Linking the Physicochemical Properties of Calcined Titania Nanoparticles with Their Biocidal Activity
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Physicochemical Characterization
2.2. Storage and Light Exposure of Modified nTiO2 Samples
2.3. Microorganisms and Viability Assay
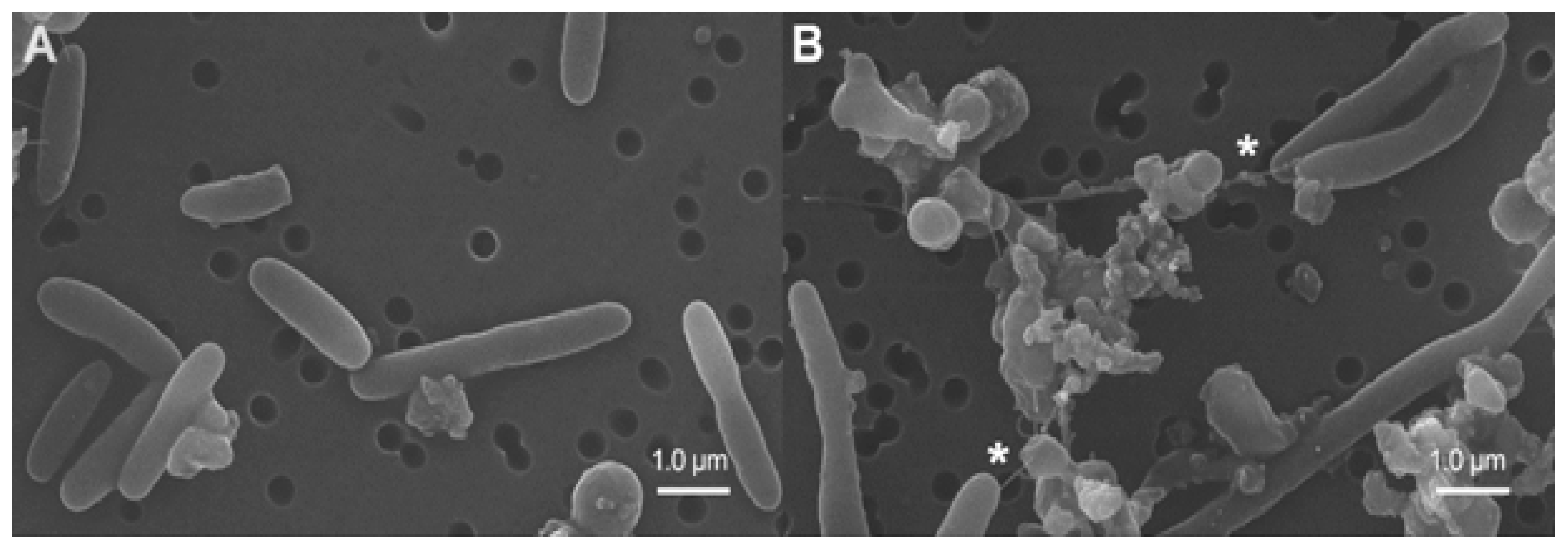
2.4. Transmission and Scanning Electron Microscopy
3. Results
3.1. Physicochemical Properties
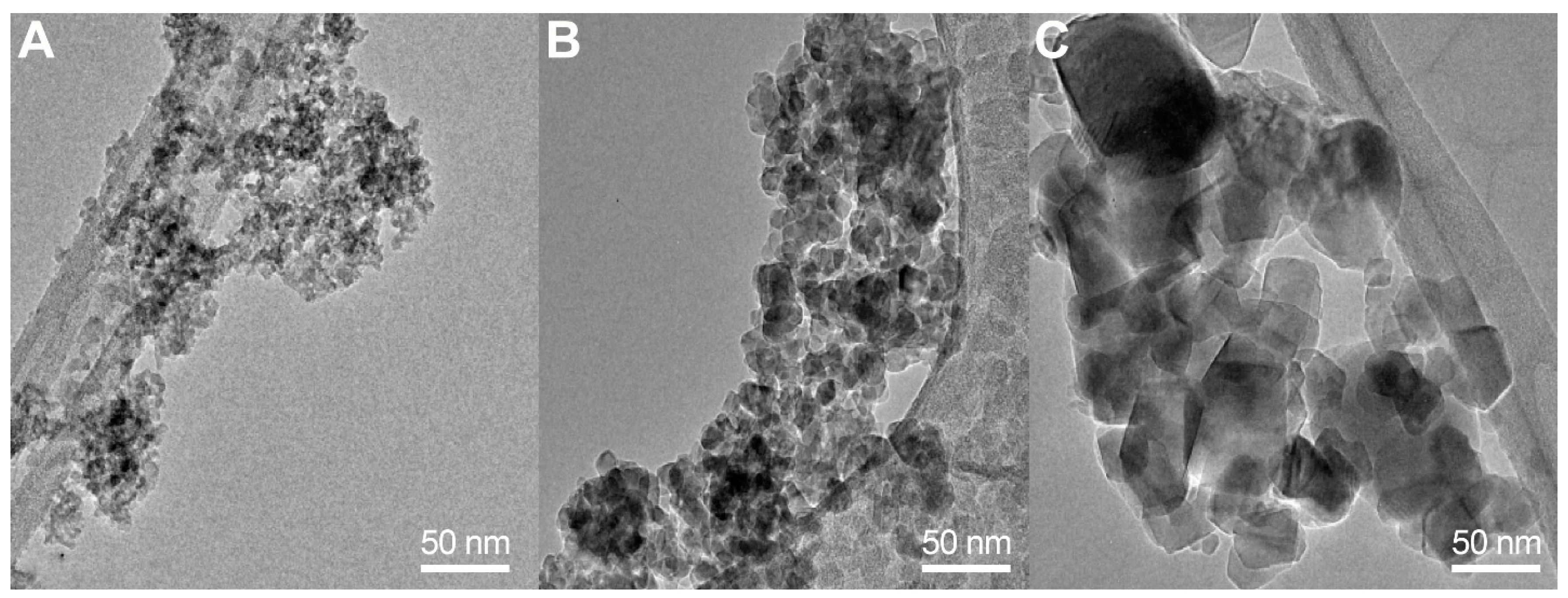
3.2. Transmission Electron Microscopy
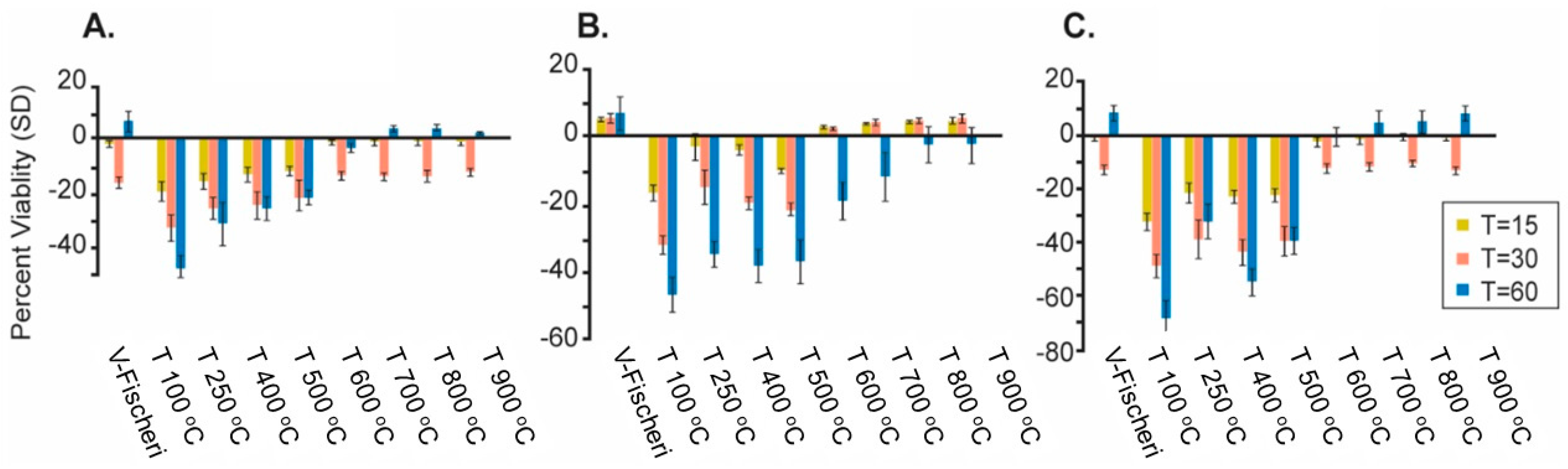
3.3. Viability Studies
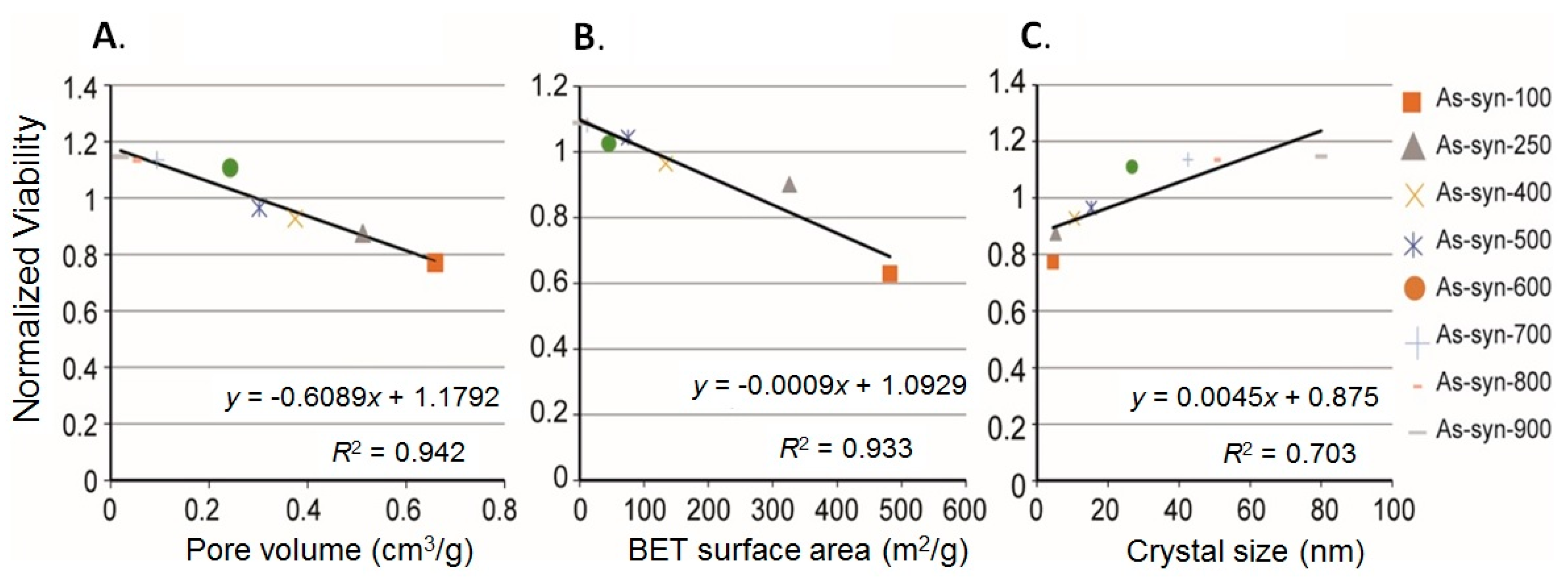
3.4. Linear Correlation
4. Discussion
4.1. Physicochemical Effects of Calcination Temperatures
4.2. Biocidal Effects
4.3. Physicochemical Properties Influence Toxicity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
References
- Jacobs, J.F.; van de Poel, I.; Osseweijer, P. Sunscreens with titanium dioxide (TiO2) nano-particles: A societal experiment. Nanoethics 2010, 4, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Kongsong, P.; Sikong, L.; Niyomwas, S.; Rachpech, V. Photocatalytic antibacterial performance of glass fibers thin film coated with n-doped SnO2/TiO2. Sci. World J. 2014, 2014, 869706. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Samarghandi, M.; Samadi, M.; Nazemi, F. Photocatalytic disinfection of coliform bacteria using UV/TiO2. J. Res. Health Sci. 2009, 9, 1–6. [Google Scholar] [PubMed]
- Schilling, K.; Bradford, B.; Castelli, D.; Dufour, E.; Nash, J.F.; Pape, W.; Schulte, S.; Tooley, I.; van den Bosch, J.; Schellauf, F. Human safety review of “nano” titanium dioxide and zinc oxide. Photochem. Photobiol. Sci. 2010, 9, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Sethi, D.; Pal, A.; Sakthivel, R.; Pandey, S.; Dash, T.; Das, T.; Kumar, R. Water disinfection through photoactive modified Titania. J. Photochem. Photobiol. B Biol. 2014, 130, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Tsuang, Y.H.; Sun, J.S.; Huang, Y.C.; Lu, C.H.; Chang, W.H.S.; Wang, C.C. Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif. Organs 2008, 32, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Tooley, I.R. Photoprotection in the Era of Nanotechnology, Seminars in Cutaneous Medicine and Surgery; Frontline Medical Communications: Parsippany, NJ, USA, 2011; pp. 210–213. [Google Scholar]
- Li, F.; Liang, Z.; Zheng, X.; Zhao, W.; Wu, M.; Wang, Z. Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production. Aquat. Toxicol. 2015, 158, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Antimicrobial effects of TiO2 and Ag2O nanoparticles against drug-resistant bacteria and leishmania parasites. Future Microbiol. 2011, 6, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Maness, P.-C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [PubMed]
- Angelstorf, J.S.; Ahlf, W.; von der Kammer, F.; Heise, S. Impact of particle size and light exposure on the effects of TiO2 nanoparticles on caenorhabditis elegans. Environ. Toxicol. Chem. 2014, 33, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Blaise, C.; Gagné, F.; Férard, J.; Eullaffroy, P. Ecotoxicity of selected nano-materials to aquatic organisms. Environ. Toxicol. 2008, 23, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Clément, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants–effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Bradley, T.; Moore, J.T.; Kuykindall, T.; Minella, L. Acute and chronic toxicity of nano-scale TiO2 particles to freshwater fish, cladocerans, and green algae, and effects of organic and inorganic substrate on TiO2 toxicity. Nanotoxicology 2009, 3, 91–97. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Cui, Y.; Li, D.; Chen, J.; Yang, L. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol. Biochem. 2010, 42, 586–591. [Google Scholar] [CrossRef]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Minetto, D.; Libralato, G.; Ghirardini, A.V. Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: An overview. Environ. Int. 2014, 66, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K. Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—A review. J. Environ. Sci. Health Part A 2009, 44, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Wang, L.; Su, M.; Zhao, X.; Hu, R.; Yu, X.; Hong, J.; Liu, D.; Xu, B.; Zhu, Y. Mechanism of TiO2 nanoparticle-induced neurotoxicity in zebrafish (Danio rerio). Environ. Toxicol. 2014, 31, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Van Gestel, C.A.; Lofts, S.; Svendsen, C.; Soares, A.M.; Loureiro, S. Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012, 31, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Burda, C. Photoelectron spectroscopic investigation of nitrogen-doped Titania nanoparticles. J. Phys. Chem. B 2004, 108, 15446–15449. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Chen, Z.; Cheng, H.-M.; Lu, G.Q.M. The role of crystal phase in determining photocatalytic activity of nitrogen doped TiO2. J. Colloid Interface Sci. 2009, 329, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Baruwati, B.; Jayalakshmi, M.; Rao, M.M.; Manorama, S.V. S-, N- and C-doped titanium dioxide nanoparticles: Synthesis, characterization and redox charge transfer study. J. Solid State Chem. 2005, 178, 3352–3358. [Google Scholar] [CrossRef]
- Virkutyte, J.; Baruwati, B.; Varma, R.S. Visible light induced photobleaching of methylene blue over melamine-doped TiO2 nanocatalyst. Nanoscale 2010, 2, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.K. Vibrio fischeri metabolism: Symbiosis and beyond. Adv. Microb. Physiol. 2012, 61, 37. [Google Scholar] [PubMed]
