Ectomycorrhizal Fungi and Mineral Interactions in the Rhizosphere of Scots and Red Pine Seedlings
Abstract
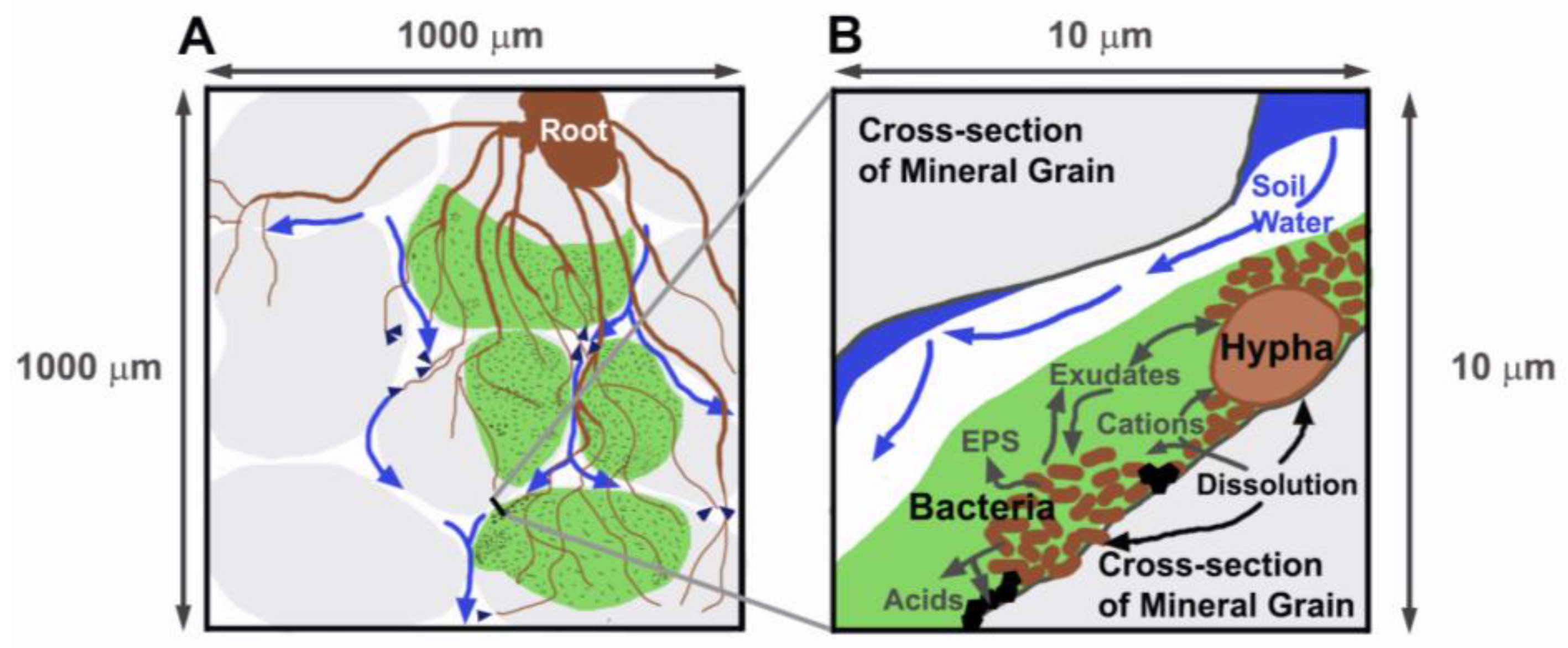
1. Introduction
2. Materials and Methods
2.1. Seed Germination and Inoculation
2.2. Experimental Setup
2.3. Chemical Analysis
2.4. Atomic Force Microscopy (AFM) Analysis
2.5. Scanning Electron Microscopy (SEM) Analysis
3. Results
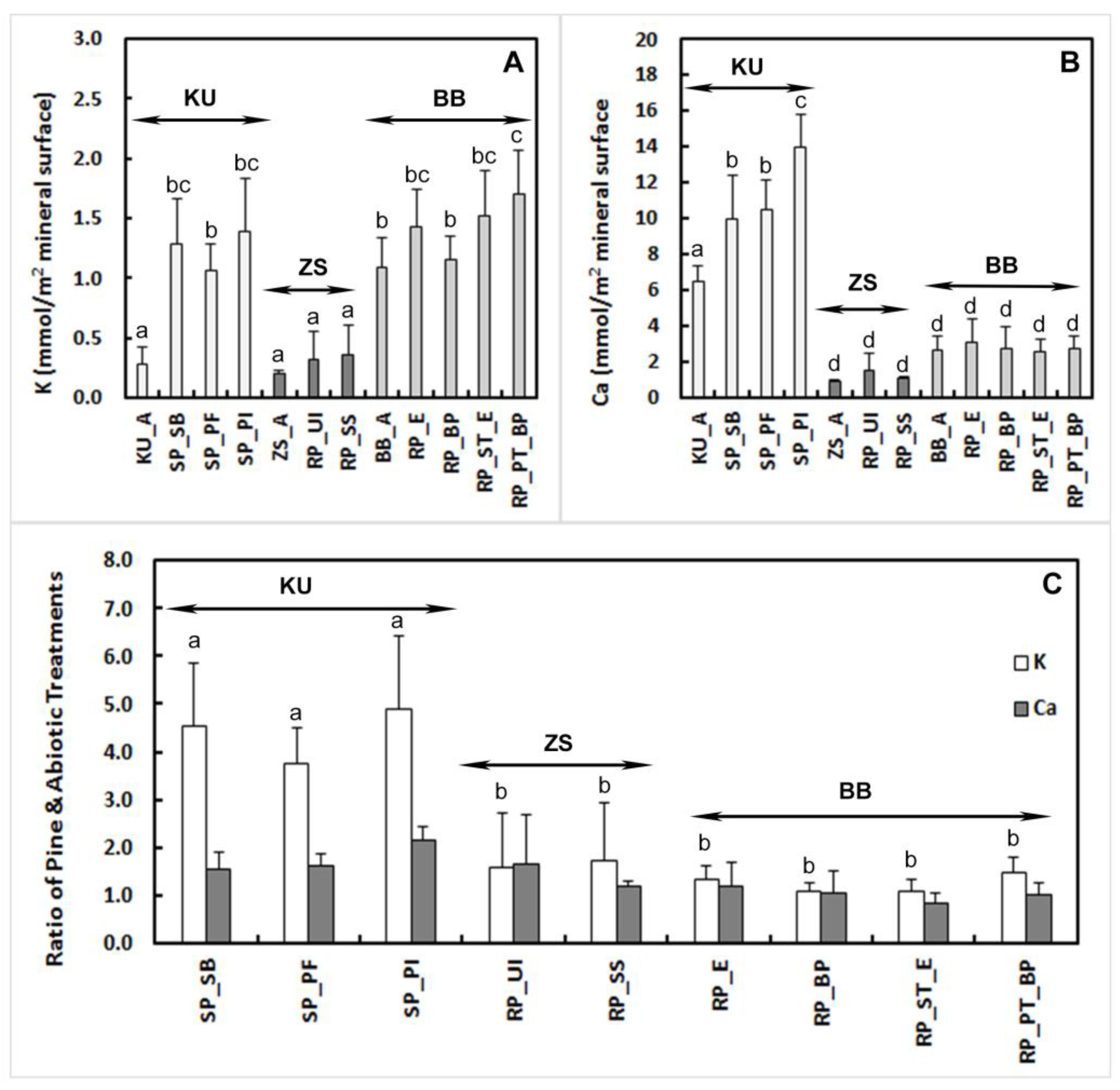
3.1. Bulk Composition
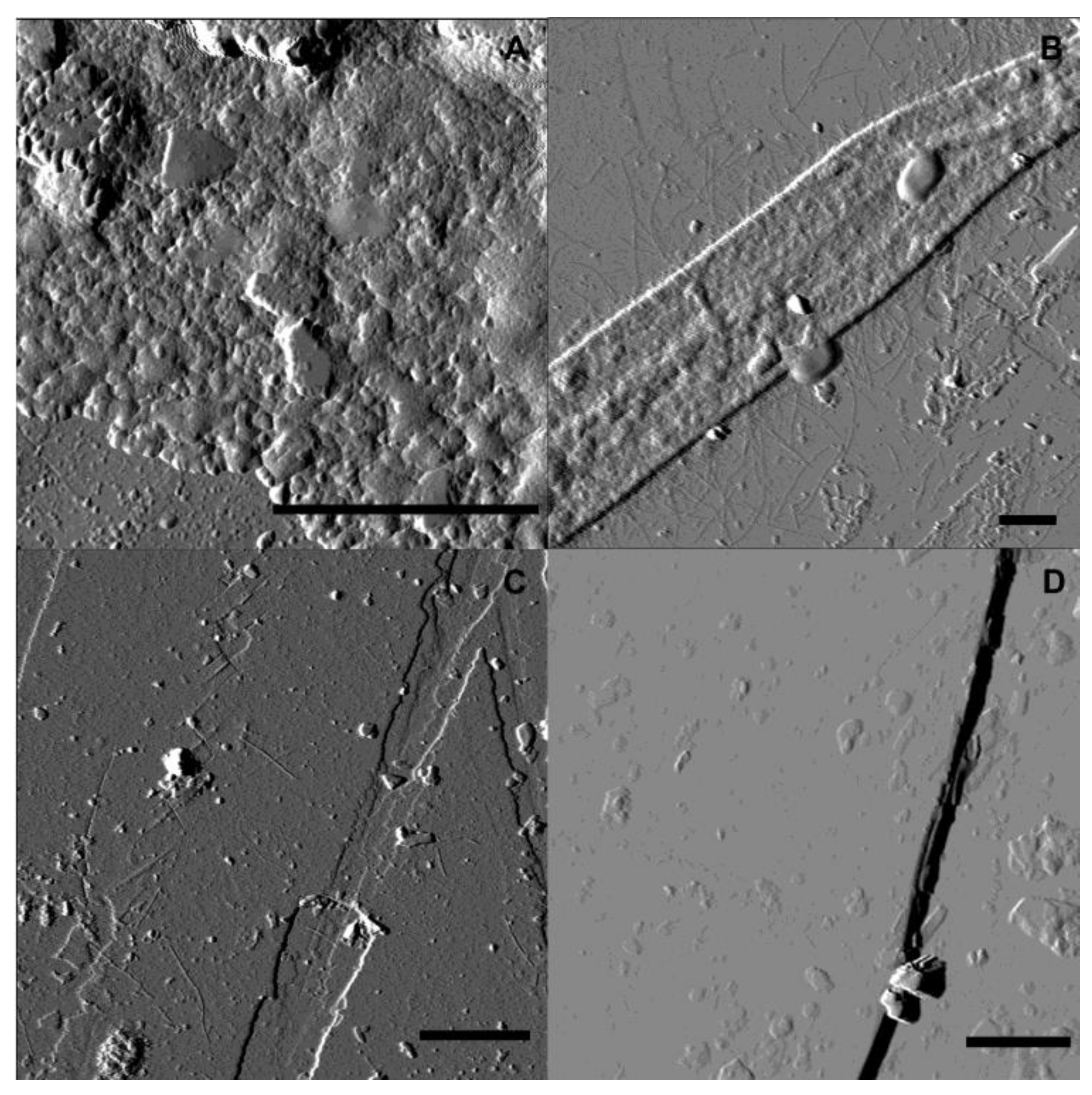
3.2. Mineral Surface Analysis
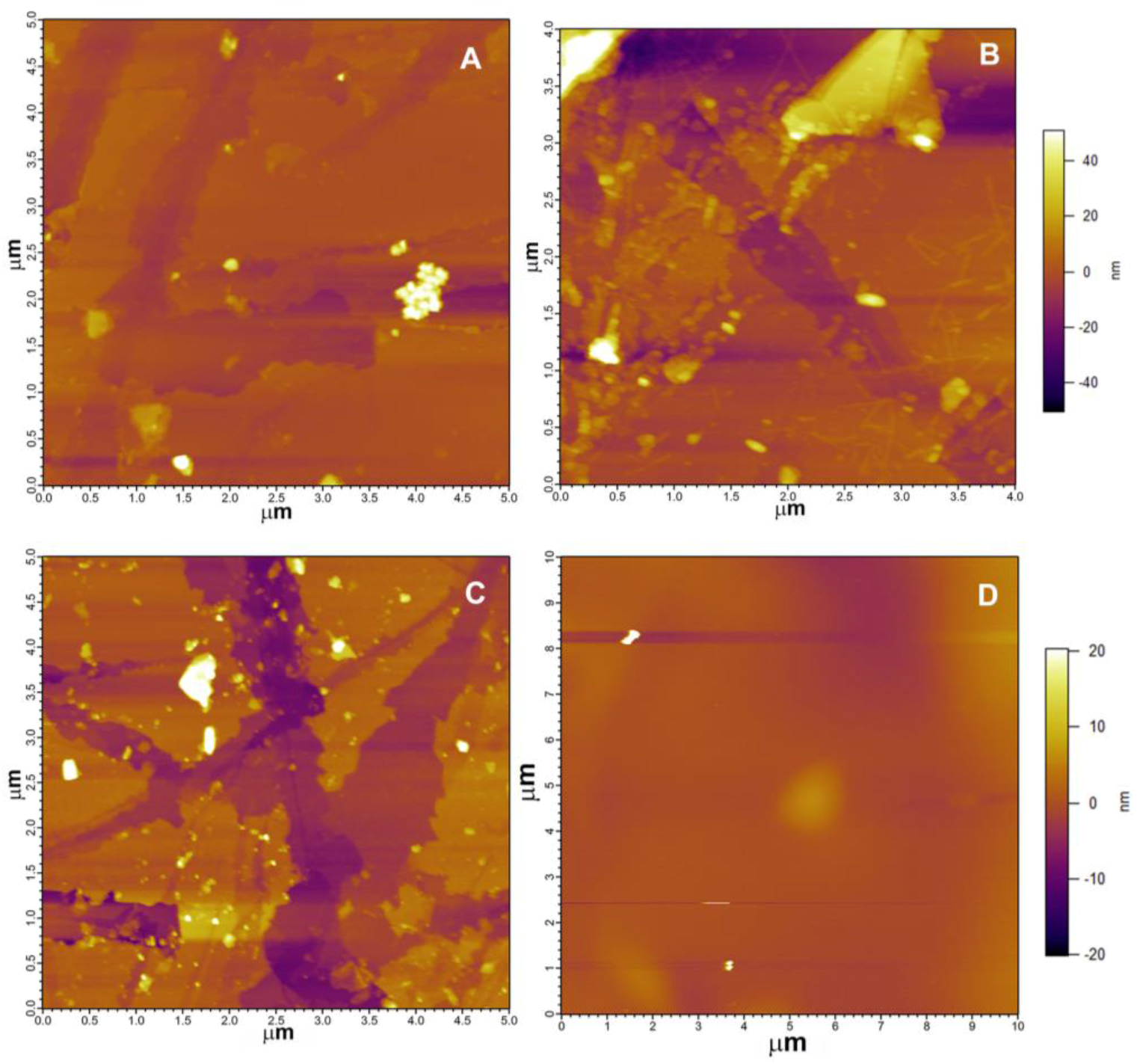
