Development of a Heating Block as an Aid for the DNA-Based Biosensing of Plant Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Device Design
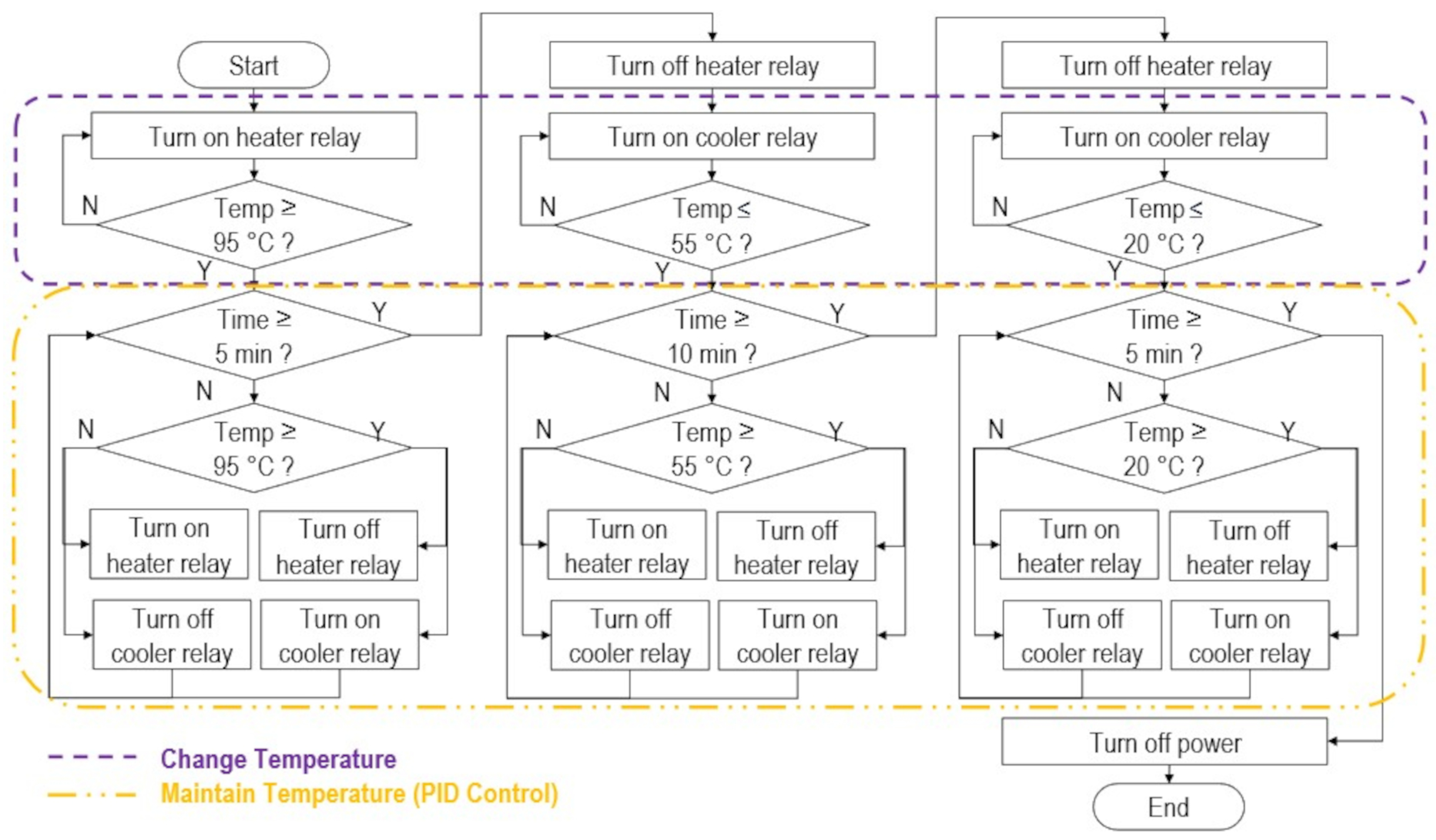
- The machine must control the temperature of the DNA to for 5 min, for 10 min, and for 5 min, corresponding to the three DNA hybridization phases, namely denaturation, annealing, and cooling, respectively.
- The machine must be user-friendly and easy to operate for farmers by having a single button that automatically sets the desired temperatures in sequence at the specified times.
- The machine must be field-deployable and easy to carry in resource-limited areas.
