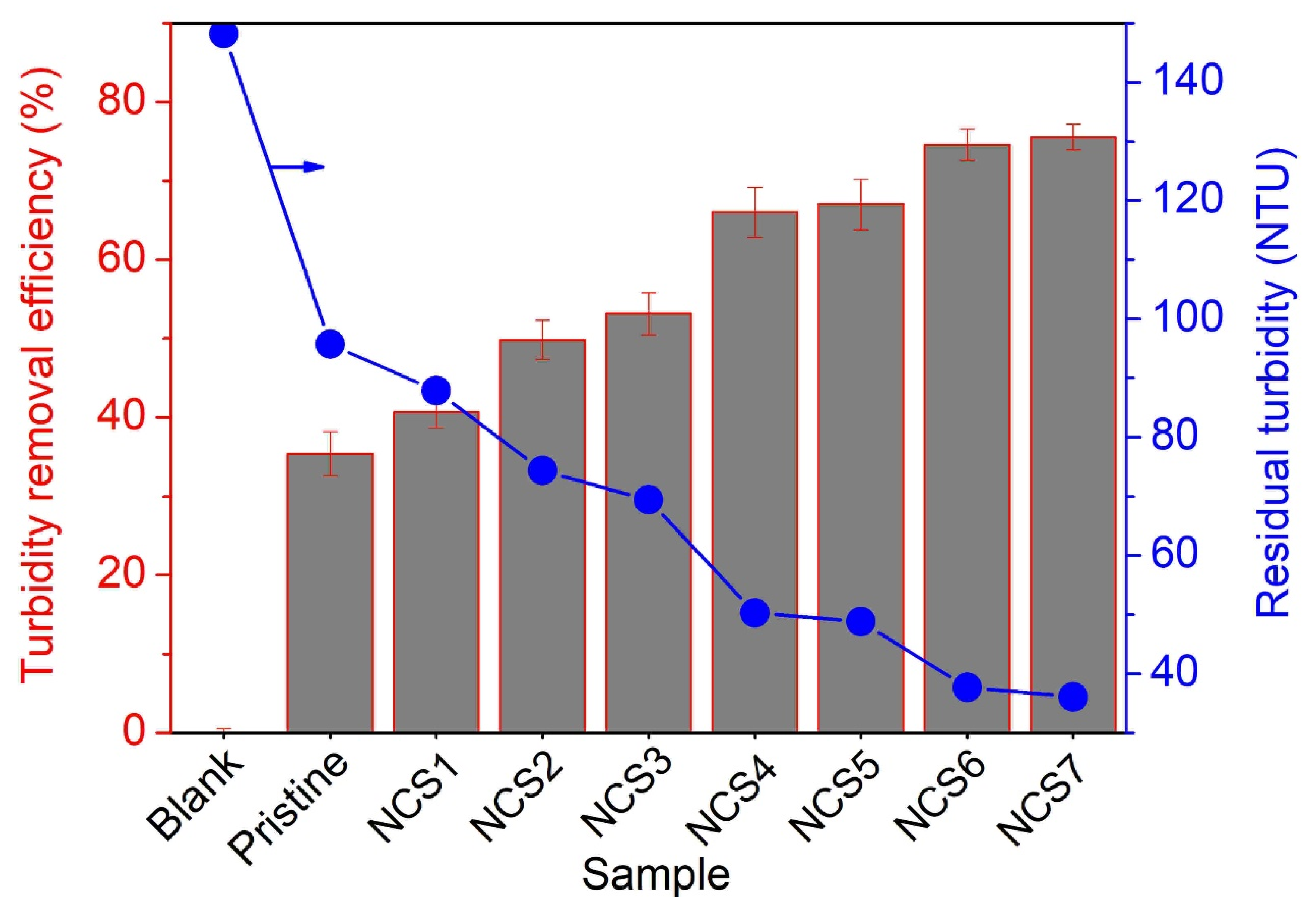
3.4.1. Turbidity Removal Efficiency
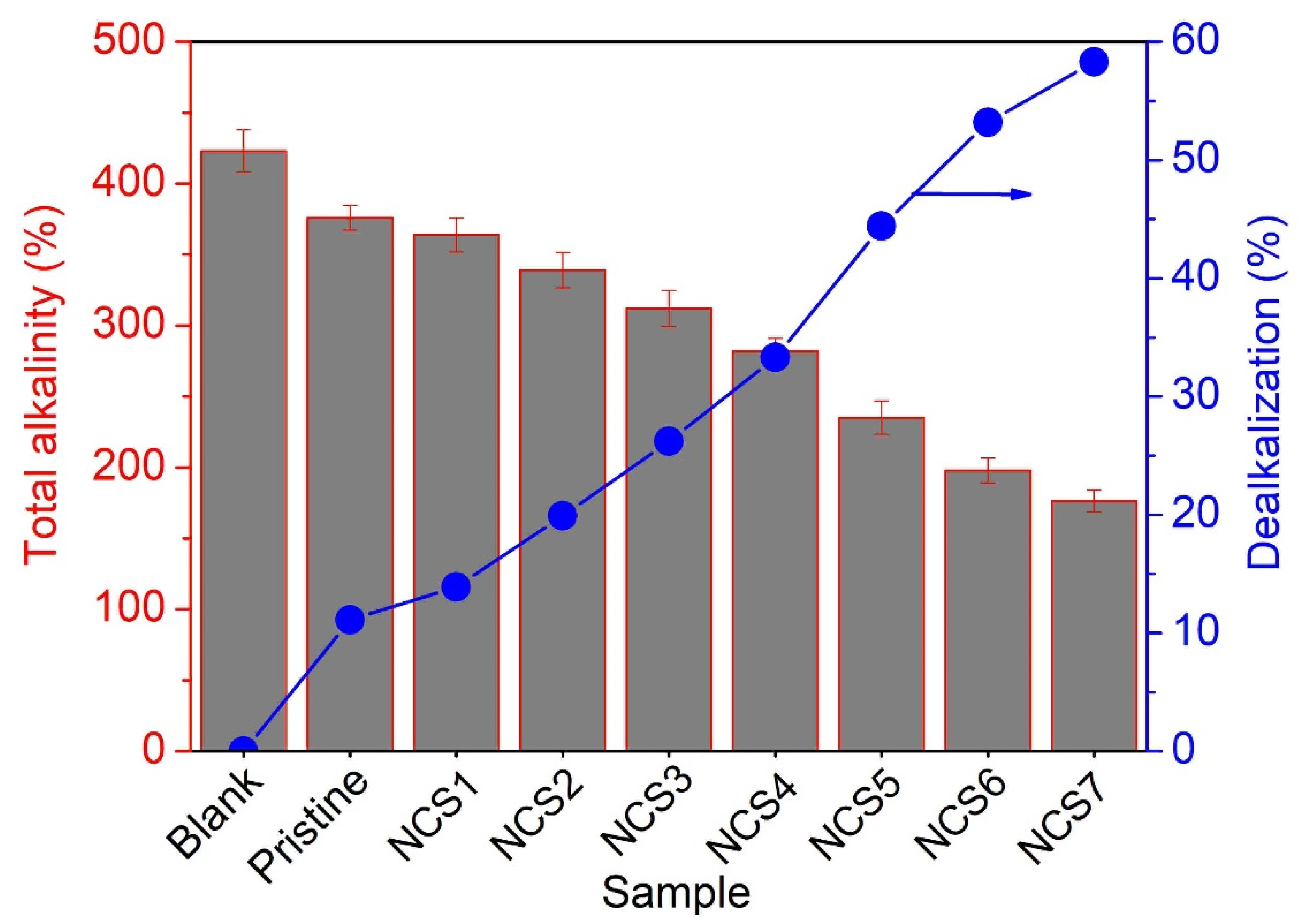
3.4.2. Dealkalization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agbovi, H.K.; Wilson, L.D. Design of amphoteric chitosan flocculants for phosphate and turbidity removal in wastewater. Carbohydr. Polym. 2018, 189, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Devi, B.M.; Latha, S.; Gomathi, T.; Sudha, P.N.; Venkatesan, J.; Anil, S. Batch adsorption and desorption studies on the removal of lead (II) from aqueous solution using nanochitosan/sodium alginate/microcrystalline cellulose beads. Int. J. Biol. Macromol. 2017, 104, 1483–1494. [Google Scholar] [CrossRef]
- Chen, Y.; Nakazawa, Y.; Matsui, Y.; Shirasaki, N.; Matsushita, T. Sulfate ion in raw water affects performance of high-basicity PACl coagulants produced by Al(OH)3 dissolution and base-titration: Removal of SPAC particles by coagulation-flocculation, sedimentation, and sand filtration. Water Res. 2020, 183, 116093. [Google Scholar] [CrossRef]
- Quinlan, P.J.; Tanvir, A.; Tam, K.C. Application of the central composite design to study the flocculation of an anionic azo dye using quaternized cellulose nanofibrils. Carbohydr. Polym. 2015, 133, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Fang, L.-F.; Cheng, L.; Jeon, S.; Kato, N.; Matsuyama, H. Novel ultrafiltration membranes with excellent antifouling properties and chlorine resistance using a poly(vinyl chloride)-based copolymer. J. Membr. Sci. 2018, 549, 101–110. [Google Scholar] [CrossRef]
- Ayoub, M.; Afify, H.; Abdelfattah, A. Chemically enhanced primary treatment of sewage using the recovered alum from water treatment sludge in a model of hydraulic clari-flocculator. J. Water Process Eng. 2017, 19, 133–138. [Google Scholar] [CrossRef]
- Ucevli, O.; Kaya, Y. A comparative study of membrane filtration, electrocoagulation, chemical coagulation and their hybrid processes for greywater treatment. J. Environ. Chem. Eng. 2021, 9, 104946. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S. Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment—A review. J. Water Process Eng. 2021, 42, 102096. [Google Scholar] [CrossRef]