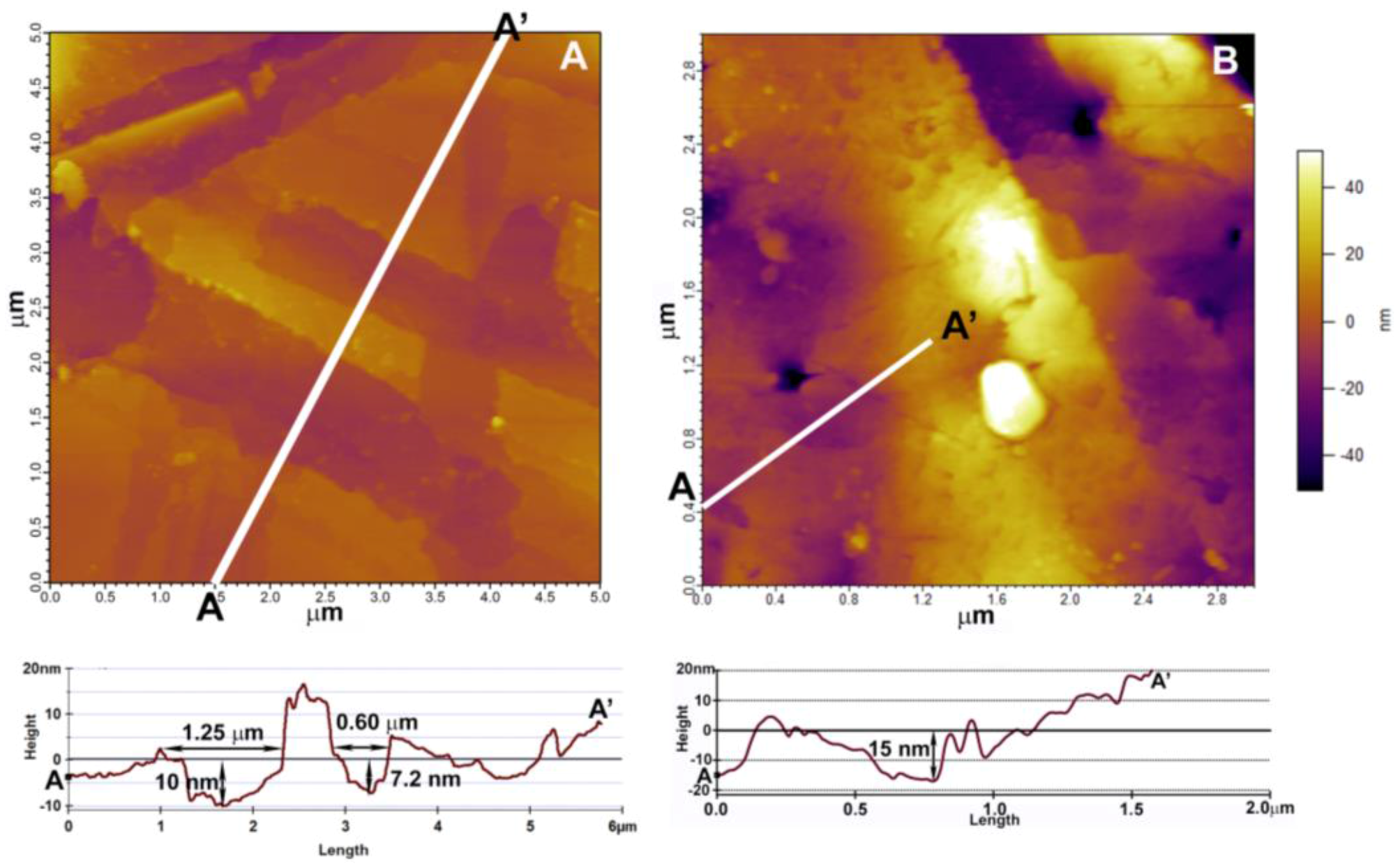
3.2.1. Atomic Force Microscopy (AFM)
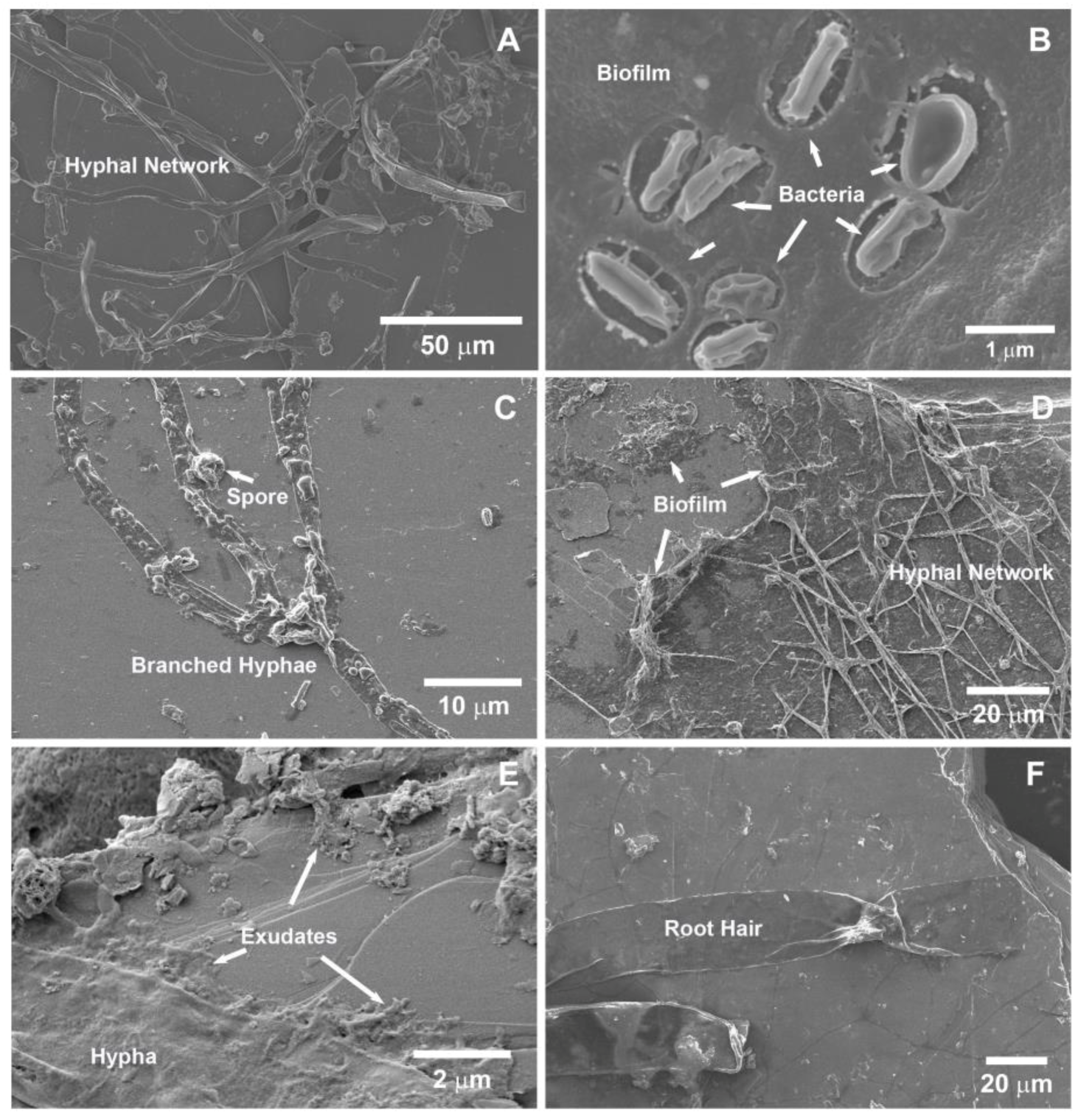
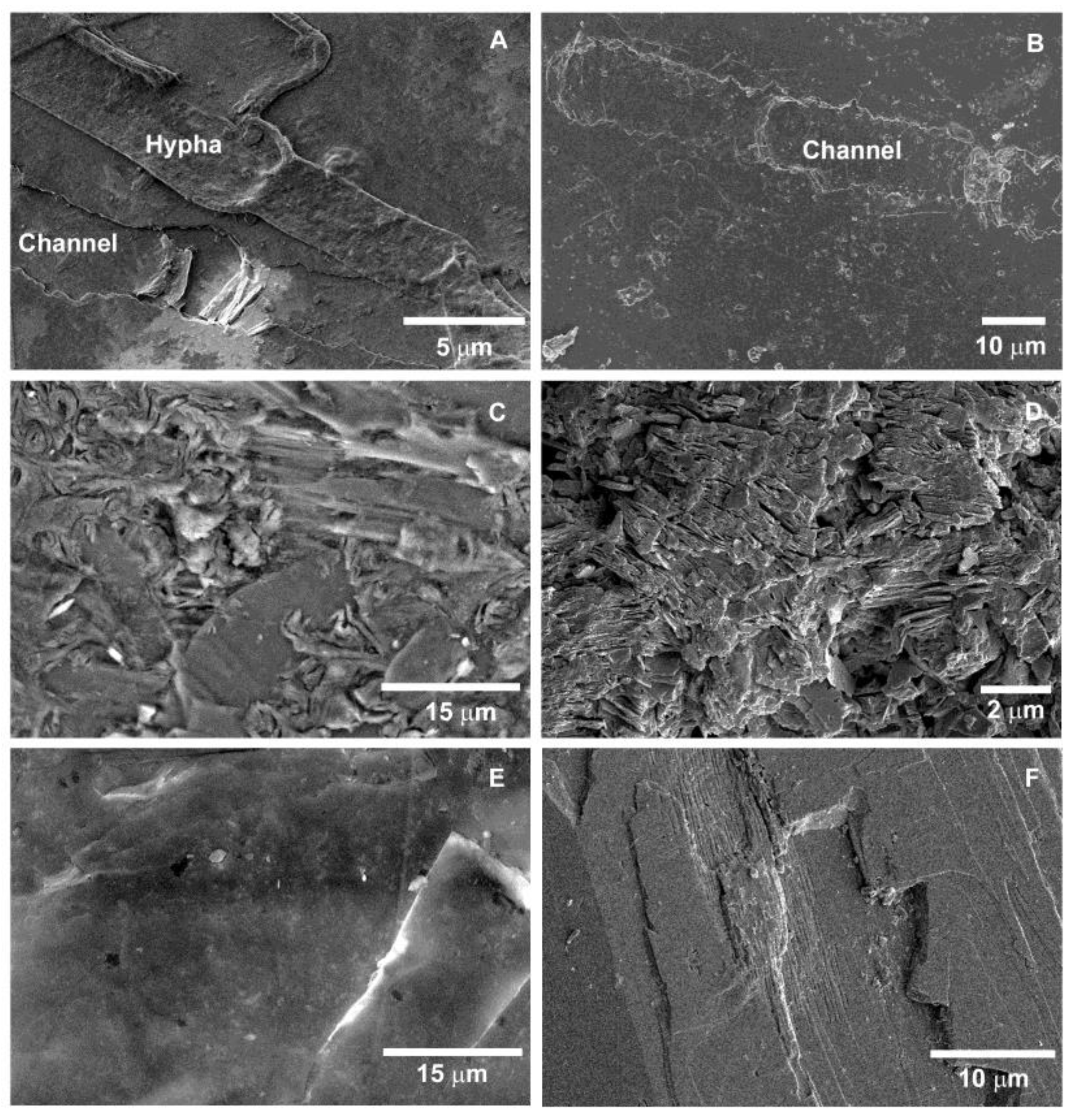
3.2.2. Scanning Electron Microscopy (SEM)
4. Discussion
4.1. Element Release Rates
4.2. Rhizospheric Mineral Alterations and Biofilm Formation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beerling, D.J.; Berner, R.A. Feedbacks and the coevolution of plants and atmospheric CO2. Proc. Natl. Acad. Sci. USA 2005, 102, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Brantley, S.L.; Goldhaber, M.B.; Ragnarsdottir, K.V. Crossing