Mathematical Modeling of the Influence of Electrical Heterogeneity on the Processes of Salt Ion Transfer in Membrane Systems with Axial Symmetry Taking into Account Electroconvection
Abstract
1. Introduction
2. Methods
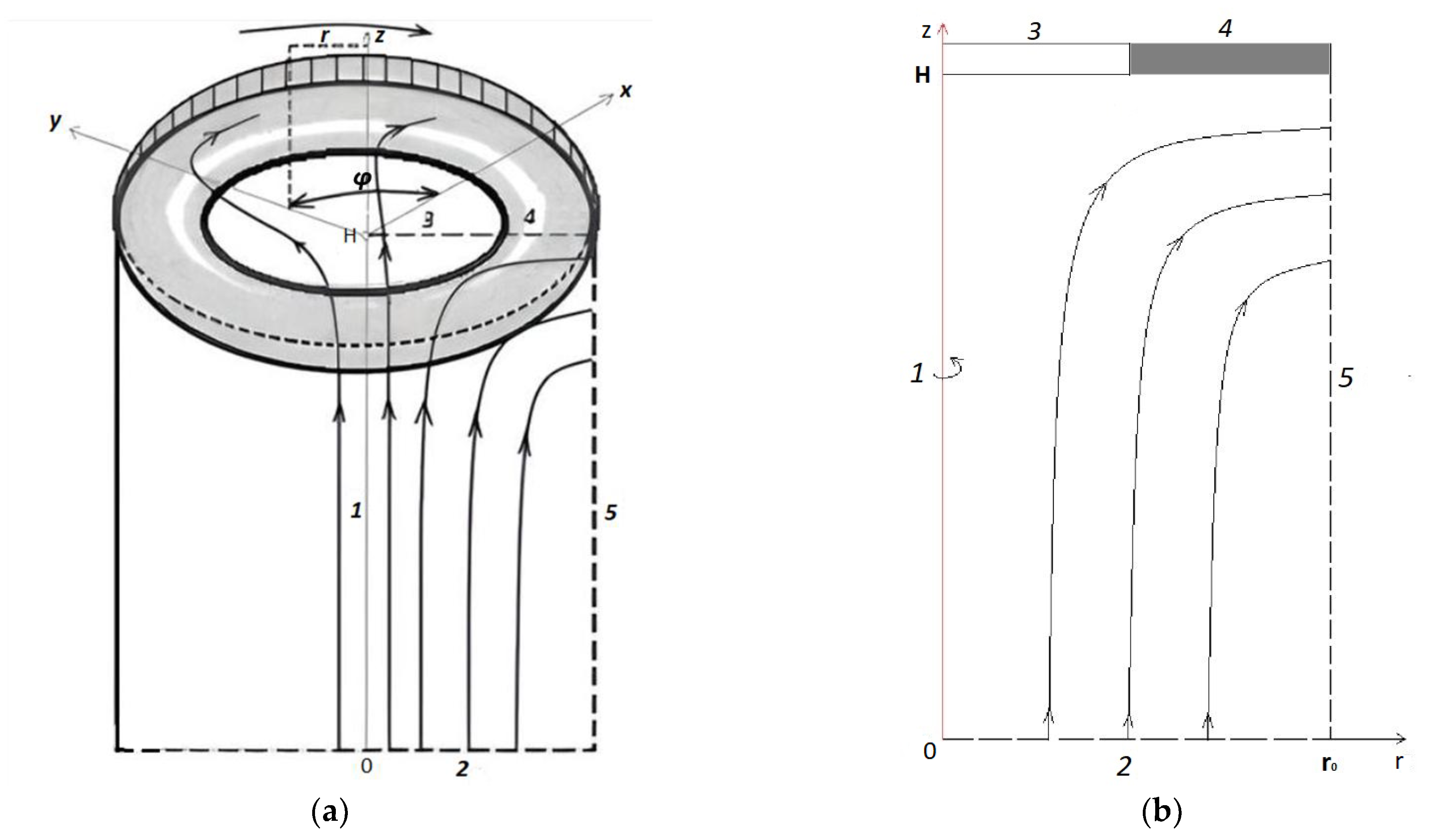
2.1. Mathematical Model of Transport for Annular Membrane Disk
2.2. Algorithm for Hybrid Numerical–Analytical Solution
- 1.