- W. Pontius, F. Chitosan as a Drinking Water Treatment Coagulant. Am. J. Civ. Eng. 2016, 4, 205. [Google Scholar] [CrossRef] [Green Version]
- Khoerunnisa, F.; Rahmah, W.; Seng Ooi, B.; Dwihermiati, E.; Nashrah, N.; Fatimah, S.; Ko, Y.G.; Ng, E.P.; Khoerunnisa, F. Chitosan/PEG/MWCNT/Iodine composite membrane with enhanced antibacterial properties for dye wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103686. [Google Scholar] [CrossRef]
- Khoerunnisa, F.; Nurhayati, M.; Dara, F.; Rizki, R.; Nasir, M.; Aziz, H.A.; Hendrawan, H.; Ng, E.P.; Kaewsaneha, C.; Opaprakasit, P. Physicochemical Properties of TPP-Crosslinked Chitosan Nanoparticles as Potential Antibacterial Agents. J. Colloid Interface Sci. 2021, 22, 225–235. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Mohammed, I.A.; Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Physicochemical modification of chitosan with fly ash and tripolyphosphate for removal of reactive red 120 dye: Statistical optimization and mechanism study. Int. J. Biol. Macromol. 2020, 161, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Saheed, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Iber, B.T.; Okomoda, V.T.; Rozaimah, S.A.; Kasan, N.A. Eco-friendly approaches to aquaculture wastewater treatment: Assessment of natural coagulants vis-a-vis chitosan. Bioresour. Technol. Rep. 2021, 15, 100702. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Huang, Z.; Luo, Z.; Qian, C.; Li, Y.; Duan, Y. Preparation of low molecular chitosan by microwave-induced plasma desorption/ionization technology. Int. J. Biol. Macromol. 2021, 187, 441–450. [Google Scholar] [CrossRef]
- Chavan, P.; Sinhmar, A.; Nehra, M.; Thory, R.; Pathera, A.K.; Sundarraj, A.A.; Nain, V. Impact on various properties of native starch after synthesis of starch nanoparticles: A review. Food Chem. 2021, 364, 130416. [Google Scholar] [CrossRef]
- Wani, T.U.; Pandith, A.H.; Sheikh, F.A. Polyelectrolytic nature of chitosan: Influence on physicochemical properties and synthesis of nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 65, 102730. [Google Scholar] [CrossRef]