disciplines and scales to understand the critical zone. Elements 2007, 3, 307–314. [Google Scholar] [CrossRef]
- Banwart, S.A.; Berg, A.; Beerling, D.J. Process-based modeling of silicate mineral weathering responses to increasing atmospheric CO2 and climate change. Glob. Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- Landeweert, R.; Hoffland, E.; Finlay, R.D.; Kuyper, T.W.; van Breemen, N. Linking plants to rocks: Ectomycorrhizal fungi mobilize nutrients from minerals. Trends Ecol. Evol. 2001, 16, 248–254. [Google Scholar] [CrossRef]
- Bornyasz, M.A.; Graham, R.C.; Allen, M.F. Ectomycorrhizae in a soil-weathered granitic bedrock regolith: Linking matrix resources to plants. Geoderma 2005, 126, 141–160. [Google Scholar] [CrossRef]
- Van Hees, P.A.W.; Rosling, A.; Lundström, U.S.; Finlay, R.D. The biogeochemical impact of ectomycorrhizal conifers on major soil elements (Al, Fe, K and Si). Geoderma 2006, 136, 364–377. [Google Scholar] [CrossRef]
- Finlay, R.; Wallander, H.; Smits, M.; Holmstrom, S.; Van Hess, P.; Lian, B.; Rosling, A. The role of fungi in biogenic weathering in boreal forest soils. Fungal Biol. Rev. 2009, 23, 101–106. [Google Scholar] [CrossRef]
