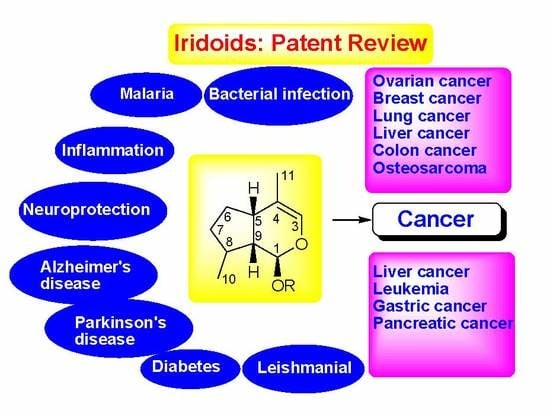
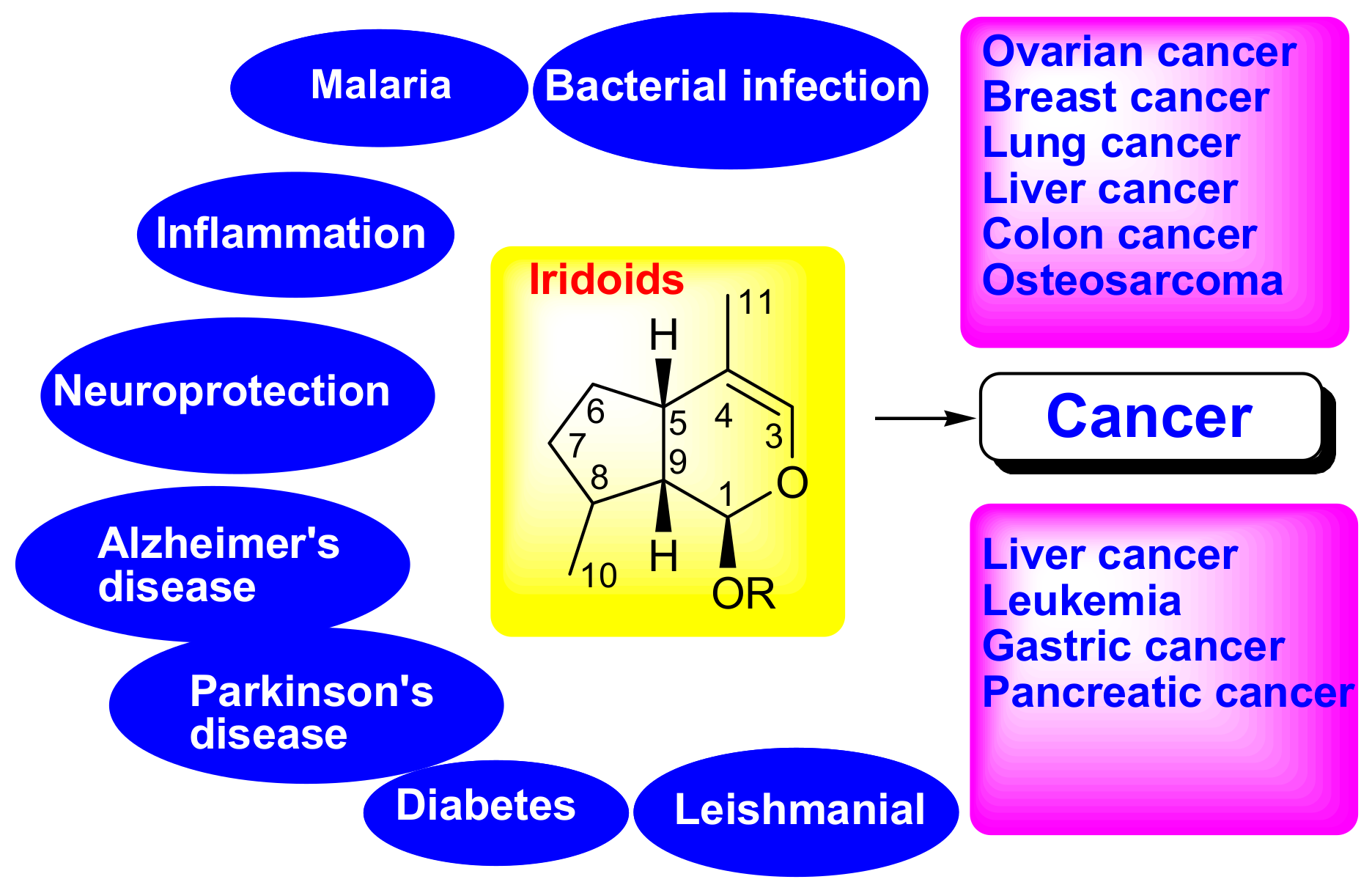
Therapeutic Potential of Iridoid Derivatives: Patent Review
Abstract
:1. Introduction
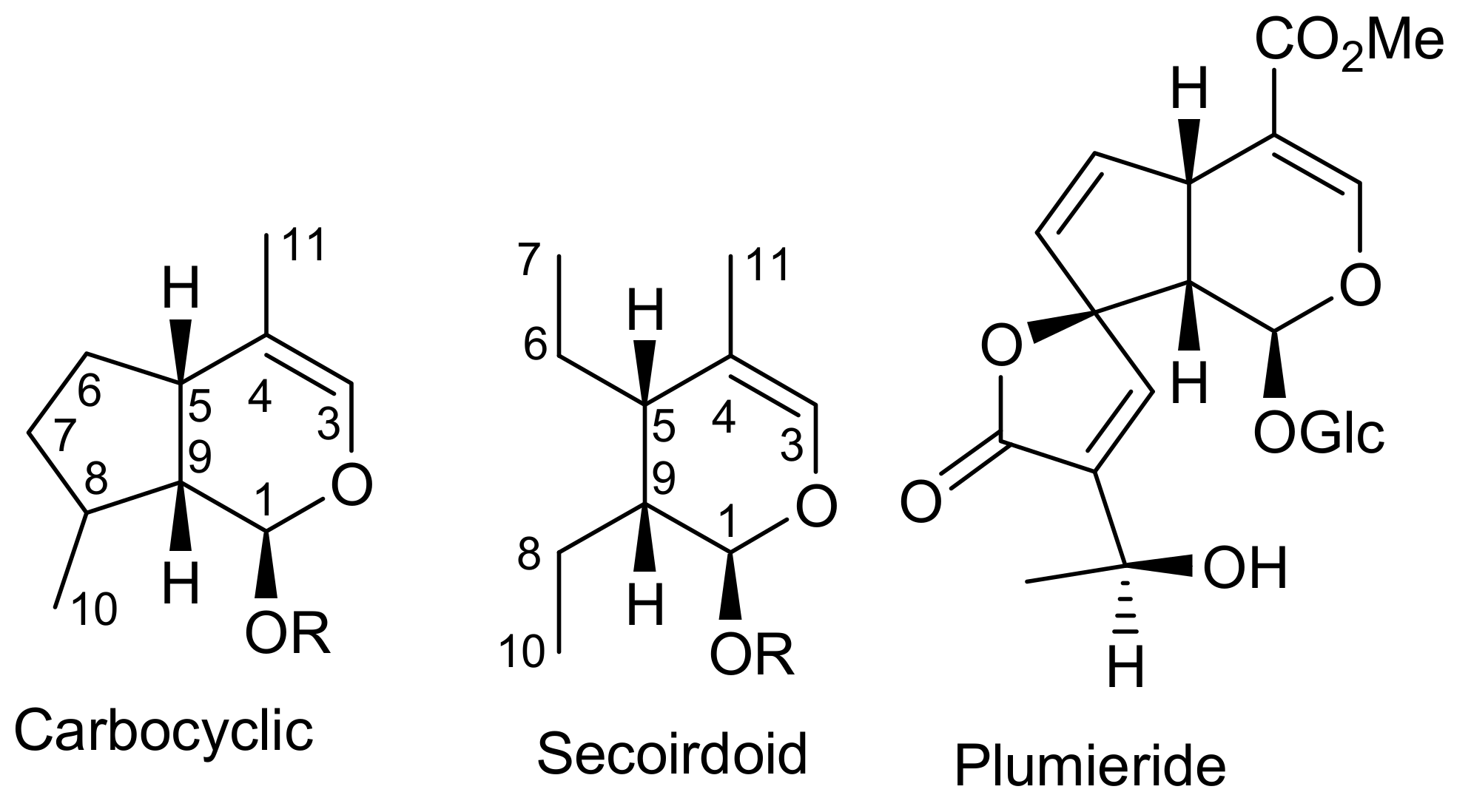
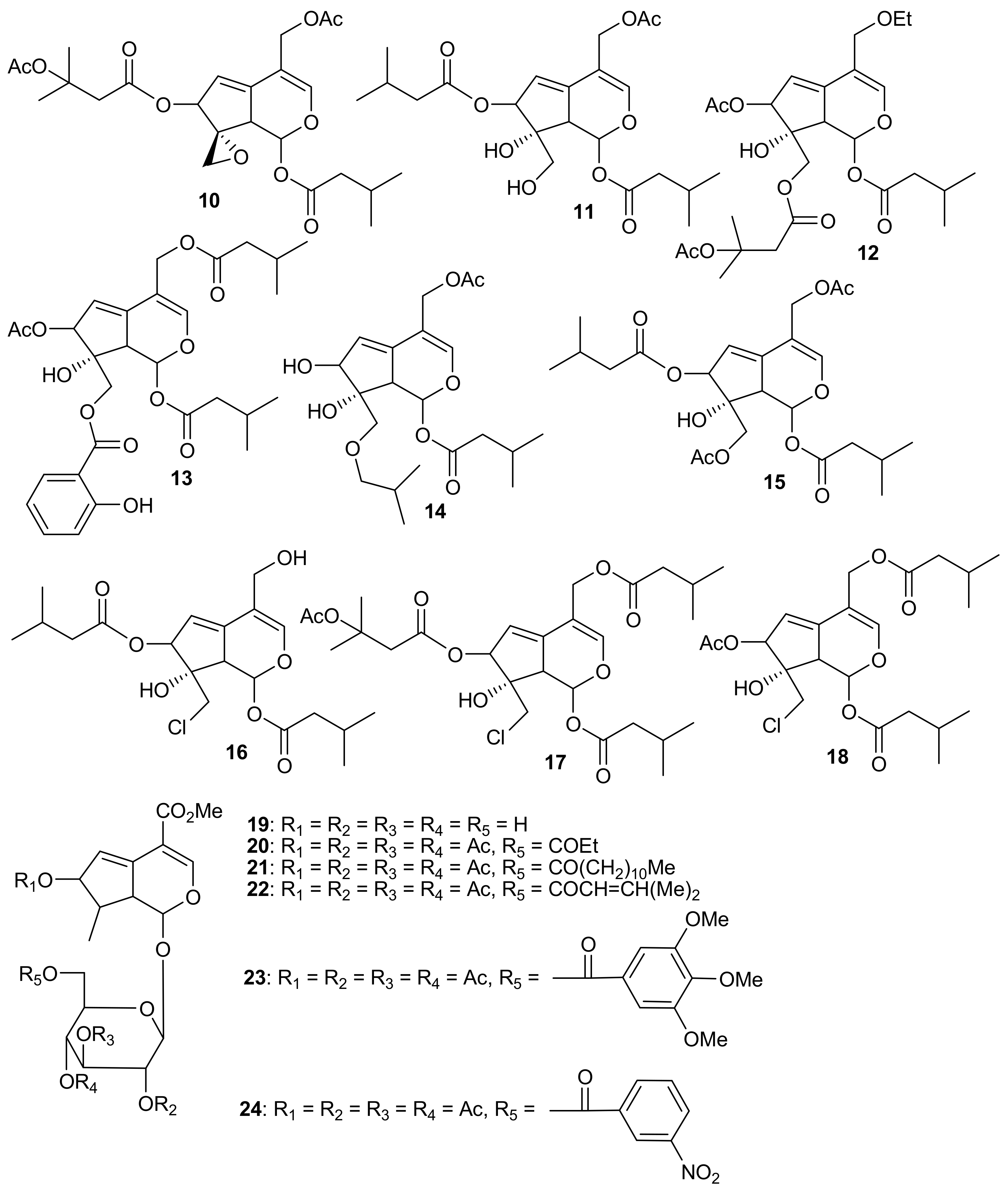
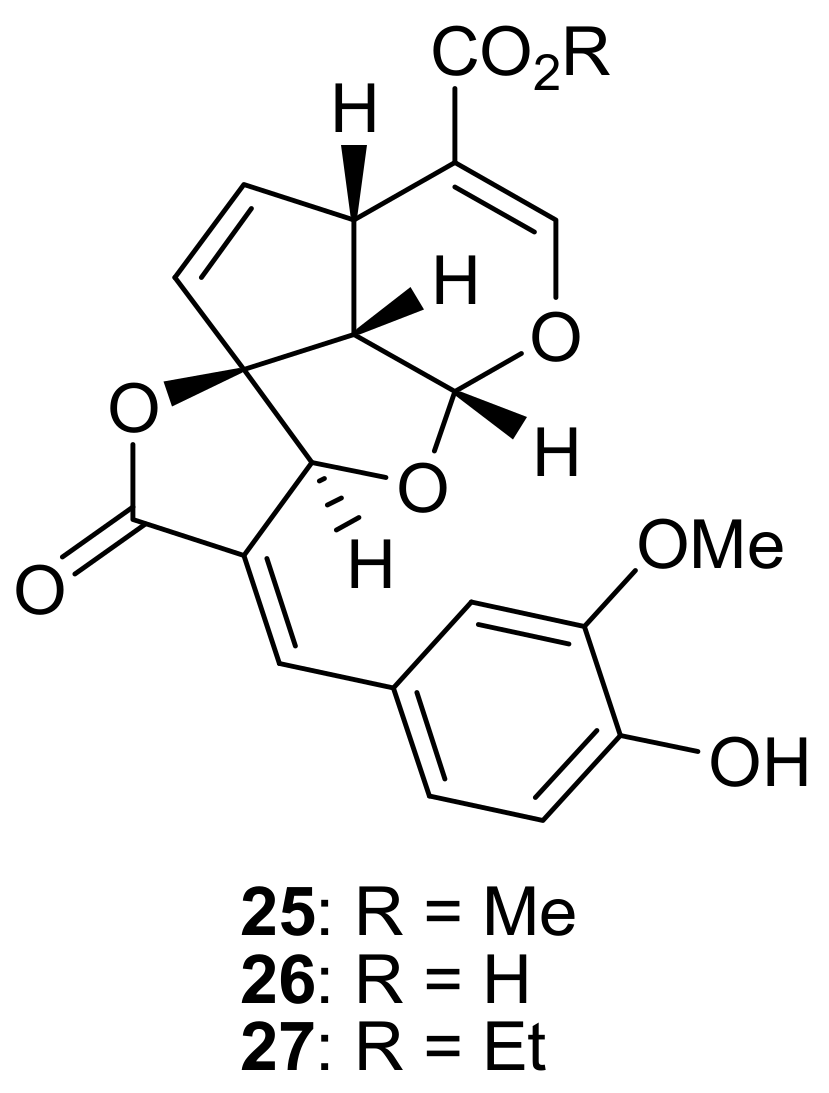
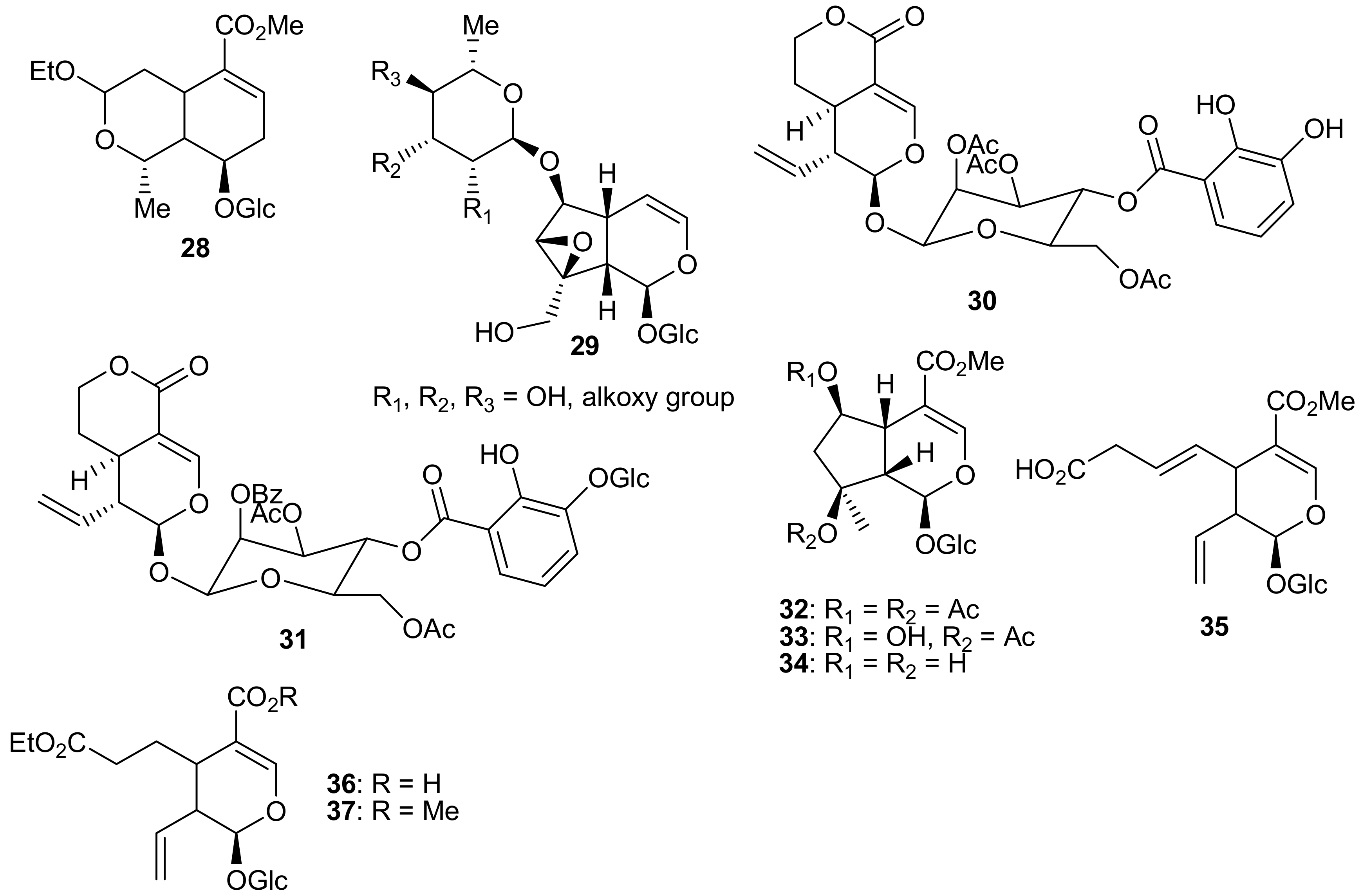
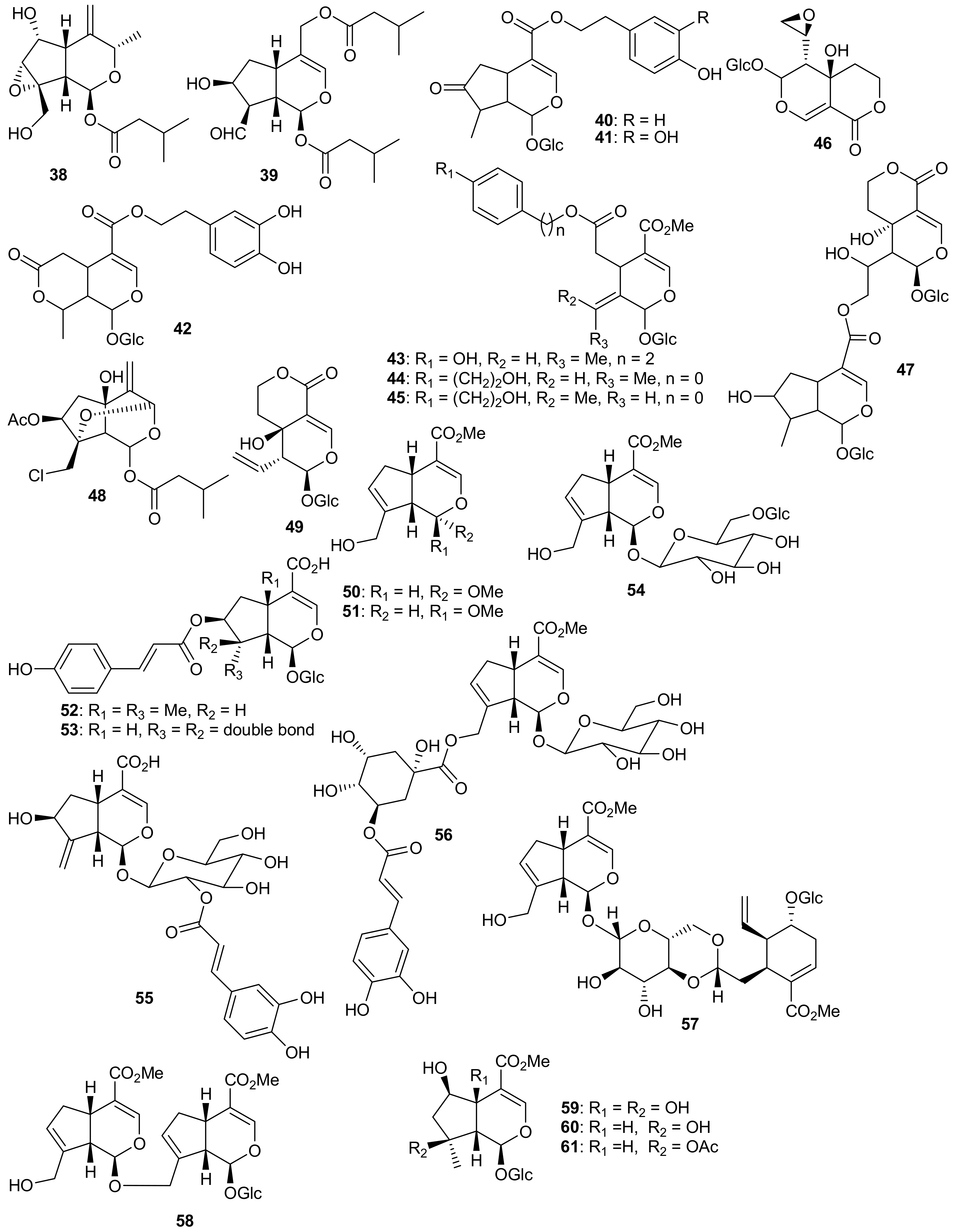
2. Patents Filed for Iridoids
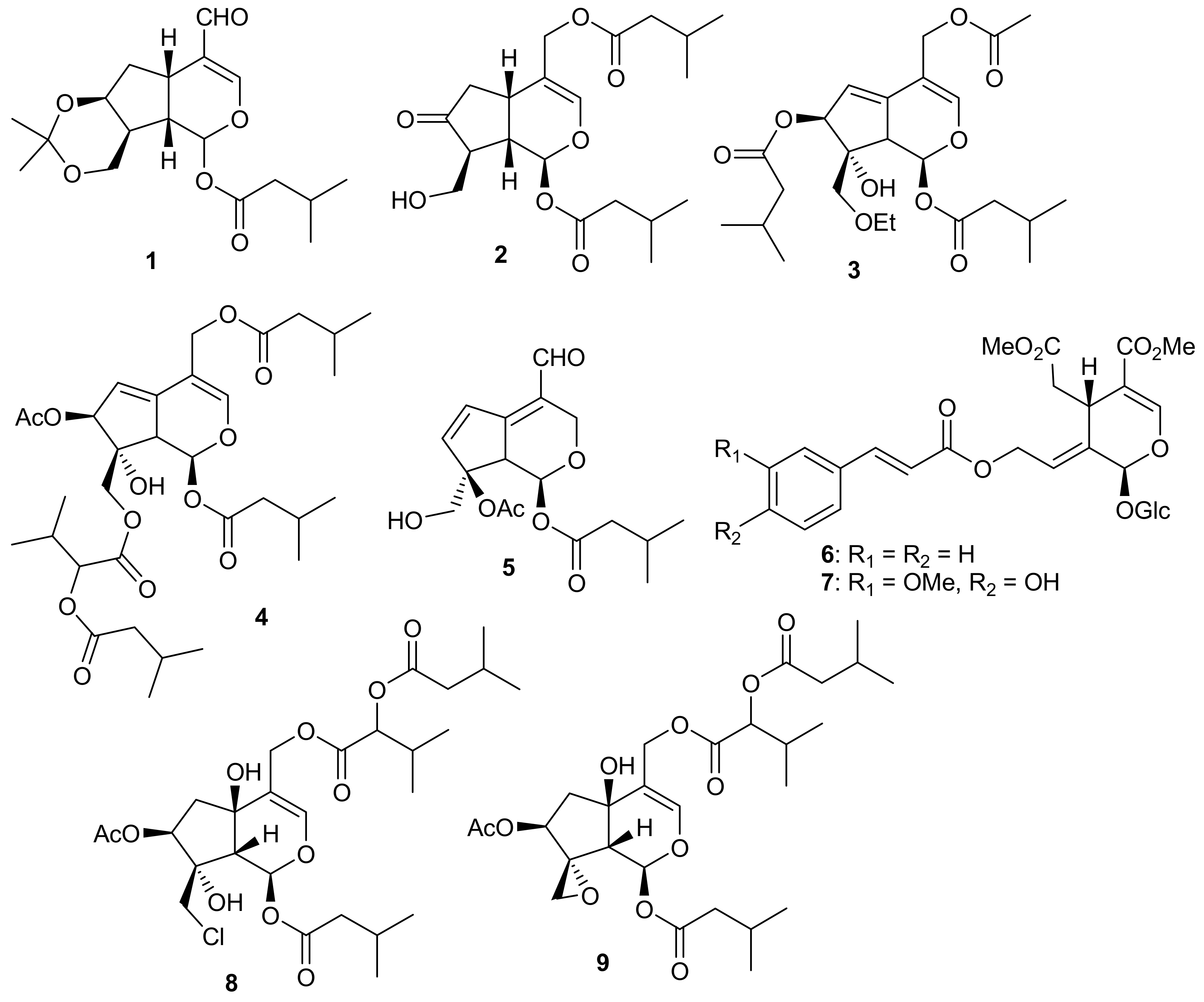
3. Iridoid Derivatives as Anticancer Agents
4. Iridoid Derivatives as Anti-Trypanosomal Agents
5. Iridoid Derivatives as Antileishmanial Agents
6. Iridoid Derivatives as Antiplasmodial Agents
7. Iridoid Derivatives as Anti-Inflammatory Agents
8. Iridoid Derivatives as Antidiabetic Agents
9. Miscellaneous
10. Pharmaceutical Compositions or Formulation Comprising Iridoids
11. Conclusions and Future Developments
Acknowledgments
Conflicts of Interest
References
- Sampaio-Santos, M.I.; Kaplan, M.A.C. Biosynthesis significance of iridoids in chemosystematics. J. Braz. Chem. Soc. 2010, 12, 144–153. [Google Scholar] [CrossRef]
- Franzyk, H. Synthetic aspects of iridoid chemistry. Fortschr. Chem. Org. Naturst. 2000, 79, 1–114. [Google Scholar]
- Halpern, O.; Schmid, H. Zur Kenntnis des Plumierids. 2. Mitteilung. Helv. Chim. Acta 1958, 41, 1109–1154. [Google Scholar] [CrossRef]
- Grignon-Dubois, M.; Rezzonico, B.; Usubillaga, A.; Vojas, L.B. Isolation of plumieride from Plumeria inodora. Chem. Nat. Comp. 2005, 41, 730–731. [Google Scholar] [CrossRef]
- Pandeti, S.; Sharma, K.; Bathula, S.R.; Tadigoppula, N. Synthesis of novel anticancer iridoid derivatives and their cell cycle arrest and caspase dependent apoptosis. Phytomedicine 2014, 15, 333–339. [Google Scholar] [CrossRef]
- Jensen, S.R.; Nielsen, B.J.; Dahlgren, R. Iridoid compounds, their occurrence and systematic importance in the angiosperms. Bot. Not. 1975, 128, 148–180. [Google Scholar]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A. Menichini F Biological and Pharmacological activities of iridoids: Recent developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine 1998, 5, 147–163. [Google Scholar] [CrossRef]