- Sivakami, M.S.; Gomathi, T.; Venkatesan, J.; Jeong, H.; Kim, S.; Sudha, P.N. International Journal of Biological Macromolecules Preparation and characterization of nano chitosan for treatment wastewaters. Int. J. Biol. Macromol. 2013, 57, 204–212. [Google Scholar] [CrossRef]
- Wonnie Ma, I.A.; Ammar, S.; Bashir, S.; Selvaraj, M.; Assiri, M.A.; Ramesh, K.; Ramesh, S. Preparation of Hybrid Chitosan/Silica Composites Via Ionotropic Gelation and Its Electrochemical Impedance Studies. Prog. Org. Coat. 2020, 145, 105679. [Google Scholar] [CrossRef]
- Oliveira, D.A.J.; Amaral, J.G.; Garcia, L.B.; dos Santos, M.S.; Silva, L.A.O.; Almeida, M.P.; Gomes, A.F.; Barros, D.R.P.; Lopes, N.P.; Pereira, G.R.; et al. Associating chitosan and microemulsion as a topical vehicle for the administration of herbal medicines. Carbohydr. Polym. 2021, 255, 117482. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, J.; Zhang, Z.; Chen, W.; Hu, Q.; Wang, Y. Convenient one-step approach based on stimuli-responsive sol-gel transition properties to directly build chitosan-alginate core-shell beads. Food Hydrocoll. 2019, 87, 253–259. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Machado, D.; Laia, C.; Pereira, L.C.J.; Coutinho, J.T.; Ferreira, I.M.M.; Novo, C.M.M.; Borges, J.P. Thermal and magnetic properties of chitosan-iron oxide nanoparticles. Carbohydr. Polym. 2016, 149, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Gokce, Y.; Cengiz, B.; Yildiz, N.; Calimli, A.; Aktas, Z. Ultrasonication of chitosan nanoparticle suspension: Influence on particle size. Colloids Surf. A Physicochem. Eng. Asp. 2014, 462, 75–81. [Google Scholar] [CrossRef]
- Ng, E.P.; Awala, H.; Ghoy, J.P.; Vicente, A.; Ling, T.C.; Ng, Y.H.; Mintova, S.; Adam, F. Effects of ultrasonic irradiation on crystallization and structural properties of EMT-type zeolite nanocrystals. Mater. Chem. Phys. 2015, 159, 38–45. [Google Scholar] [CrossRef]
- Boufi, S.; Bel Haaj, S.; Magnin, A.; Pignon, F.; Impéror-Clerc, M.; Mortha, G. Ultrasonic assisted production of starch nanoparticles: Structural characterization and mechanism of disintegration. Ultrason. Sonochem. 2018, 41, 327–336. [Google Scholar] [CrossRef]