- Lopez-Roldan, R.; Kazlauskaite, L.; Ribo, J.; Riva, M.C.; González, S.; Cortina, J.L. Evaluation of an automated luminescent bacteria assay for in situ aquatic toxicity determination. Sci. Total Environ. 2012, 440, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Menz, J.; Schneider, M.; Kümmerer, K. Toxicity testing with luminescent bacteria-characterization of an automated method for the combined assessment of acute and chronic effects. Chemosphere 2013, 93, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, M.; Kasemets, K.; Heinlaan, M.; Kurvet, I.; Kahru, A. High throughput kinetic Vibrio fischeri bioluminescence inhibition assay for study of toxic effects of nanoparticles. Toxicol. In Vitro 2008, 22, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Venkataraman, C.; Mukherji, S. A review on advantages of implementing luminescence inhibition test (Vibrio fischeri) for acute toxicity prediction of chemicals. Environ. Int. 2006, 32, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.C.; Costa, S.P.; Lima, J.L.; Saraiva, M.L.M. Automated high-throughput Vibrio fischeri assay for (eco) toxicity screening: Application to ionic liquids. Ecotoxicol. Environ. Saf. 2012, 80, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Stolper, P.; Fabel, S.; Weller, M.G.; Knopp, D.; Niessner, R. Whole-cell luminescence-based flow-through biodetector for toxicity testing. Anal. Bioanal. Chem. 2008, 390, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Kahru, A.; Kasemets, K.; Kurvet, I.; Waterlot, C.; Sepp, K.; Dubourguier, H.-C.; Douay, F. Rapid screening for soil ecotoxicity with a battery of luminescent bacteria tests. Altern. Lab. Anim.: ATLA 2007, 35, 101–110. [Google Scholar] [PubMed]