- The Peclet number shows the ratio of the coefficients of kinematic viscosity and diffusion. With a characteristic channel height and an outer radius of the membrane disk of 1 mm, we obtain an estimate of the Peclet number (Table 1):
- 2.
- Dimensionless number
- 3.
- The Reynolds number Re, which is the ratio of the inertial force to the viscous friction force .
- 4.
- The dimensionless parameter is the ratio of electrical force to inertial force. Let us calculate it for a membrane radius of m (Table 3):
- 1.
- The quasi-equilibrium SCR is formed almost instantly, in about 10−5 seconds;
- 2.
- The thickness of the quasi-equilibrium SCR does not depend on the radius (r) of the membrane disk, except for the vicinity of ;
- 3.
- The quasi-equilibrium SCR is also quasi-stationary, i.e., it is practically independent of time;
- 4.
- The axial and radial velocities in the quasi-equilibrium SCR are close to zero, and the azimuthal can be considered practically constant and equal to , i.e., the electrolyte solution near the membrane in the quasi-equilibrium SCR rotates as a whole.
- Find an analytical solution in the quasi-equilibrium SCR;
- Determine the boundary conditions on the left boundary of the quasi-equilibrium SCR for the numerical solution;
- Splice the analytical and numerical solutions, i.e., find the constants included in the analytical solution and determine the boundary between the analytical and numerical solutions.
- Solution in the pre-limit case
- 2.
- Solution in the overlimit case
- 3.
- Splicing solutions and determining constants
- We numerically solve the boundary value problem of the model without the RICC and find , .
- We find the potential jump numerically. Next, we find the potential jump for the basic model using the following relation:
- 3.
- We find the analytical solution in the RICC using Formulas (18)–(20) for the sub-limit current mode and using Formulas (21) and (22) for the overlimit current mode.
- 4.
- Using steps (1) and (3), we obtain the solution to the boundary value problem of the basic model.
3. Results
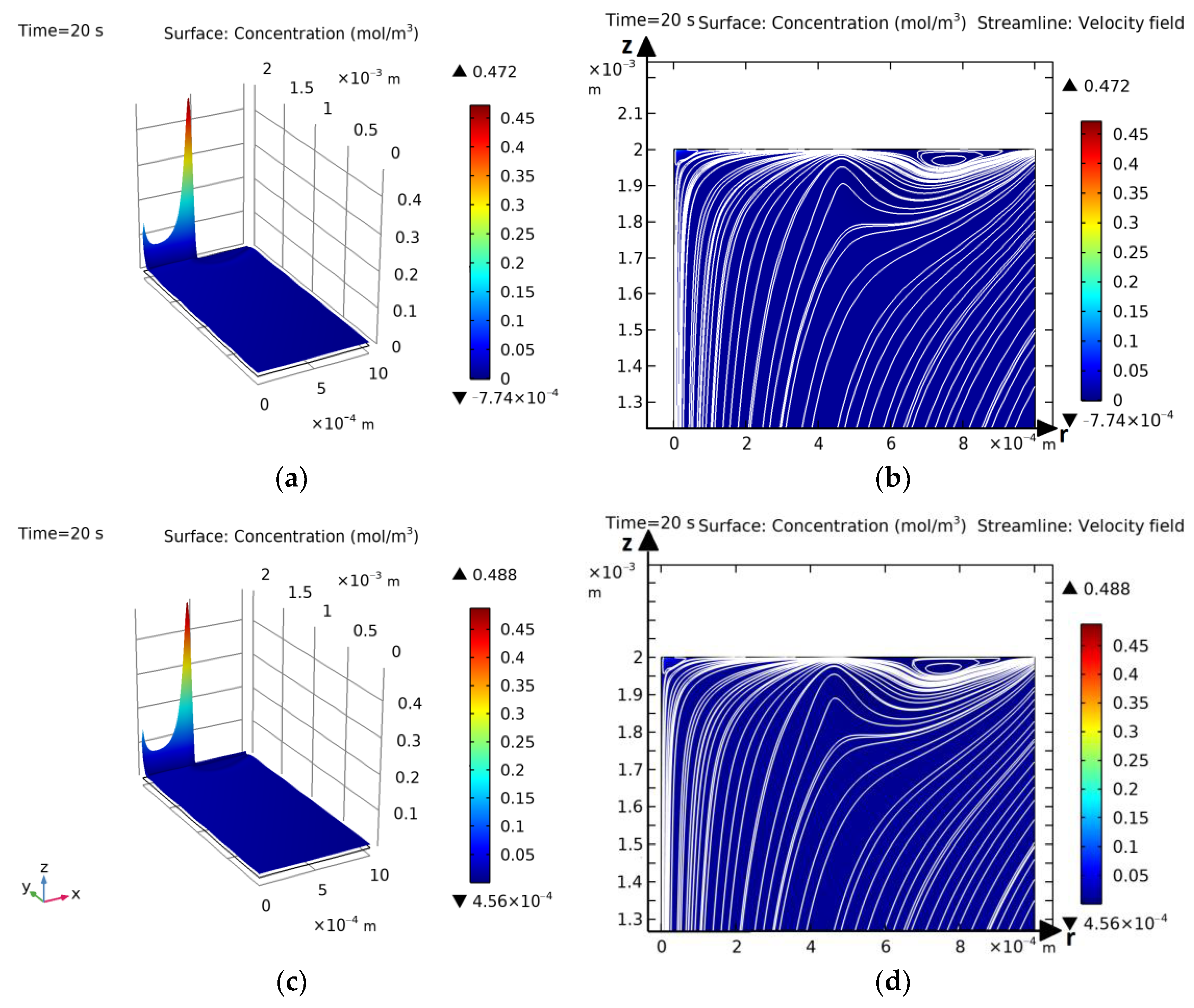
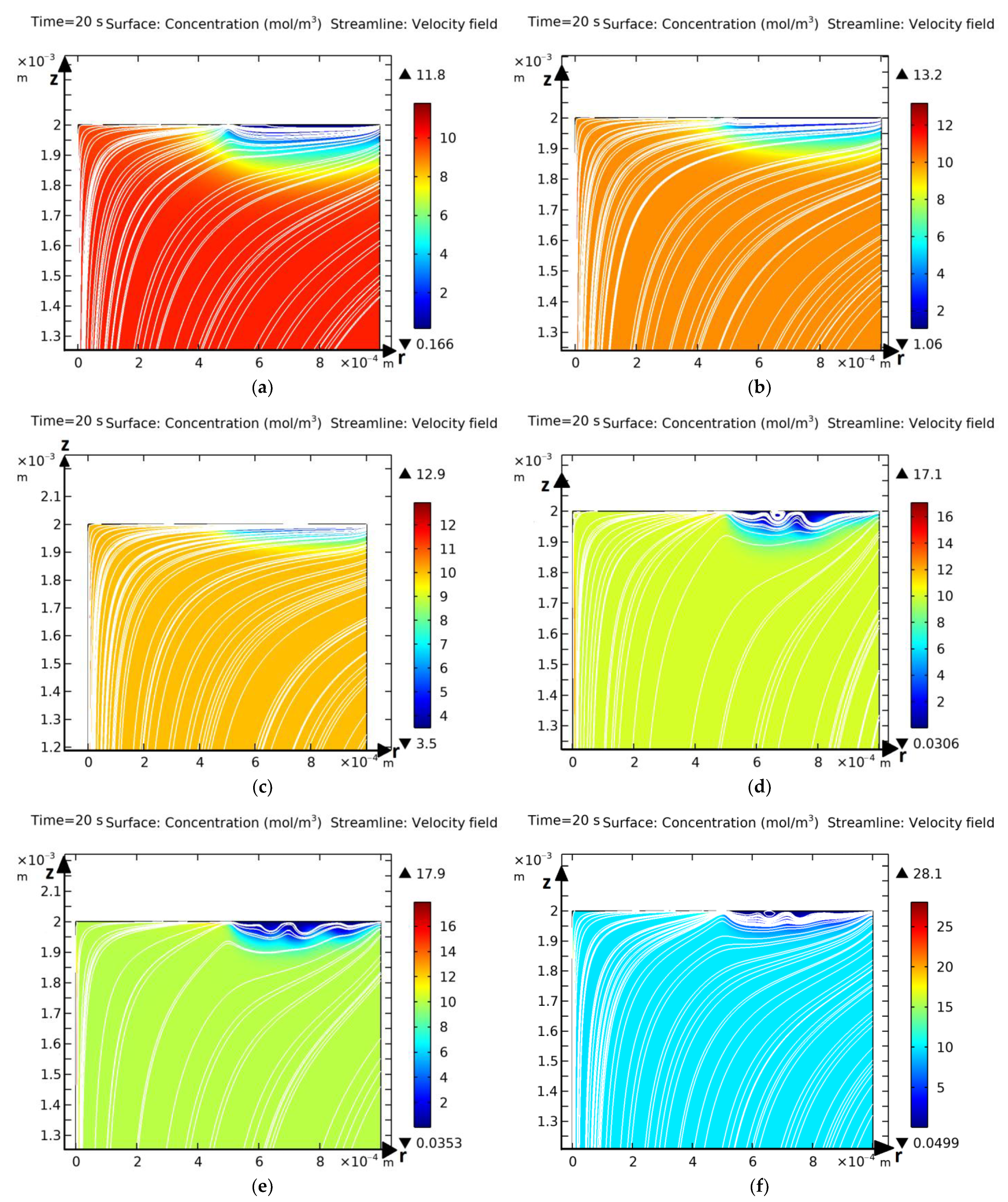