- Lobo, C.; Castellari, J.; Colman Lerner, J.; Bertola, N.; Zaritzky, N. Functional iron chitosan microspheres synthesized by ionotropic gelation for the removal of arsenic (V) from water. Int. J. Biol. Macromol. 2020, 164, 1575–1583. [Google Scholar] [CrossRef]
- Xu, D.; Li, L.; Wu, Y.; Zhang, X.; Wu, M.; Li, Y.; Gai, Z.; Li, B.; Zhao, D.; Li, C. Influence of ultrasound pretreatment on the subsequent glycation of dietary proteins. Ultrason. Sonochem. 2020, 63, 104910. [Google Scholar] [CrossRef]
- Dai, C.; Yin, Z.; Wang, P.; Miao, Q.; Chen, J. Analysis on ground surface in ultrasonic face grinding of silicon carbide (SiC) ceramic with minor vibration amplitude. Ceram. Int. 2021, 47, 21959–21968. [Google Scholar] [CrossRef]
- Roohi, R.; Abedi, E.; Hashemi, S.M.B.; Marszałek, K.; Lorenzo, J.M.; Barba, F.J. Ultrasound-assisted bleaching: Mathematical and 3D computational fluid dynamics simulation of ultrasound parameters on microbubble formation and cavitation structures. Innov. Food Sci. Emerg. Technol. 2019, 55, 66–79. [Google Scholar] [CrossRef]
- Wong, S.F.; Deekomwong, K.; Wittayakun, J.; Ling, T.C.; Muraza, O.; Adam, F.; Ng, E.P. Crystal growth study of K-F nanozeolite and its catalytic behavior in Aldol condensation of benzaldehyde and heptanal enhanced by microwave heating. Mater. Chem. Phys. 2017, 196, 295–301. [Google Scholar] [CrossRef]
- Sangeetha, V.; Sudha, P.N. Synthesis and characterisation of nano chitosan/PVP/SF for environmental engineering applications. Int. J. Ambient Energy 2021, 42, 150–155. [Google Scholar] [CrossRef]
- Baklagina, Y.G.; Klechkovskaya, V.V.; Kononova, S.V.; Petrova, V.A.; Poshina, D.N.; Orekhov, A.S.; Skorik, Y.A. Polymorphic Modifications of Chitosan. Crystallogr. Rep. 2018, 63, 303–313. [Google Scholar] [CrossRef]
- Wu, T.; Wu, C.; Xiang, Y.; Huang, J.; Luan, L.; Chen, S.; Hu, Y. Kinetics and mechanism of degradation of chitosan by combining sonolysis with H2O2/ascorbic acid. RSC Adv. 2016, 6, 76280–76287. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Joseph, D.; Cruz-romero, M.; Collins, T.; Cummins, E.; Kerry, J.P.; Morris, M.A. Food Hydrocolloids Synthesis of monodisperse chitosan nanoparticles. Food Hydrocoll. 2018, 83, 355–364. [Google Scholar]