- Wu, J. New Iridoid, Its Preparation Method and Medical Application Thereof. CN105503892A, 20 April 2016. [Google Scholar]
- Yao, T. A Kind of New Iridoid and Its Preparation Method and Medicinal Use. CN105367536A, 2 March 2016. [Google Scholar]
- Yang, B.; Zhao, H.; Gu, M.; Song, H. Preparation of Iridoid Ester Compounds for Treatment of Cancer. CN104387362A, 4 March 2015. [Google Scholar]
- Thusoo, S.; Gupta, S.; Sudan, R.; Kour, J.; Bhagat, S.; Hussain, R.; Bhagat, M. Antioxidant activity of essential oil and extracts of Valeriana jatamansi roots. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, C.; Lin, Y.; Lan, M.; Zhang, R.; Xu, K.; Zhang, T. Iridoid Compound, and Preparation Method and Use Thereof as Antitumor Agent. CN104086520A, 8 October 2014. [Google Scholar]
- Yang, B.; Gu, M.; Cheng, R.; Wang, W.; Chen, J.; Zhang, R. Method for Preparing Epoxy Iridoid Ester Extract of Valeriana jatamansii. CN103599144A, 26 February 2014. [Google Scholar]
- Meng, D.; Zhang, L.; Chen, C.; Xu, L. Preparation Method of Iridoid in Jasminum lanceolarium and Its Application. CN102942605A, 27 February 2013. [Google Scholar]
- Zhang, W.; Su, J.; Lin, S.; Shan, L.; Liu, R.; Li, H.; Xu, X. Application of Iridoid Compounds in Preparation of Medicine for Treating Ovarian Cancer. CN101829080A, 15 September 2010. [Google Scholar]
- Zhang, W.; Lin, S.; Shan, L.; Su, J.; Liu, R.; Li, H.; Shen, Y.; Xu, X. Iridoid Compounds Extracted from Valeriana officinalis and Its Application as Antitumor Agents. CN101444501A, 3 June 2009. [Google Scholar]
- Khanuja, S.P.S.; Srivastava, S.K.; Garg, A.; Khan, M.; Darokar, M.P.; Pal, A. Isolation of Loganin Analogs from Fruit Pulp of Strychnos nux-vomica and Method for their Preparation as Antitumor Agents. WO2007060686A1, 31 May 2007. [Google Scholar]