- Paulovits, G.; Kováts, N.; Ács, A.; Ferincz, Á.; Kovacs, A.; Kakasi, B.; Nagy, S.; Kiss, G. Ecotoxicological characterisation of sedimentation in the kis-balaton water protection system. Acta Biol. Hung. 2012, 63, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Villa, S.; Migliorati, S.; Monti, G.S.; Vighi, M. Toxicity on the luminescent bacterium Vibrio fischeri (Beijerinck). II: Response to complex mixtures of heterogeneous chemicals at low levels of individual components. Ecotoxicol. Environ. Saf. 2012, 86, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Binaeian, E.; Attar, H.; Safekordi, A.A.; Saber, R.; Chaichi, M.J. Study on toxicity of seven manufactured nano particles and two phenolic compounds to bacteria Vibrio fischeri using homemade luminometer. Asian J. Chem. 2012, 24, 5211. [Google Scholar]
- Garcia, A.; Recillas, S.; Sánchez, A.; Font, X. The luminescent bacteria test to determine the acute toxicity of nanoparticle suspensions. Nanotoxic. Methods Protoc. 2012, 255–259. [Google Scholar]
- Baun, A.; Hartmann, N.B.; Grieger, K.; Kusk, K.O. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: A brief review and recommendations for future toxicity testing. Ecotoxicology 2008, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, Y.J.; Varma, R.S.; Dionysiou, D.D. Thermally stable nanocrystalline TiO2 photocatalysts synthesized via sol-gel methods modified with ionic liquid and surfactant molecules. Chem. Mater. 2006, 18, 5377–5384. [Google Scholar] [CrossRef]
- Diehl, A.; Levin, Y. Smoluchowski equation and the colloidal charge reversal. J. Chem. Phys. 2006, 125, 54902. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Chuang, C.-H.; Hill, R.J.; Burda, C. Nanoparticle ζ-potentials. Acc. Chem. Res. 2011, 45, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Safety Data Sheet. Available online: http://www.carolina.com/pdf/msds/photobrothdeghs.pdf (accessed on 19 December 2016).