3.1. Computational Experiments for Verification of Hybrid Numerical Method
Comparison of Results for the Annular Disk Model Obtained Using the Hybrid Method and the Independent Finite Element Method
3.2. Basic Laws of Occurrence and Development of Electroconvection and Mass Transfer for the Model with an Annular Membrane
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEM | Cation exchange membrane |
| RICC | Region of increasing cation concentration |
| NS | Numerical solution |
| EMS | Electromembrane systems |
| CVC | Current–voltage characteristic |
| SCR | Space charge region |
References
- Stockmeier, F.; Felder, D.; Eser, S.; Habermann, M.; Peric, P.; Musholt, S.; Albert, K.; Linkhorst, J.; Wessling, M. Localized Electroconvection at Ion-Exchange Membranes with Heterogeneous Surface Charge. Preprint 2021. [Google Scholar] [CrossRef]
- Bräsel, B.; Yoo, S.-W.; Huber, S.; Wessling, M.; Linkhorst, J. Evolution of particle deposits at communicating membrane pores during crossflow filtration. J. Membr. Sci. 2023, 686, 121977. [Google Scholar] [CrossRef]
- Seo, M.; Kim, W.; Lee, H.; Kim, S.J. Non-negligible effects of reinforcing structures inside ion exchange membrane on sta-bilization of electroconvective vortices. Desalination 2022, 538, 115902. [Google Scholar] [CrossRef]
- Ugrozov, V.V.; Filippov, A.N. Resistance of an Ion-Exchange Membrane with a Surface-Modified Charged Layer. Colloid J. 2022, 84, 761–768. [Google Scholar] [CrossRef]
- Kovalenko, A.V.; Yzdenova, A.M.; Sukhinov, A.I.; Chubyr, N.O.; Urtenov, M.K. Simulation of galvanic dynamic mode in membrane hydrocleaning systems taking into account space charge. AIP Conf. Proc. 2019, 2188, 050021. [Google Scholar] [CrossRef]
- Urtenov, M.K.; Kovalenko, A.V.; Sukhinov, A.I.; Chubyr, N.O.; Gudza, V.A. Model and numerical experiment for calculating the theoretical current-voltage characteristic in electro-membrane systems. Iop Conf. Ser. Mater. Sci. Eng. 2019, 680, 012030. [Google Scholar] [CrossRef]