- Jones, A.J.; Grkovic, T.; Sykes, M.L.; Vicky, M.; Avery, V.M. Trypanocidal Activity of Marine Natural Products. Mar. Drugs 2013, 11, 4058–4082. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, S.; Ohta, N.; Suzuki, M.; Shoyama, Y.; Morinaga, O.; Uto, T.; Tung, N.H.; Koram, K.A.; Bosompem, K.M.; Nyarko, A.K. Novel Tetracyclic Spirolactone Iridoid Compounds for Treatment of Trypanosomiasis. WO2015105198A1, 16 July 2015. [Google Scholar]
- Hussain, H.; Al-Harrasi, A.; Al-Rawahi, A.; Green, I.R.; Gibbons, S. Fruitful decade for antileishmanial compounds from 2002 to late 2011. Chem. Rev. 2014, 114, 10369–10428. [Google Scholar] [CrossRef]
- Buskes, M.J.; Harvey, K.L.; Prinz, B.; Crabb, B.S.; Gilson, P.R.; Wilson, D.J.D.; Abbott, B.M.A. Exploration of 3-methylisoquinoline-4-carbonitriles as protein kinase A inhibitors of Plasmodium falciparum. Bioorg. Med. Chem. 2016, 24, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Pabbisetty, D.; Illendula, A.; Muraleedharan, K.M.; Chittiboyina, A.G.; Williamson, J.S.; Avery, M.A.; Avery, B.A. Determination of antimalarial compound, ARB-89 (7-hydroxy-artemisinin carbamate) in rat serum by UPLC/MS/MS and its application in pharmacokinetics. J. Chromat. B 2012, 889–890, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Urbán, P.; Ranucci, E.; Busquets, X.F. Polyamidoamine nanoparticles as nanocarriers for the drug delivery to malaria parasite stages in the mosquito vector. Nanomedicine 2015, 10, 3401–3414. [Google Scholar] [CrossRef]
- Manohar, S.; Khan, S.I.; Rawat, D.S. Synthesis, antimalarial activity and cytotoxicity of 4-aminoquinoline-triazine conjugates. Bioorg. Med. Chem. Lett. 2010, 20, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zheng, X.; Wang, L.; Wang, Q.; Chen, H.; Jiang, Y. Preparation Method of 7-O-ethyl-morroniside. CN105968150A, 28 September 2016. [Google Scholar]
- Li, Y.; Xu, J.; Zhang, L.; Zhu, T.; Yuan, P.; Chen, X. Pharmaceutical Application of Iridoid Glycoside Compounds. CN105326850A, 17 February 2016. [Google Scholar]
- Cheng, Y.; Wang, S.; Wang, Y. Iridoid Compound with Anti-Inflammatory Activity, Its Preparation Method and Application in Preparing Antiinflammatory Medicine. CN103113432A, 22 May 2013. [Google Scholar]