- Adam, F.; Appaturi, J.N.; Thankappan, R.; Nawi, M.A.M. Silica-tin nanotubes prepared from rice husk ash by sol–gel method: Characterization and its photocatalytic activity. Appl. Surf. Sci. 2010, 257, 811–816. [Google Scholar] [CrossRef]
- Gaber, A.; Abdel-Rahim, M.; Abdel-Latief, A.; Abdel-Salam, M.N. Influence of calcination temperature on the structure and porosity of nanocrystalline SnO2 synthesized by a conventional precipitation method. Int. J. Electrochem. Sci. 2014, 9, 81–95. [Google Scholar]
- Barmo, C.; Ciacci, C.; Canonico, B.; Fabbri, R.; Cortese, K.; Balbi, T.; Marcomini, A.; Pojana, G.; Gallo, G.; Canesi, L. In vivo effects of N-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2013, 132, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Buonocore, F.; Frenzilli, G.; Corsolini, S.; Brunelli, A.; Guidi, P.; Kocan, A.; Mariottini, M.; Mottola, F.; Nigro, M. Influence of titanium dioxide nanoparticles on 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin bioconcentration and toxicity in the marine fish european sea bass (Dicentrarchus labrax). Environ. Pollut. 2015, 196, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Dineshram, R.; Subasri, R.; Somaraju, K.; Jayaraj, K.; Vedaprakash, L.; Ratnam, K.; Joshi, S.; Venkatesan, R. Biofouling studies on nanoparticle-based metal oxide coatings on glass coupons exposed to marine environment. Colloids Surf. B Biointerfaces 2009, 74, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.; Lewis, C.; Dolciotti, I.; Johnston, B.D.; Moger, J.; Regoli, F. Sublethal toxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete. Environ. Pollut. 2010, 158, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of TiO2 nanoparticles with the marine microalga Nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, J.; Cai, Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar. Pollut. Bull. 2011, 63, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Pelaez, M.; Likodimos, V.; Kontos, A.G.; Falaras, P.; O’Shea, K.; Dionysiou, D.D. Innovative visible light-activated sulfur doped TiO2 films for water treatment. Appl. Catal. B Environ. 2011, 107, 77–87. [Google Scholar] [CrossRef]
- Puddu, V.; Choi, H.; Dionysiou, D.D.; Puma, G.L. TiO2 photocatalyst for indoor air remediation: Influence of crystallinity, crystal phase, and UV radiation intensity on trichloroethylene degradation. Appl. Catal. B Environ. 2010, 94, 211–218. [Google Scholar] [CrossRef]
- Liou, J.-W.; Chang, H.-H. Bactericidal effects and mechanisms of visible light-responsive titanium dioxide photocatalysts on pathogenic bacteria. Arch. Immunol. Ther. Exp. 2012, 60, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Duan, W.; Wang, Q.; Li, X. The damage of outer membrane of Escherichia coli in the presence of TiO2 combined with UV light. Colloids Surf. B Biointerfaces 2010, 78, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Swetha, S.; Kumari Singh, M.; Minchitha, K.; Geetha Balakrishna, R. Elucidation of cell killing mechanism by comparative analysis of photoreactions on different types of bacteria. Photochem. Photobiol. 2012, 88, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cui, D.; Liu, M.; Gong, H.; Huang, Y.; Han, F. Corn porous starch: Preparation, characterization and adsorption property. Int. J. Biol. Macromol. 2012, 50, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, R.; Strus, M.; Buchalska, M.; Heczko, P.B.; Macyk, W. Visible light induced photocatalytic inactivation of bacteria by modified titanium dioxide films on organic polymers. Photochem. Photobiol. Sci. 2015, 14, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Tallósy, S.P.; Janovák, L.; Ménesi, J.; Nagy, E.; Juhász, Á.; Balázs, L.; Deme, I.; Buzás, N.; Dékány, I. Investigation of the antibacterial effects of silver-modified TiO2 and ZnO plasmonic photocatalysts embedded in polymer thin films. Environ. Sci. Pollut. Res. 2014, 21, 11155–11167. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.-S.; Chu, W.-C.; Sun, D.-S.; Huang, H.-S.; Chen, J.-H.; Tsai, P.-J.; Lin, N.-T.; Yu, M.-S.; Hsu, S.-F.; Wang, S.-L. Visible-light-induced bactericidal activity of a nitrogen-doped titanium photocatalyst against human pathogens. Appl. Environ. Microbiol. 2006, 72, 6111–6116. [Google Scholar] [CrossRef] [PubMed]