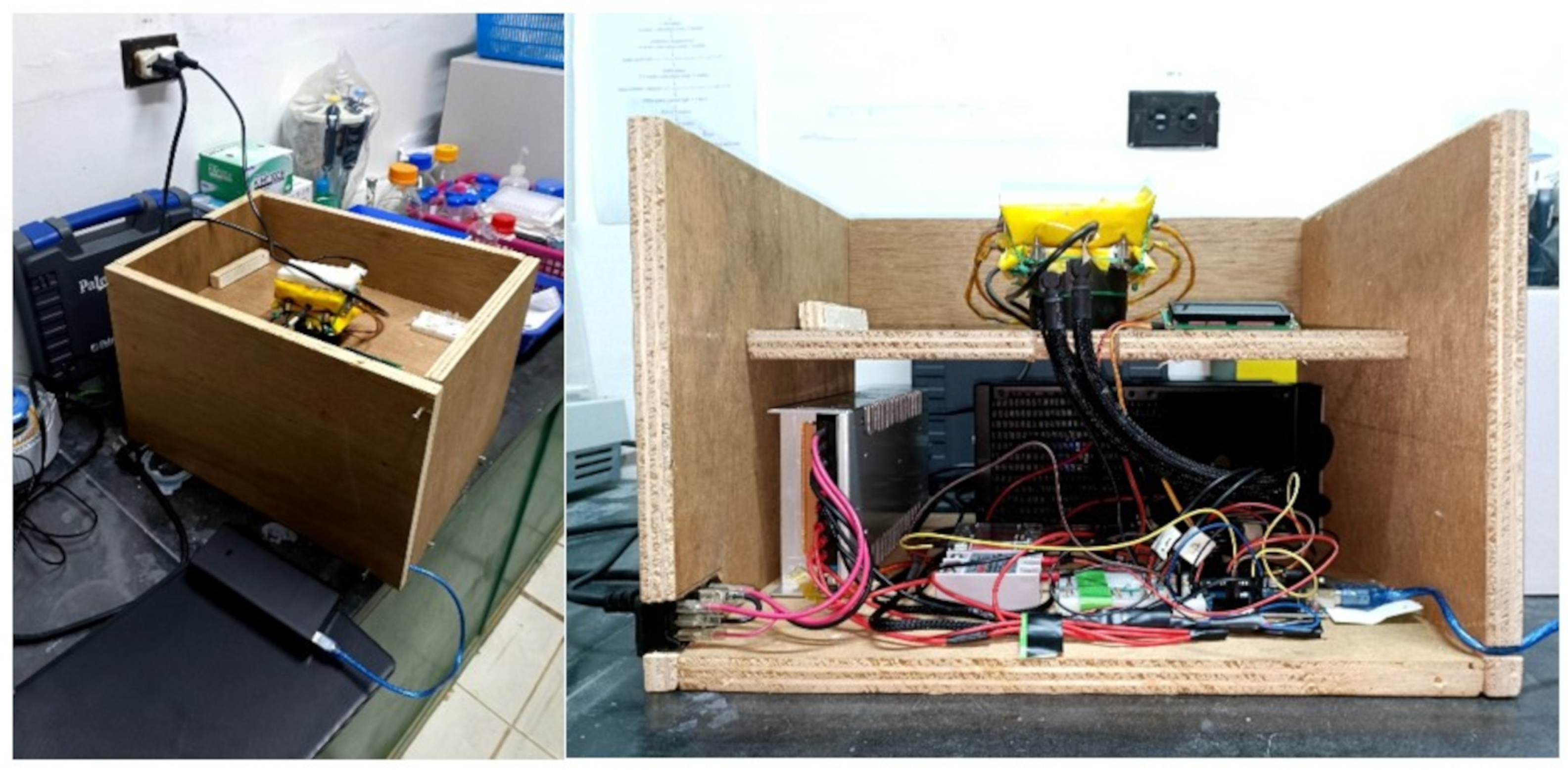
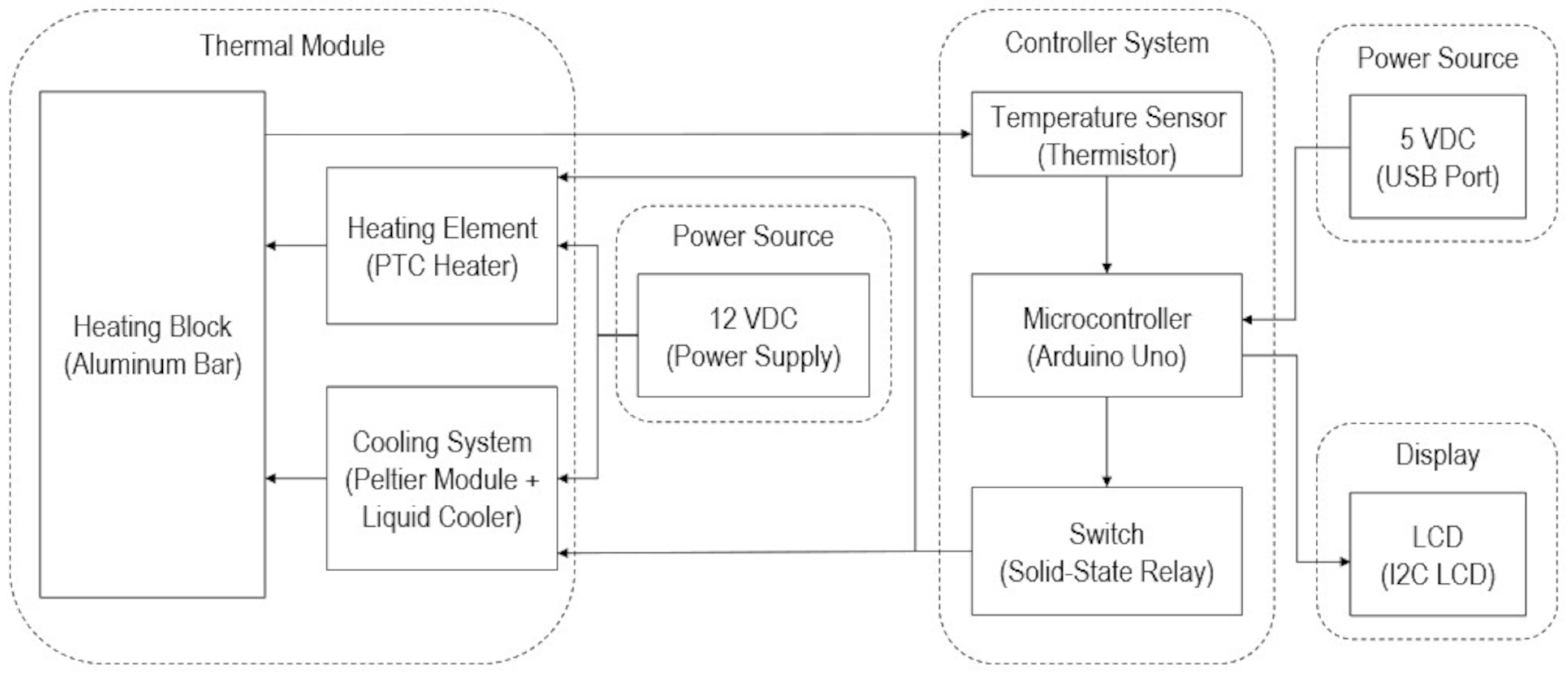
2.2. Thermal Module Design and Fabrication
2.3. Temperature Controller Design and Fabrication
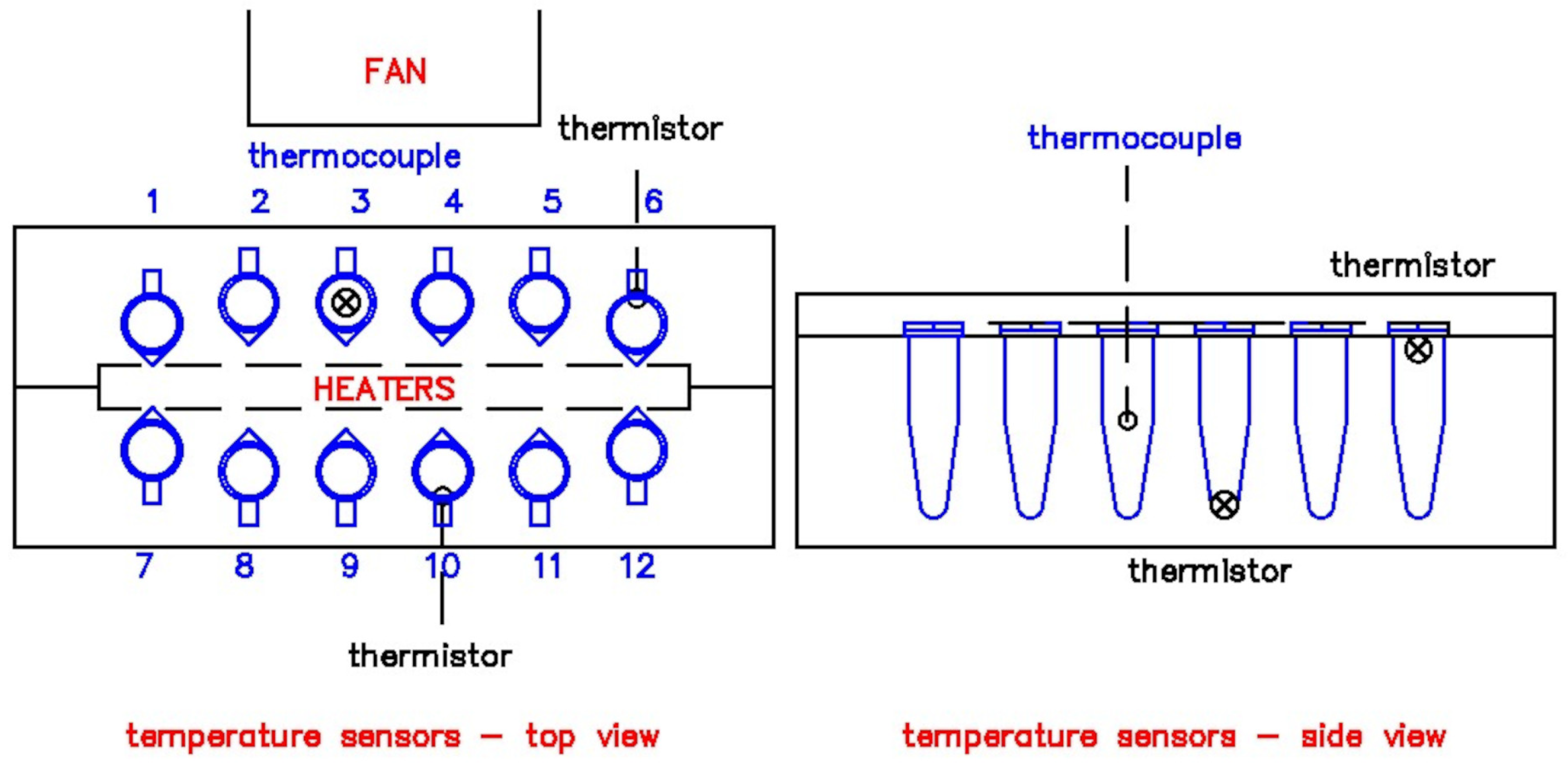
2.4. Device Testing
3. Results and Discussion
3.1. Simulated Device Performance
3.2. Actual Device Performance
4. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGORA | Accelerating Growth to One Research and Extension in Action |
| AI | Artificial Intelligence |
| ANOVA | Analysis of variance |
| ARDS | Agricultural and Rural Development Scholarship |
| BMSL | Biomaterials and Sensors Laboratory |
| CEAT | College of Engineering and Agro-Industrial Technology |
| DC | Direct current |
| DNA | Deoxyribonucleic Acid |
| DOST-SEI | Department of Science and Technology-Science Education Institute |
| ELISAs | Enzyme-Linked Immunosorbent Assays |
| FISH | Fluorescence In Situ Hybridization |
| ICropS | Institute of Crop Science |
| LCD | liquid crystal display |
| PCR | polymerase chain reaction |
| PFTE | polytetrafluoroethylene |
| PID | proportional-integral-derivative |
| POC | point-of-care |
| PTC | positive thermal 114 coefficient |
| RMSE | root mean square error |
| SPR | surface plasmon resonance |
| UPLB | University of the Philippines Los Baños |
| USB | universal serial bus |
| VOC | volatile organic compound |
Appendix A
References
- Buja, I.; Sabella, E.; Monteduro, A.G.; Chiriacò, M.S.; De Bellis, L.; Luvisi, A.; Maruccio, G. Advances in plant disease detection and monitoring: From traditional assays to in-field diagnostics. Sensors 2021, 21, 2129. [Google Scholar] [CrossRef]
- Franco, A.J.D.; Merca, F.E.; Rodriguez, M.S.; Balidion, J.F.; Migo, V.P.; Amalin, D.M.; Alocilja, E.C.; Fernando, L.M. DNA-based electrochemical nanobiosensor for the detection of Phytophthora palmivora (Butler) Butler, causing black pod rot in cacao (Theobroma cacao L.) pods. Physiol. Mol. Plant Pathol. 2019, 107, 14–20. [Google Scholar] [CrossRef]
- Gautam, A.K.; Kumar, S. Techniques for the detection, identification, and diagnosis of agricultural pathogens and diseases. In Natural Remedies for Pest, Disease and Weed Control; Academic Press: Cambridge, MA, USA, 2020; pp. 135–142. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Sharief, S.A.; Caliskan-Aydogan, O.; Alocilja, E.C. Carbohydrate-coated nanoparticles for PCR-less genomic detection of Salmonella from fresh produce. Food Control 2023, 150, 109770. [Google Scholar] [CrossRef]