- Karan, M.; Kaur, I.; Chopra, D. Anti-Inflammatory Activity of the Iridoid Glycosides. WO2010032269A2, 25 March 2010. [Google Scholar]
- Gambhire, M.N.; Wankhede, S.S.; Juvekar, A.R. Antiinflammatory activity of aqueous extract of Barleria cristata leaves. J. Young Pharm. 2009, 1, 220–224. [Google Scholar]
- Kwak, W.J.; Han, C.K.; Chang, H.W.; Kim, H.P.; Kang, S.S.; Son, K.H. Loniceroside C, an antiinflammatory saponin from Lonicera japonica. Chem. Pharm. Bull. 2003, 51, 333–335. [Google Scholar] [CrossRef]
- Rahman, A.; Kang, S.C. In vitro control of food-borne and food spoilage bacteria by essential oil and ethanol extracts of Lonicera japonica Thunb. Food Chem. 2009, 16, 670–675. [Google Scholar] [CrossRef]
- Song, S.; Peng, Y.; Liu, Z.; Li, L.; Liu, Q.; Huang, X. Iridoid Glycoside Compounds in Lonicera japonica and Its Preparation Method and Application. CN105017353A, 4 November 2015. [Google Scholar]
- Ma, W.; Wang, K.J.; Cheng, C.S.; Yan, G.Q.; Lu, W.L.; Ge, J.F.; Cheng, Y.X.; Li, N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J. Ethnopharmacol. 2014, 153, 840–845. [Google Scholar] [CrossRef]
- Kang, J.; Yang, N.; Wang, H.; Bai, Y.; Wang, S.; Wang, Z. Application of Cornus officinalis Iridoid Glycoside in Antidiabetic Agent. CN104784195A, 22 July 2015. [Google Scholar]
- Wu, J. Preparation of Iridoid Compound with Endothelial Cell Protection Effect. CN105503893A, 20 April 2016. [Google Scholar]
- Yang, H. Extraction of Iridoid Compound from Eucommia ulmoides for Myocardial Protection. CN105481820A, 13 April 2016. [Google Scholar]
- Yin, J.; Zhou, Z.; Han, N.; Liu, Z. New Application of Iridoids and Its Metabolites in the Preparation of Drug-Resistant Bacterial Infection Medicine. CN105687221A, 22 June 2016. [Google Scholar]
- Li, G.; Chen, Y.; Zhang, Z.; Dong, J.; Zuo, M.; Zeng, L.; Zhang, J.; Wu, K. An Iridoid Compound and Its Manufacture Method, Preparations and Application. CN105017354A, 4 November 2015. [Google Scholar]