- Górska, P.; Zaleska, A.; Kowalska, E.; Klimczuk, T.; Sobczak, J.W.; Skwarek, E.; Janusz, W.; Hupka, J. TiO2 photoactivity in vis and UV light: The influence of calcination temperature and surface properties. Appl. Catal. B Environ. 2008, 84, 440–447. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. Identification of the mechanisms that drive the toxicity of TiO2 particulates: The contribution of physicochemical characteristics. Part. Fibre Toxicol. 2009, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Semmler-Behnke, M.; Seitz, J.; Scymczak, W.; Wenk, A.; Mayer, P.; Takenaka, S.; Oberdörster, G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 2009, 21, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Rabolli, V.; Thomassen, L.C.; Princen, C.; Napierska, D.; Gonzalez, L.; Kirsch-Volders, M.; Hoet, P.H.; Huaux, F.; Kirschhock, C.E.; Martens, J.A. Influence of size, surface area and microporosity on the in vitro cytotoxic activity of amorphous silica nanoparticles in different cell types. Nanotoxicology 2010, 4, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Wiesenthal, A.; Hunter, L.; Wang, S.; Wickliffe, J.; Wilkerson, M. Nanoparticles: Small and mighty. Int. J. Dermatol. 2011, 50, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Grassian, V.H.; O’Shaughnessy, P.T.; Adamcakova-Dodd, A.; Pettibone, J.M.; Thorne, P.S. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ. Health Perspect. 2007, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, M.; Sunahara, G.I. In vitro cytotoxicity and genotoxicity studies of titanium dioxide (TiO2) nanoparticles in Chinese hamster lung fibroblast cells. Toxicol. In Vitro 2013, 27, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Fontana, L.; Bergamaschi, A. Toxicological effects of titanium dioxide nanoparticles: A review of in vitro mammalian studies. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 481–508. [Google Scholar] [PubMed]
- Musee, N.; Thwala, M.; Nota, N. The antibacterial effects of engineered nanomaterials: Implications for wastewater treatment plants. J. Environ. Monit. 2011, 13, 1164–1183. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Charbonneau, M.; Cloutier, Y.; Tardif, R.; Truchon, G. Rat pulmonary responses to inhaled nano-TiO2: Effect of primary particle size and agglomeration state. Part. Fibre Toxicol. 2013, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Oberdorster, G. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Inhal. Toxicol. 1995, 8, 73–89. [Google Scholar] [CrossRef]
- Rabolli, V.; Thomassen, L.C.; Uwambayinema, F.; Martens, J.A.; Lison, D. The cytotoxic activity of amorphous silica nanoparticles is mainly influenced by surface area and not by aggregation. Toxicol. Lett. 2011, 206, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Gurr, J.-R.; Wang, A.S.; Chen, C.-H.; Jan, K.-Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Semmler, M.; Möller, W. Dosimetry and toxicology of ultrafine particles. J. Aerosol Med. 2004, 17, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Nel, A. Air pollution-related illness: Effects of particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Finkelstein, J.; Johnston, C.; Gelein, R.; Cox, C.; Baggs, R.; Elder, A. Ultrafine particles as inducers of acute lung injury: Mechanisms and correlation with age and disease. Health Effects Inst. 2000, 96, 1–88. [Google Scholar]