- Bankole, O.E.; Verma, D.K.; Chávez González, M.L.; Ceferino, J.G.; Sandoval-Cortés, J.; Aguilar, C.N. Recent trends and technical advancements in biosensors and their emerging applications in food and bioscience. Food Biosci. 2022, 47, 101695. [Google Scholar] [CrossRef]
- Choi, W.; Min, Y.W.; Lee, K.Y.; Jun, S.; Lee, H.G. Dielectrophoresis-based microwire biosensor for rapid detection of Escherichia coli K-12 in ground beef. LWT 2020, 132, 109230. [Google Scholar] [CrossRef]
- Fatema, K.N.; Liu, Y.; Cho, K.Y.; Oh, W.C. Comparative study of electrochemical biosensors based on highly efficient mesoporous ZrO2-Ag-G-SiO2 and In2O3-G-SiO2 for rapid recognition of E. coli O157:H7. ACS Omega 2020, 5, 22719–22730. [Google Scholar] [CrossRef]
- Soares, R.R.A.; Hjort, R.G.; Pola, C.C.; Parate, K.; Reis, E.L.; Soares, N.F.F.; McLamore, E.S.; Claussen, J.C.; Gomes, C.L. Laser-induced graphene electrochemical immunosensors for rapid and label-free monitoring of Salmonella enterica in chicken broth. ACS Sens. 2020, 5, 1900–1911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, L.; Huang, F.; Wang, S.; Wang, L.; Liu, N.; Lin, J. A capillary biosensor for rapid detection of Salmonella using Fe-nanocluster amplification and smart phone imaging. Biosens. Bioelectron. 2019, 127, 142–149. [Google Scholar] [CrossRef]
- Chen, L.; Leng, Y.K.; Liu, B.; Liu, J.; Wan, S.P.; Wu, T.; Yuan, J.; Shao, L.; Gu, G.; Fu, Y.Q.; et al. Ultrahigh-sensitivity label-free optical fiber biosensor based on a tapered singlemode- no core-singlemode coupler for Staphylococcus aureus detection. Sens. Actuators B Chem. 2020, 320, 128283. [Google Scholar] [CrossRef]
- Ozcan, S.M.; Sesal, N.C.; Sener, M.K.; Koca, A. An alternative strategy to detect bacterial contamination in milk and water: A newly designed electrochemical biosensor. Eur. Food Res. Technol. 2020, 246, 1317–1324. [Google Scholar] [CrossRef]
- Lee, T.; Park, S.Y.; Jang, H.; Kim, G.H.; Lee, Y.; Park, C.; Mohammadniaei, M.; Lee, M.; Min, J. Fabrication of electrochemical biosensor consisted of multi-functional DNA structure/porous au nanoparticle for avian influenza virus (H5N1) in chicken serum. Mater. Sci. Eng. C 2019, 99, 511–519. [Google Scholar] [CrossRef]
- Chalupowicz, D.; Veltman, B.; Droby, S.; Eltzov, E. Evaluating the use of biosensors for monitoring of Penicillium digitatum infection in citrus fruit. Sens. Actuators B Chem. 2020, 311, 127896. [Google Scholar] [CrossRef]
- Patkar, R.S.; Vinchurkar, M.; Ashwin, M.; Adami, A.; Giacomozzi, F.; Lorenzelli, L.; Baghini, M.S.; Rao, V.R. Microcantilever based dual mode biosensor for agricultural applications. IEEE Sens. J. 2019, 20, 6826–6832. [Google Scholar] [CrossRef]