- Zhang, Z.; Chen, Y.; Li, G.; Dong, J.; Zhang, J.; Wu, K.; Zuo, M.; Zeng, L. Iridoid Compound (Septemfidoside), and Its Preparation Method, Application in Preparing Drugs for Preventing and Treating TMV (Tobacco Mosaic Virus) and Pesticide Formulation. CN104910224A, 16 September 2015. [Google Scholar]
- Yan, Z.; Zuo, C.; Lin, Y.; Lan, M.; Li, S.; Chen, C.; Chen, C.; Zhang, R.; Zhang, T. Iridoid-Like New Compounds, Their Manufacture Method, Neuroprotective Effect and Application. CN103145724A, 12 June 2013. [Google Scholar]
- Liu, C.; Yang, T.; Wang, Q.; Tao, Y.; Liu, P.; Shen, L.; Li, S.; Peng, Y. Use of Iridoids and Iridoid Derivatives for Treating Hepatic Fibrosis and Hepatic Cirrhosis. CN103040854A, 17 April 2013. [Google Scholar]
- Liu, K.; Zou, X.; Xu, H.; Lang, Y.; Fan, H. Method for Preparation of Iridoid Genipin Methyl ether and Its Medical Application. CN102875518A, 16 January 2013. [Google Scholar]
- Luo, Y.; Ma, S.; Fu, H.; Fu, J.; Chen, W.; Huang, B.; Wang, S. Two Iridoid Glycoside Compounds and Production Method and Applications Thereof. CN104892698A, 9 September 2015. [Google Scholar]
- Fan, X.; Chen, M. A Novel Pharmaceutical Application of Iridoid Glycoside. CN104510747A, 15 April 2015. [Google Scholar]
- Xiao, W.; Yao, X.; Li, H.; Yu, Y.; Wang, Z.; Yao, Z.; Dai, Y.; Gao, H. Iridoid Glycoside Compound and Its Preparation Method and Application. CN104098632A, 15 October 2014. [Google Scholar]
- Xiao, W.; Yao, X.; Li, H.; Yu, Y.; Wang, Z.; Yao, Z.; Dai, Y.; Gao, H. Iridoid Glycoside Compound and Its Preparation Method and Application. CN104098630A, 15 October 2014. [Google Scholar]
- Xiao, W.; Yao, X.; Li, H.; Yu, Y.; Wang, Z.; Yao, Z.; Dai, Y.; Gao, H. Iridoid Glycoside Dimer Compound, Its Preparation Method and Application. CN104072551A, 1 October 2014. [Google Scholar]
- Xiao, W.; Yao, X.; Li, H.; Yu, Y.; Wang, Z.; Yao, Z.; Dai, Y.; Gao, H. Iridoid Glycoside Compound, Its Preparation Method and Application. CN103254259A, 21 August 2013. [Google Scholar]
- Rao, Z.; Lou, Z.; Guo, Y.; Chen, W.; Wang, B.; Niu, G.; Feng, J.; Li, J. Application of Iridoid Compounds in Preparation of Anti-SARS Medicament. CN103120699A, 29 May 2013. [Google Scholar]