- Jampafuang, Y.; Tongta, A.; Waiprib, Y. Impact of crystalline structural differences between α-and β-chitosan on their nanoparticle formation via ionic gelation and superoxide radical scavenging activities. Polymers 2019, 11, 2010. [Google Scholar] [CrossRef] [Green Version]
- Muley, A.B.; Chaudhari, S.A.; Mulchandani, K.H.; Singhal, R.S. Extraction and characterization of chitosan from prawn shell waste and its conjugation with cutinase for enhanced thermo-stability. Int. J. Biol. Macromol. 2018, 111, 1047–1058. [Google Scholar] [CrossRef]
- Maniammal, K.; Madhu, G.; Biju, V. X-ray diffraction line profile analysis of nanostructured nickel oxide: Shape factor and convolution of crystallite size and microstrain contributions. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 85, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Vivekanandan, K.E.; Swethavinayagam, S.; Venkatesan, D.; Rebecca, L.J. Preparation of Chitosan Nanoparticles and its Synergistic Effects against Gram Positive and Gram Negative Microorganisms. J. Pure Appl. Microbiol. 2019, 13, 2317–2324. [Google Scholar] [CrossRef] [Green Version]
- Morsy, M.; Mostafa, K.M.; Amyn, H.A.M.; El-Ebissy, A.A.-H.; Salah, A.M.; Youssef, M.A. Synthesis and characterization of freeze dryer chitosan nanoparticles as multifunctional eco-friendly finish for fabricating easy care and antibacterial cotton textiles. Egypt. J. Chem. 2019, 62, 1277–1293. [Google Scholar]
- Kaur, P.; Choudhary, A.; Thakur, R. Synthesis of chitosan-silver nanocomposites and their antibacterial activity. Int. J. Sci. Eng. Res. 2013, 4, 869. [Google Scholar]
- Bainus, A.; Darmawan, W.B.; Yulianti, D.; Husin, L.H. Between fear and survival: Human security issues in Citarum River Basin, Indonesia. J. Hum. Secur. 2021, 17, 4–14. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan nanocomposite coatings for food, paints, and water treatment applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef] [Green Version]
- Meraz, K.A.S.; Vargas, S.M.P.; Maldonado, J.T.L.; Bravo, J.M.C.; Guzman, M.T.O.; Maldonado, E.A.L. Eco-friendly innovation for nejayote coagulation–flocculation process using chitosan: Evaluation through zeta potential measurements. Chem. Eng. J. 2016, 284, 536–542. [Google Scholar] [CrossRef]