- Berto, M.; Vecchi, E.; Baiamonte, L.; Condò, C.; Sensi, M.; Di Lauro, M.; Sola, M.; De Stradis, A.; Biscarini, F.; Minafra, A.; et al. Label free detection of plant viruses with organic transistor biosensors. Sens. Actuators B Chem. 2019, 281, 150–156. [Google Scholar] [CrossRef]
- Jarocka, U.; Wąsowicz, M.; Radecka, H.; Malinowski, T.; Michalczuk, L.; Radecki, J. Impedimetric immunosensor for detection of plum pox virus in plant extracts. Electroanalysis 2011, 23, 2197–2204. [Google Scholar] [CrossRef]
- Huang, X.; Xu, J.; Ji, H.F.; Li, G.; Chen, H. Quartz crystal microbalance based biosensor for rapid and sensitive detection of maize chlorotic mottle virus. Anal. Methods 2014, 6, 4530–4536. [Google Scholar] [CrossRef]
- Regiart, M.; Rinaldi-Tosi, M.; Aranda, P.R.; Bertolino, F.A.; Villarroel-Rocha, J.; Sapag, K.; Messina, G.A.; Raba, J.; Fernández-Baldo, M.A. Development of a nanostructured immunosensor for early and in situ detection of Xanthomonas arboricola in agricultural food production. Talanta 2017, 175, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Wongkaew, P.; Poosittisak, S. Diagnosis of sugarcane white leaf disease using the highly sensitive DNA based voltammetric electrochemical determination. Am. J. Plant Sci. 2014, 5, 2256–2268. [Google Scholar] [CrossRef]
- Khater, M.; De La Escosura-Muñiz, A.; Quesada-González, D.; Merkoçi, A. Electrochemical detection of plant virus using gold nanoparticle-modified electrodes. Anal. Chim. Acta 2019, 1046, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Henríquez, L.; Brenes-Acuña, M.; Castro-Rojas, A.; Cordero-Salmerón, R.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Biosensors for the detection of bacterial and viral clinical pathogens. Sensors 2020, 20, 6926. [Google Scholar] [CrossRef]
- Dyussembayev, K.; Sambasivam, P.; Bar, I.; Brownlie, J.C.; Shiddiky, M.J.; Ford, R. Biosensor technologies for early detection and quantification of plant pathogens. Front. Chem. 2021, 9, 636245. [Google Scholar] [CrossRef]
- Nesakumar, N.; Lakshmanakumar, M.; Srinivasan, S.; Jayalatha Jbb, A.; Balaguru Rayappan, J.B. Principles and recent advances in biosensors for pathogens detection. ChemistrySelect 2021, 6, 10063–10091. [Google Scholar] [CrossRef]