- Chen, G. Preparation Method of Anticancer Iridoid Glycoside Extract of Colocasia antiquorum. CN105878622A, 24 August 2016. [Google Scholar]
- Bai, N.; Guo, S.; Liu, Q.; Zhang, Y.; Zhang, L.; Bai, L.; Zhang, X.; Tian, X. Fraxinus rhynchophylla Seed Chemical Composition Standardized Extract and Its Preparation Method and Application. CN105748496A, 13 July 2016. [Google Scholar]
- Mascenco, N.; Lupascu, G.; Gurev, A.; Barba, A.; Gorincioi, E.; Gavzer, S. Process for Treating Winter Wheat Against Fusarium oxysporum. MD819Y20141031, 31 October 2014. [Google Scholar]
- Liu, S.; Liu, Y.; Zhang, T.; Ji, R. Extract of Japanese Honeysuckle Stem or Flower or Leaf of Its Original Plants Lonicera and Its Preparation Method and Application. CN105311085A, 10 February 2016. [Google Scholar]
- Yang, X.; Zhu, S.; Wang, Y.; Dong, Z.; Hou, J.; Cheng, X.; Jiao, X.; Wang, Y.; Jiang, H.; Zhen, P. Paederia scandens Extract, Its Preparation Method and Application. CN105287790A, 3 February 2016. [Google Scholar]
- Wu, T.; Shen, X.; Shen, L.; Zhou, H.; Li, M.; Yue, X.; Zhang, Y.; Yan, R.; Zhang, X.; Zhang, M. Paederia scandens Extract and Its Application. CN104398619A, 11 March 2015. [Google Scholar]
- Wu, T.; Shen, X.; Li, Y.; Shen, L.; Wang, X.; Zhou, H.; Miao, L.; Zhang, L.; An, Y.; Yin, B. Paederia scandens Water Extract, Its Preparation and Application. CN102846784A, 2 January 2013. [Google Scholar]
- Wang, J.; Qin, J.; Zhang, Q.; Chen, G.; Shi, W. Application of Gentian Total Iridoid Glycosides in the Manufacture of a Medicament for the Treatment of Liver Injury. CN105878362A, 28 April 2016. [Google Scholar]
- Wang, Z.; Zhang, Y.; Pan, Y. Gardenia Emulsified Vinegar oil and Gardenia Sauce Vinegar and Their Production Method. CN104886546A, 9 September 2015. [Google Scholar]
- Carollo, C.A.; Vercosa, D.; Marques, M.R.; Kadri, M.C.T. Tabebuia-Derived Extracts Enriched in Glycosylated Iridoids for Anti-Inflammatory Use. BR2011000685A2, 21 July 2015. [Google Scholar]