- Jin, C.; Tang, Y.; Yang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Huang, Y.Y.; Yang, Y.J. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol. Trace Elem. Res. 2011, 141, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; TeeSy, C.; Franzi, L.; Weir, A.; Westerhoff, P.; Evans, J.E.; Pinkerton, K.E. Biological response to nano-scale titanium dioxide (TiO2): Role of particle dose, shape, and retention. J. Toxicol. Environ. Health Part A 2013, 76, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, K.; Fenoglio, I.; Carella, E.; Kolling, J.; Albrecht, C.; Boots, A.W.; Förster, I.; Schins, R.P. Distinctive toxicity of TiO2 rutile/anatase mixed phase nanoparticles on Caco-2 cells. Chem. Res. Toxicol. 2012, 25, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Jones, M.A.; Lin, Y.-S.; Haynes, C.L. Functional assessment of metal oxide nanoparticle toxicity in immune cells. ACS Nano 2010, 4, 3363–3373. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Metzler, D.; Cha, D.K.; Huang, C. The short-term toxic effects of TiO2 nanoparticles toward bacteria through viability, cellular respiration, and lipid peroxidation. Environ. Sci. Pollut. Res. 2015, 22, 17917–17924. [Google Scholar] [CrossRef] [PubMed]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; O’Shea, K.E.; Dionysiou, D.D.; Hiskia, A. Assessment of the roles of reactive oxygen species in the UV and visible light photocatalytic degradation of cyanotoxins and water taste and odor compounds using C-TiO2. Water Res. 2016, 90, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Likodimos, V.; Khan, J.A.; Nadagouda, M.N.; Andersen, J.; Falaras, P.; Rosales-Lombardi, P.; Dionysiou, D.D. UV-visible light-activated Ag-decorated, monodisperse TiO2 aggregates for treatment of the pharmaceutical oxytetracycline. Environ. Sci. Pollut. Res. 2014, 21, 11781–11793. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.-J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of nano titanium dioxides in HaCaT keratinocytes-generation of reactive oxygen species and cell damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Pathakoti, K.; Morrow, S.; Han, C.; Pelaez, M.; He, X.; Dionysiou, D.D.; Hwang, H.-M. Photoinactivation of Escherichia coli by sulfur-doped and nitrogen–fluorine-codoped TiO2 nanoparticles under solar simulated light and visible light irradiation. Environ. Sci. Technol. 2013, 47, 9988–9996. [Google Scholar] [CrossRef] [PubMed]

| TiO2 Sample | BET Area (m2/g) | Pore Volume (cm3/g) | Zeta Potential (mV) | Crystal Size (nm) a | Crystal Phase b |
|---|---|---|---|---|---|
| As-syn-100 | 482 | 0.659 | −28.7 | 4.5 | Amp |
| As-syn-250 | 326 | 0.512 | −27.8 | 5.2 | Amp |
| As-syn-400 | 134 | 0.375 | −22.8 | 10.5 | A |
| As-syn-500 | 75.4 | 0.302 | −27.9 | 15.3 | A |
| As-syn-600 | 45.4 | 0.243 | −25.5 | 26.7 | A |
| As-syn-700 | 11.8 | 0.094 | −26.9 | 42.5 | A:R = 4:6 |
| As-syn-800 | 3.45 | 0.046 | −28.3 | 50.0 | R |
| As-syn-900 | 0.95 | 0.02 | −24.7 | 80.0 | R |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Pelaez, M.; Betancourt, D.; Choi, H.; Dionysiou, D.D.; Veronesi, B. Linking the Physicochemical Properties of Calcined Titania Nanoparticles with Their Biocidal Activity. Inventions 2016, 1, 26. https://doi.org/10.3390/inventions1040026
Han C, Pelaez M, Betancourt D, Choi H, Dionysiou DD, Veronesi B. Linking the Physicochemical Properties of Calcined Titania Nanoparticles with Their Biocidal Activity. Inventions. 2016; 1(4):26. https://doi.org/10.3390/inventions1040026
Chicago/Turabian StyleHan, Changseok, Miguel Pelaez, Doris Betancourt, Hyeok Choi, Dionysios D. Dionysiou, and Bellina Veronesi. 2016. "Linking the Physicochemical Properties of Calcined Titania Nanoparticles with Their Biocidal Activity" Inventions 1, no. 4: 26. https://doi.org/10.3390/inventions1040026
APA StyleHan, C., Pelaez, M., Betancourt, D., Choi, H., Dionysiou, D. D., & Veronesi, B. (2016). Linking the Physicochemical Properties of Calcined Titania Nanoparticles with Their Biocidal Activity. Inventions, 1(4), 26. https://doi.org/10.3390/inventions1040026




_Dionysiou.jpg)