- Thakur, M.; Wang, B.; Verma, M.L. Development and applications of nanobiosensors for sustainable agricultural and food industries: Recent developments, challenges and perspectives. Environ. Technol. Innov. 2022, 26, 102371. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Flynn, C.D.; Chang, D. Artificial intelligence in point-of-care biosensing: Challenges and opportunities. Diagnostics 2024, 14, 1100. [Google Scholar] [CrossRef] [PubMed]
- Nashruddin, S.N.A.B.M.; Salleh, F.H.M.; Yunus, R.M.; Zaman, H.B. Artificial intelligence—powered electrochemical sensor: Recent advances, challenges, and prospects. Heliyon 2024, 10, e37964. [Google Scholar] [CrossRef] [PubMed]
- Goumas, G.; Vlachothanasi, E.N.; Fradelos, E.C.; Mouliou, D.S. Biosensors, artificial intelligence biosensors, false results and novel future perspectives. Diagnostics 2025, 15, 1037. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Laghari, A.A.; Alsafyani, M.; Baqasah, A.M.; Kryvinska, N.; Almadhor, A.; Alroobaea, R.; Gregus, M., Jr. A cost-effective approach using generative AI and gamification to enhance biomedical treatment and real-time biosensor monitoring. Sci. Rep. 2025, 15, 17305. [Google Scholar] [CrossRef]
- Ramola, A.; Shakya, A.K.; Bergman, A. Finite element method-based modeling of a novel square photonic crystal fiber surface plasmon resonance sensor with a Au–TiO2 interface and the relevance of artificial intelligence techniques in sensor optimization. Photonics 2025, 12, 565. [Google Scholar] [CrossRef]
- Akkaş, T.; Reshadsedghi, M.; Şen, M.; Kılıç, V.; Horzum, N. The role of artificial intelligence in advancing biosensor technology: Past, present, and future perspectives. Adv. Mater. 2025, 37, 2504796. [Google Scholar] [CrossRef] [PubMed]
- Etli, D.; Djurovic, A.; Lark, J. The future of personalized healthcare: AI-driven wearables for real-time health monitoring and predictive analytics. Curr. Res. Health Sci. 2024, 2, 10–14. [Google Scholar]
- Althobiti, M.; Nhung, T.T.T.; Verma, S.; Albugami, R.R.; Kumar, R. Artificial intelligence and biosensors: Transforming cancer diagnostics. Med. Nov. Technol. Devices 2025, 27, 100378. [Google Scholar] [CrossRef]
- Dester, E.; Kao, K.; Alocilja, E.C. Detection of Unamplified E. coli O157 DNA Extracted from Large Food Samples Using a Gold Nanoparticle Colorimetric Biosensor. Biosensors 2022, 12, 274. [Google Scholar] [CrossRef]
- Agrawal, N. Design and Characterization of Convective Thermal Cyclers for High-Speed DNA Analysis. Ph.D. Thesis, Texas A and M University, College Station, TX, USA, 2009. [Google Scholar]
- Atwood, J.G.; Fawcett, A.; Ferrara, K.S.; Hetherington, P.M.; Noreiks, R.W.; Olsen, D.E.; Widomski, J.R.; Wittmer, C.M. Thermal Cycler for PCR. U.S. Patent No. 8246243B2, 21 August 2012. [Google Scholar]