- Hu, J.; Yang, J.; Nian, Y.; Dong, F.; Jiang, H.; Zhou, J.; Yang, L. Application of Rhizoma Valerianae Latifoliae Iridoid Fraction in Preparing n-Type Calcium Channel Inhibitor. CN104721180A, 24 June 2015. [Google Scholar]
- Xiao, J.; Wu, Y.; Zhou, C.; Kang, X.; Chen, L. Pharmaceutical Composition Containing Eucommia ulmoides Iridoid and Application Thereof. CN104523849A, 22 April 2015. [Google Scholar]
- Xiao, J.; Wu, Y.; Yang, W.; Chen, J.; Li, X. Eucommia ulmoides Targeted Formulation for Treating Colon Cancer and Its Preparation Method. CN104434950A, 25 March 2015. [Google Scholar]
- Xiao, J.; Wu, Y.; Yang, W.; Chen, L.; Kang, X. Eucommia ulmoides Bark Composition Having Weight Loss Effect and Its Preparation. CN104435068A, 25 March 2015. [Google Scholar]
- Xiao, J.; Wu, Y.; Chen, L.; Kang, X.; Mao, J. One Kind of Eucommia Component Composition Having Cardioprotective Effect and Its Preparations. CN104435067A, 25 March 2015. [Google Scholar]
- Shi, R.; Wang, Y.; Mei, Y. Drug Having Antibacterial and Synergistic Antimicrobial Activity and Preparation and Application of the Same. CN104306492A, 28 January 2015. [Google Scholar]
- Xu, H.; Di, L.; Li, W.; Li, W.; Zhao, T.; Wang, H.; Lv, G. Drug Effective Component Compatible Composition for Preventing and Treating Diabetes and Its Vascular Disease. CN104173568A, 3 December 2014. [Google Scholar]
- Li, M.; Jia, Z.; Wang, J.; Tao, R. Application of Lamiophlomis Rotata Total Iridoid Glycoside Extract in Preparing Medicine for Treating Constipation. CN104127486A, 5 November 2014. [Google Scholar]
- Kuang, H.; Wang, Z.; Wang, Q.; Yu, Y.; Yang, B.; Zhai, Y.; Song, P.; Xue, J. A Kind of Gentianella Acuta Extract for the Treatment of Arrhythmia, Preparation Method and Use Thereof. CN103638096A, 19 March 2014. [Google Scholar]
- Sun, W.; Liang, J.; Zhu, S.; Yu, J. Method for Extracting Cornus Officinalis Total Glycoside Used for Manufacturing Antihypoxic Drug. CN102614243A, 1 July 2012. [Google Scholar]