- Filippov, A.N.; Akberova, E.M.; Vasil’eva, V.I. Study of the Thermochemical Effect on the Transport and Structural Charac-teristics of Heterogeneous Ion-Exchange Membranes by Combining the Cell Model and the Fine-Porous Membrane Model. Polymers 2023, 15, 3390. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.; Fan, S.; Wang, Y.; Chen, J.; Chen, Y.; Bai, K.; Deng, L.; Xiao, Z. Catalytic nanofiber composite membrane by combining electrospinning precursor seeding and flowing synthesis for immobilizing ZIF-8 derived Ag nanoparticles. J. Membr. Sci. 2022, 643, 120045. [Google Scholar] [CrossRef]
- Stockmeier, F.; Stüwe, L.; Kneppeck, C.; Musholt, S.; Albert, K.; Linkhorst, J.; Wessling, M. On the interaction of electroconvection at a membrane interface with the bulk flow in a spacer-filled feed channel. J. Membr. Sci. 2023, 678, 121589. [Google Scholar] [CrossRef]
- Zaltzman, B.; Rubinstein, I. Electro-osmotic slip and electroconvection at heterogeneous ion-exchange membranes. J. Fluid Mech. 2007, 579, 173–226. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. How the fine structure of the electric double layer and the flow affect morphological instability in electrodeposition. Phys. Rev. Fluids 2023, 8, 093701. [Google Scholar] [CrossRef]
- Choi, J.; Cho, M.; Shin, J.; Kwak, R.; Kim, B. Electroconvective instability at the surface of one-dimensionally patterned ion exchange membranes. J. Membr. Sci. 2024, 691, 122256. [Google Scholar] [CrossRef]
- Gupta, A.; Zuk, P.J.; Stone, H.A. Charging Dynamics of Overlapping Double Layers in a Cylindrical Nanopore. Phys. Rev. Lett. 2020, 125, 076001. [Google Scholar] [CrossRef] [PubMed]
- Kozmai, A.; Mareev, S.A.; Butylskii, D.; Ruleva, V.; Pismenskaya, N.; Nikonenko, V. Low-frequency impedance of ion-exchange membrane with electrically heterogeneous surface. Electrochim. Acta 2023, 451, 142285. [Google Scholar] [CrossRef]
- Vasilieva, V.I.; Meshcheryakova, E.E.; Falina, I.V.; Kononenko, N.A.; Brovkina, M.A.; Akberova, E.M. Effect of Heterogeneous Ion-Exchange Membranes Composition on Their Structure and Transport Properties. Membr. Membr. Technol. 2023, 5, 139–147. [Google Scholar] [CrossRef]
- Kazakovtseva, E.V.; Kovalenko, A.V.; Pismenskiy, A.V.; Urtenov, M.A.H. Hybrid numerical-analytical method for solving the problems of salt ion transport in membrane systems with axial symmetry. J. Samara State Tech. Univ. Ser. Phys. Math. Sci. 2024, 28, 130–151. [Google Scholar] [CrossRef]