- Diep, T.T.; Bizley, S.; Ray, P.P.; Edwards, A.D. MicroMI: A portable microbiological mobile incubator that uses inexpensive lithium power banks for field microbiology. HardwareX 2021, 10, e00242. [Google Scholar] [CrossRef] [PubMed]
- Wight, J.; Varin, M.P.; Robertson, G.J.; Huot, Y.; Lang, A.S. Microbiology in the field: Construction and validation of a portable incubator for real-time quantification of coliforms and other bacteria. Front. Public Health 2020, 8, 607997. [Google Scholar] [CrossRef]
- Nasser, G.A.; Abdel-Mawgood, A.L.; Abouelsoud, A.A.; Mohamed, H.; Umezu, S.; El-Bab, A.M.F. New cost effective design of PCR heating cycler system using Peltier plate without the conventional heating block. J. Mech. Sci. Technol. 2021, 35, 3259–3268. [Google Scholar] [CrossRef]
- Zou, Q.; Miao, Y.; Chen, Y.; Sridhar, U.; Chong, C.S.; Chai, T.; Tie, Y.; Teh, C.H.L.; Lim, T.M.; Heng, C. Micro-assembled multi-chamber thermal cycler for low-cost reaction chip thermal multiplexing. Sens. Actuators A Phys. 2002, 102, 114–121. [Google Scholar] [CrossRef]
- Sun, H.; Olsen, T.; Zhu, J.; Tao, J.; Ponnaiya, B.; Amundson, S.A.; Brenner, D.J.; Lin, Q. A bead-based microfluidic approach to integrated single-cell gene expression analysis by quantitative RT-PCR. RSC Adv. 2015, 5, 4886–4893. [Google Scholar] [CrossRef]
- Priye, A.; Wong, S.; Bi, Y.; Carpio, M.; Chang, J.; Coen, M.; Cope, D.; Harris, J.; Johnson, J.; Keller, A.; et al. Lab-on-a-drone: Toward pinpoint deployment of smartphone-enabled nucleic acid-based diagnostics for mobile health care. Anal. Chem. 2016, 88, 4651–4660. [Google Scholar] [CrossRef]
- Kim, Y.T.; Chen, Y.; Choi, J.Y.; Kim, W.J.; Dae, H.M.; Jung, J.; Seo, T.S. Integrated microdevice of reverse transcription-polymerase chain reaction with colorimetric immunochromatographic detection for rapid gene expression analysis of influenza A H1N1 virus. Biosens. Bioelectron. 2012, 33, 88–94. [Google Scholar] [CrossRef]
- Bu, M.; Perch-Nielsen, I.R.; Sørensen, K.S.; Skov, J.; Sun, Y.; Bang, D.D.; Pedersen, M.E.; Hansen, M.F.; Wolff, A. A temperature control method for shortening thermal cycling time to achieve rapid polymerase chain reaction (PCR) in a disposable polymer microfluidic device. J. Micromech. Microeng. 2013, 23, 74002. [Google Scholar] [CrossRef]
- Sun, K.; Whiteside, B.; Hebda, M.; Fan, Y.; Zhang, Y.; Xie, Y.; Liang, K. A low-cost and hand-hold PCR microdevice based on water-cooling technology. Biomed. Microdevices 2023, 25, 12. [Google Scholar] [CrossRef]