- Zhang, L.; Hu, J.J.; Lin, J.W.; Fang, W.S.; Du, G.H. Anti-Inflammatory and Analgesic Effects of Ethanol and Aqueous Extracts of Pterocephalus hookeri (C.B. Clarke) Höeck. J. Ethnopharmacol. 2009, 123, 510–514. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Lai, X.; Gu, R. Total Iridoid Glycoside Extract of Pterocephalus hookeri, and Manufacture Method and Applications Thereof. CN102526141A, 4 July 2012. [Google Scholar]
- Liang, G.; Liang, Z.; Jiao, H.; Pan, T.; Lv, W.; Huang, J. Borojoa Active Extract and Its Preparation Method and Application in Antimicrobial Agent and Antioxidant. CN102351927A, 15 February 2012. [Google Scholar]
- Yang, M.; Zhang, H.; Wang, J.; Liu, H.; Zhao, C.; Zheng, Q.; Yue, P.; Lu, Q. Pharmaceutical Liposome Comprising Geniposide Oriridoid Glycosides of Gardenia jasminoides and Its Application. CN102309449A, 11 January 2012. [Google Scholar]
- Miao, M.; Bai, M.; Miao, Y. Application of Total Saponins of Verbena officinalis in Drug for Preventing and Treating Cerebral Ischemia. CN102309622A, 11 January 2012. [Google Scholar]
- Rakotondramasy, V.C.; Mouriès, C.; Cachet, X.; Neghra, A.; El Mourabet, M.; Tillequin, F.; Koch, M.; Deguin, B. A novel series of cytotoxic iridoid glucosides derived from aucubin: Design, synthesis and structureeactivity relationships. Eur. J. Med. Chem. 2010, 45, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, H.; Green, I.R.; Saleem, M.; Raza, M.L.; Nazir, M. Therapeutic Potential of Iridoid Derivatives: Patent Review. Inventions 2019, 4, 29. https://doi.org/10.3390/inventions4020029
Hussain H, Green IR, Saleem M, Raza ML, Nazir M. Therapeutic Potential of Iridoid Derivatives: Patent Review. Inventions. 2019; 4(2):29. https://doi.org/10.3390/inventions4020029
Chicago/Turabian StyleHussain, Hidayat, Ivan R. Green, Muhammad Saleem, Muhammad Liaquat Raza, and Mamona Nazir. 2019. "Therapeutic Potential of Iridoid Derivatives: Patent Review" Inventions 4, no. 2: 29. https://doi.org/10.3390/inventions4020029
APA StyleHussain, H., Green, I. R., Saleem, M., Raza, M. L., & Nazir, M. (2019). Therapeutic Potential of Iridoid Derivatives: Patent Review. Inventions, 4(2), 29. https://doi.org/10.3390/inventions4020029