- Xu, Y.; Dang, Q.; Liu, C.; Yan, J.; Fan, B.; Cai, J.; Li, J. Preparation and characterization of carboxyl-functionalized chitosan magnetic microspheres and submicrospheres for Pb2+ removal. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 353–364. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Adhikari, T.; Sarkar, B.; Barman, A.; Paul, R.; Patra, A.K.; Sharma, P.C.; Kumar, P. Fe-exchanged nano-bentonite outperforms Fe3O4 nanoparticles in removing nitrate and bicarbonate from wastewater. J. Hazard. Mater. 2019, 376, 141–152. [Google Scholar] [CrossRef]
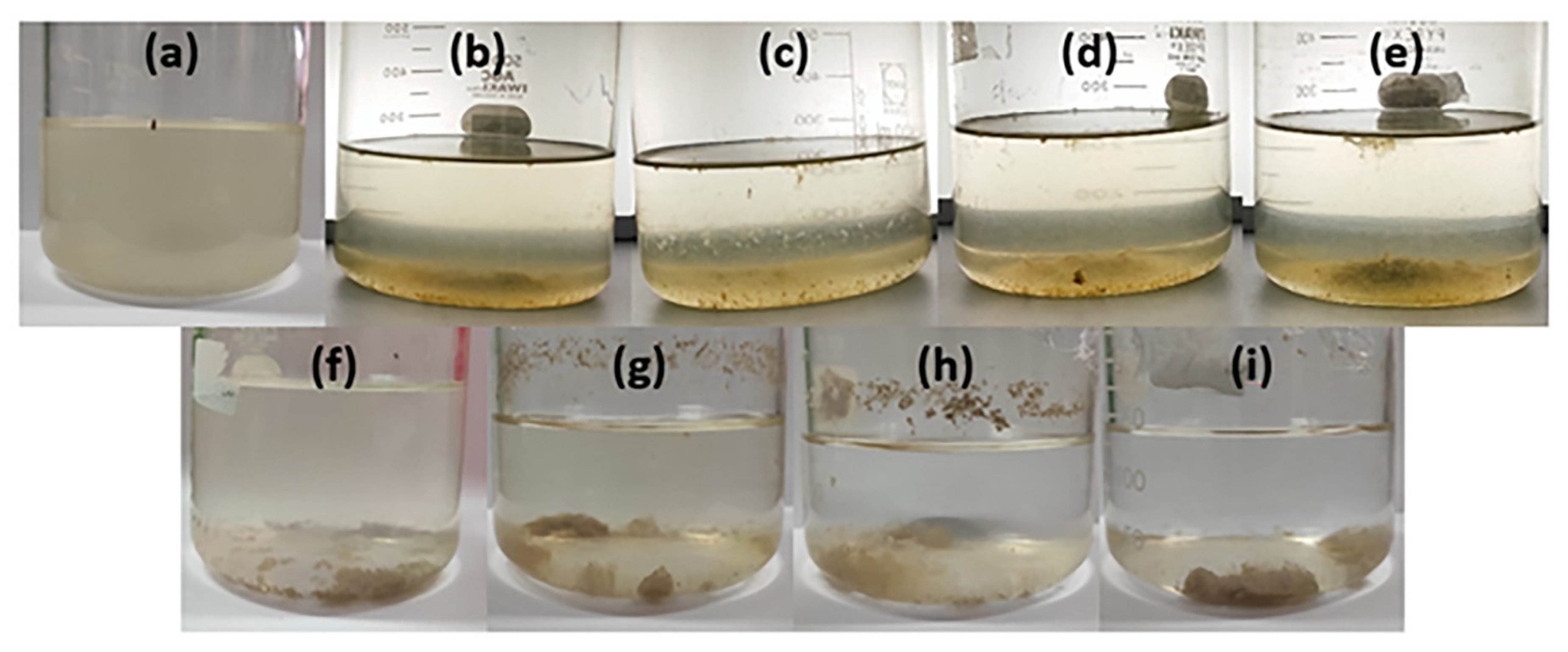
| Sample Code | Duration (min) | Amplitude (%) |
|---|---|---|
| NCS1 | 5 | 40 |
| NCS2 | 20 | 40 |
| NCS3 | 60 | 40 |
| NCS4 | 120 | 40 |
| NCS5 | 120 | 50 |
| NCS6 | 120 | 60 |
| NCS7 | 120 | 70 |
| Sample Code | Duration (min) | Amplitude (%) | Mean Diameter (nm) |
|---|---|---|---|
| NCS1 | 5 | 40 | 426.75 ± 136.12 |
| NCS2 | 20 | 40 | 336.44 ± 82.08 |
| NCS3 | 60 | 40 | 220.08 ± 20.60 |
| NCS4 | 120 | 40 | 109.59 ± 10.11 |
| NCS5 | 120 | 50 | 110.32 ± 36.27 |
| NCS6 | 120 | 60 | 79.33 ± 21.61 |
| NCS7 | 120 | 70 | 77.47 ± 28.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoerunnisa, F.; Yolanda, Y.D.; Nurhayati, M.; Zahra, F.; Nasir, M.; Opaprakasit, P.; Choo, M.-Y.; Ng, E.-P. Ultrasonic Synthesis of Nanochitosan and Its Size Effects on Turbidity Removal and Dealkalization in Wastewater Treatment. Inventions 2021, 6, 98. https://doi.org/10.3390/inventions6040098
Khoerunnisa F, Yolanda YD, Nurhayati M, Zahra F, Nasir M, Opaprakasit P, Choo M-Y, Ng E-P. Ultrasonic Synthesis of Nanochitosan and Its Size Effects on Turbidity Removal and Dealkalization in Wastewater Treatment. Inventions. 2021; 6(4):98. https://doi.org/10.3390/inventions6040098
Chicago/Turabian StyleKhoerunnisa, Fitri, Yustika Desti Yolanda, Mita Nurhayati, Firdha Zahra, Muhamad Nasir, Pakorn Opaprakasit, Min-Yee Choo, and Eng-Poh Ng. 2021. "Ultrasonic Synthesis of Nanochitosan and Its Size Effects on Turbidity Removal and Dealkalization in Wastewater Treatment" Inventions 6, no. 4: 98. https://doi.org/10.3390/inventions6040098