- Luo, K.; Cheng, W.; Chen, Y.; Zhang, Q.; Liang, C.; Li, J.; Wang, W. A portable low-cost polymerase chain reaction device. HardwareX 2025, 21, e00635. [Google Scholar] [CrossRef] [PubMed]
- Pudasaini, S.; Thapa, G.; Marasini, B.P.; Giri, B. Smartphone-operated affordable PCR thermal cycler for the detection of antimicrobial resistant bacterial genes. PLoS Glob. Public Health 2023, 3, e0001120. [Google Scholar] [CrossRef]
- Kadja, T.; Liu, C.; Sun, Y.; Chodavarapu, V.P. Low-cost, real-time polymerase chain reaction system for point-of-care medical diagnosis. Sensors 2022, 22, 2320. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Tong, J.; Li, J.; Shao, G.; Xie, B.; Zhuang, J.; Bi, G.; Mu, Y. A portable, high-throughput real-time quantitative PCR device for point-of-care testing. Anal. Biochem. 2023, 674, 115200. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Duran, J.; Killinen, W.; Wagner, J.; Kulesza, C.; Chatterley, C.; Li, Y. A field-deployable and low-cost PCR (FLC-PCR) thermocycler for the rapid detection of environmental E. coli. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 2209–2212. [Google Scholar] [CrossRef]
- Sowmya, V.S.; Dharsini, S.P.; Dharshini, R.P.; Aravind, P. Application of various PID controller tuning techniques for a temperature system. Int. J. Comput. Appl. 2014, 103, 32–34. [Google Scholar] [CrossRef]

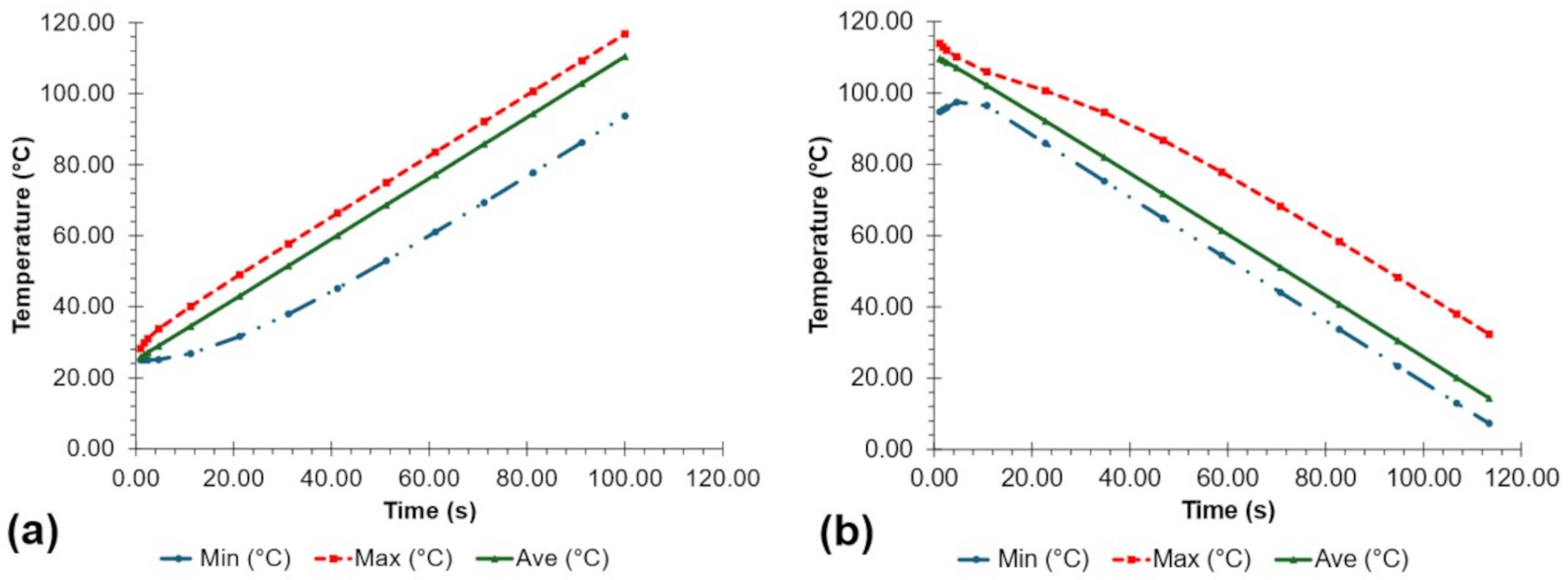
| Temperature | Calibration Equation | RMSE |
|---|---|---|
| 0.3956 | ||
| 0.1639 | ||
| 0.4964 |
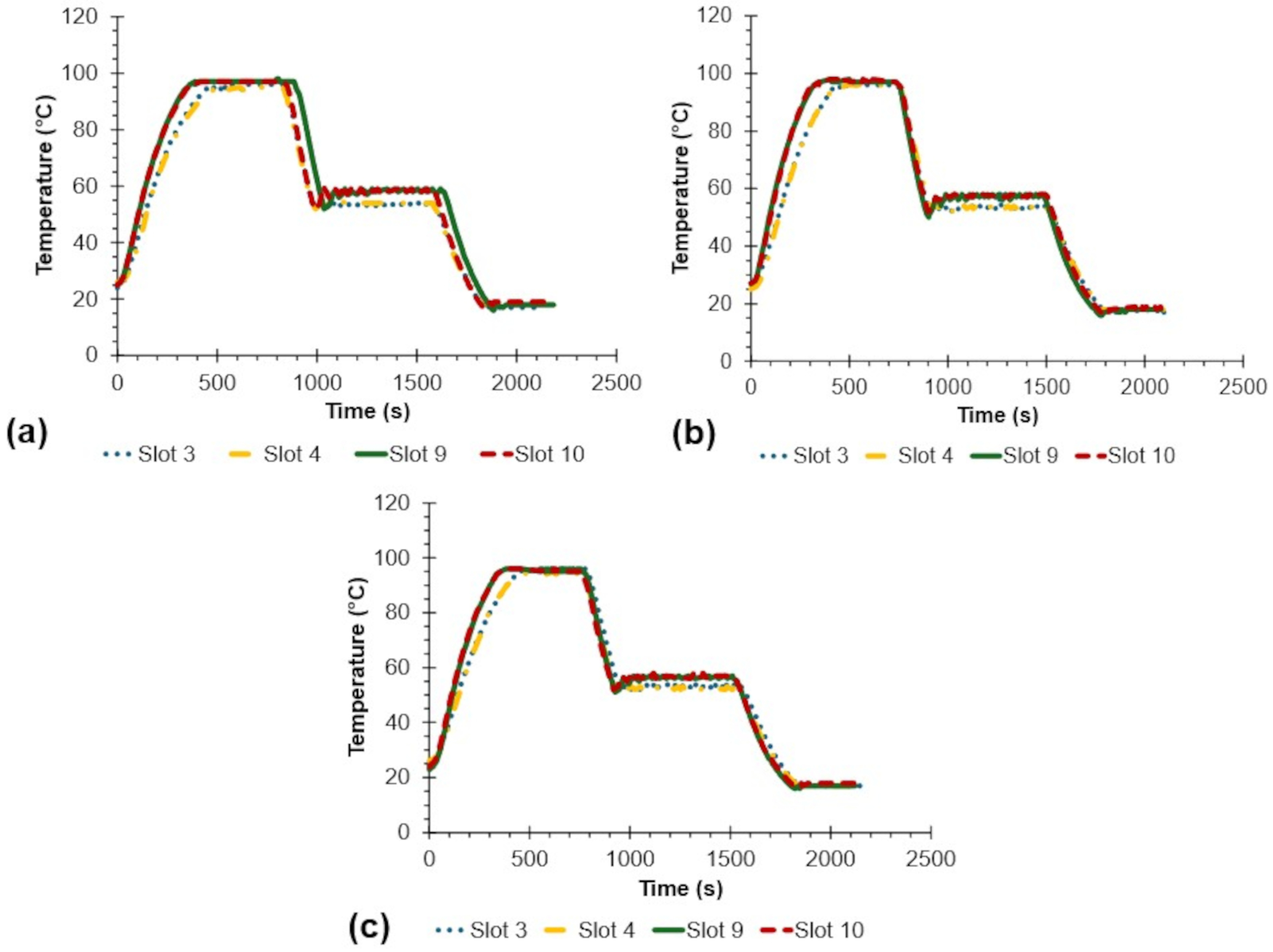
| Sample | Average () | SD () | RMSE () | Max () | Min () | Statistical Difference to Set Point * |
|---|---|---|---|---|---|---|
| 95 Setpoint (denaturation) | ||||||
| Slot 3 | 95.83 | 0.41 | 0.92 | 96.00 | 94.00 | ns |
| Slot 4 | 95.06 | 0.70 | 0.70 | 96.00 | 94.00 | ns |
| Slot 9 | 96.53 | 0.79 | 1.73 | 98.00 | 95.00 | ns |
| Slot 10 | 96.59 | 0.98 | 1.87 | 98.00 | 95.00 | ns |
| Average | 96.00 | 0.97 | 1.40 | 98.00 | 94.00 | - |
| 55 Setpoint (annealing) | ||||||
| Slot 3 | 53.33 | 0.57 | 1.76 | 59.00 | 52.00 | s |
| Slot 4 | 53.25 | 0.70 | 1.89 | 55.00 | 52.00 | s |
| Slot 9 | 56.88 | 1.52 | 2.42 | 59.00 | 50.00 | ns |
| Slot 10 | 57.16 | 1.32 | 2.53 | 60.00 | 52.00 | ns |
| Average | 55.15 | 2.17 | 2.18 | 60.00 | 50.00 | - |
| 20 Setpoint (cooling) | ||||||
| Slot 3 | 17.18 | 0.30 | 2.83 | 18.00 | 17.00 | s |
| Slot 4 | 17.94 | 0.77 | 2.20 | 19.00 | 17.00 | s |
| Slot 9 | 17.51 | 0.52 | 2.54 | 18.00 | 16.00 | s |