- Grafov, B.M.; Chernenko, A.A. Passage of direct current through a binary electrolyte solution. J. Phys. Chem. 1963, 37, 664. [Google Scholar]
- Newman, J. Electrochemical Systems. World 1977. 463p. Available online: https://books.google.com.hk/books/about/Electrochemical_Systems.html?id=vArZu0HM-xYC&redir_esc=y (accessed on 10 June 2025).
- Doolan, E.; Miller, J.; Shields, W. Uniform Numerical Methods for Solving Boundary Layer Problems; Mir: Moscow, Russia, 1983; p. 199. [Google Scholar]



| 1 | 25 | 100 | |
|---|---|---|---|
| Pe |
| ω0 | π/2 | π | 2π | 10π |
|---|---|---|---|---|
| Re | 1.56 | 3.12 | 6.25 | 31.22 |
| C0, mol/m3 | 0.1 | 1 | 10 | 100 | |
|---|---|---|---|---|---|
| ω0, rad/s | |||||
| 1 | |||||
| 105 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakovtseva, E.; Kirillova, E.; Kovalenko, A.; Urtenov, M. Mathematical Modeling of the Influence of Electrical Heterogeneity on the Processes of Salt Ion Transfer in Membrane Systems with Axial Symmetry Taking into Account Electroconvection. Inventions 2025, 10, 50. https://doi.org/10.3390/inventions10040050
Kazakovtseva E, Kirillova E, Kovalenko A, Urtenov M. Mathematical Modeling of the Influence of Electrical Heterogeneity on the Processes of Salt Ion Transfer in Membrane Systems with Axial Symmetry Taking into Account Electroconvection. Inventions. 2025; 10(4):50. https://doi.org/10.3390/inventions10040050
Chicago/Turabian StyleKazakovtseva, Ekaterina, Evgenia Kirillova, Anna Kovalenko, and Mahamet Urtenov. 2025. "Mathematical Modeling of the Influence of Electrical Heterogeneity on the Processes of Salt Ion Transfer in Membrane Systems with Axial Symmetry Taking into Account Electroconvection" Inventions 10, no. 4: 50. https://doi.org/10.3390/inventions10040050
APA StyleKazakovtseva, E., Kirillova, E., Kovalenko, A., & Urtenov, M. (2025). Mathematical Modeling of the Influence of Electrical Heterogeneity on the Processes of Salt Ion Transfer in Membrane Systems with Axial Symmetry Taking into Account Electroconvection. Inventions, 10(4), 50. https://doi.org/10.3390/inventions10040050