| Slot 10 | 18.37 | 0.53 | 1.72 | 19.00 | 17.00 | s |
| Average | 17.75 | 0.71 | 2.36 | 19.00 | 16.00 | - |
| Sample | Average * () | SD () | Time (s) | Time (min) |
|---|---|---|---|---|
| Ambient to | ||||
| Slot 3 | 0.16 a | 0.09 | 489 | 8.15 |
| Slot 4 | 0.15 a | 0.09 | 484 | 8.06 |
| Slot 9 | 0.15 a | 0.13 | 505 | 8.42 |
| Slot 10 | 0.16 a | 0.13 | 480 | 8.00 |
| Average | 0.16 | 0.11 | 490 | 8.16 |
| to | ||||
| Slot 3 | −0.29 b | 0.09 | 156 | 2.61 |
| Slot 4 | −0.28 b | 0.10 | 157 | 2.61 |
| Slot 9 | −0.30 b | 0.12 | 158 | 2.63 |
| Slot 10 | −0.29 b | 0.13 | 156 | 2.61 |
| Average | −0.29 | 0.11 | 157 | 2.61 |
| to | ||||
| Slot 3 | −0.14 c | 0.05 | 285 | 4.75 |
| Slot 4 | −0.13 c | 0.05 | 284 | 4.74 |
| Slot 9 | −0.15 c | 0.08 | 281 | 4.68 |
| Slot 10 | −0.15 c | 0.08 | 285 | 4.75 |
| Average | −0.14 | 0.07 | 284 | 4.73 |
| Prototype | Average Maintained Temperature, | Average Thermal Rate, | Estimated Cost * (USD $) |
|---|---|---|---|
| Fabricated device in this study | , , | to : , to : , to : | 272 |
| Luo et al. (2025) [47] | heating: , cooling: | 120 | |
| Pudasaini et al. (2023) [48] | heating: , cooling: | 50 | |
| Kadja et al. (2022) [49] | - | to : , to : , to : | 340 |
| Yin et al. (2023) [50] | >2.5 | 387 | |
| Ferguson et al. (2020) [51] | - | average: , to : , to : , to : | <200 |
| Nasser et al. (2021) [40] | , , | heating: , cooling: 8 | 125 |
| Sun et al. (2023) [42] | , , | heating: , cooling: | 171 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diola, B.M.L.; Borja, A.A.; Sanchez, P.R.P.; Purificacion, M.V.; Gallegos, R.K.B. Development of a Heating Block as an Aid for the DNA-Based Biosensing of Plant Pathogens. Inventions 2025, 10, 94. https://doi.org/10.3390/inventions10060094
Diola BML, Borja AA, Sanchez PRP, Purificacion MV, Gallegos RKB. Development of a Heating Block as an Aid for the DNA-Based Biosensing of Plant Pathogens. Inventions. 2025; 10(6):94. https://doi.org/10.3390/inventions10060094
Chicago/Turabian StyleDiola, Bertrand Michael L., Adrian A. Borja, Paolo Rommel P. Sanchez, Marynold V. Purificacion, and Ralph Kristoffer B. Gallegos. 2025. "Development of a Heating Block as an Aid for the DNA-Based Biosensing of Plant Pathogens" Inventions 10, no. 6: 94. https://doi.org/10.3390/inventions10060094
APA StyleDiola, B. M. L., Borja, A. A., Sanchez, P. R. P., Purificacion, M. V., & Gallegos, R. K. B. (2025). Development of a Heating Block as an Aid for the DNA-Based Biosensing of Plant Pathogens. Inventions, 10(6), 94. https://doi.org/10.3390/inventions10060094