- Turpault, M.-P.; Nys, C.; Calvaruso, C. Rhizosphere impact on the dissolution of test minerals in a forest ecosystem. Geoderma 2009, 153, 147–154. [Google Scholar] [CrossRef]
- Koele, N.; Turpault, M.P.; Hildebrand, E.E.; Uroz, S.; Frey-Klett, P. Interactions between mycorrhizal fungi and mycorrhizosphere bacteria during mineral weathering: Budget analysis and bacterial quantification. Soil Biol. Biochem. 2009, 41, 1935–1942. [Google Scholar] [CrossRef]
- Koele, N.; Dickie, I.A.; Blum, J.D.; Gleason, J.D.; de Graaf, L. Ecological significance of mineral weathering in ectomycorrhizal and arbuscular mycorrhizal ecosystems from a field-based comparison. Soil Biol. Biochem. 2014, 69, 63–70. [Google Scholar] [CrossRef]
- Calvaruso, C.; Collignon, C.; Kies, A.; Turpault, M.P. Seasonal evolution of the rhizosphere effect on major and trace elements in soil solutions of Norway Spruce (Picea abies Karst) and beech (Fagus sylvatica) in an acidic forest soil. Open J. Soil Sci. 2014, 4, 323–336. [Google Scholar] [CrossRef]
- Meheruna, A.; Akagi, T. Role of fine roots in the plant-induced weathering of andesite for several plant species. Geochem. J. 2006, 40, 57–67. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Hinsinger, P. Plant-induced changes in soil processes and properties. In Soil Conditions and Plant Growth; Gregory, P.J., Nortcliff, S., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2013; pp. 323–365. ISBN 978-1-4051-9770-0. [Google Scholar] [CrossRef]
- Brantley, S.L.; Megonigal, J.P.; Scatena, F.N.; Balogh-Brunstad, Z.; Barnes, R.T.; Bruns, M.A.; Van Cappellen, P.; Dontsova, K.; Hartnett, H.E.; Hartshorn, A.S.; et al. Twelve testable hypotheses on the geobiology of weathering. Geobiology 2011, 9, 140–165. [Google Scholar] [CrossRef] [PubMed]
- McNear, D.H., Jr. The rhizosphere-roots, soil and everything in between. Nat. Educ. Knowl. 2013, 4, 1. [Google Scholar]
- National Research Council (NRC). Basic Research Opportunities in Earth Sciences; The National Academies Press: Washington, DC, USA, 2001; p. 154. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2011; ISBN 978-0-12-384905-2. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2010; ISBN 978-0-12-370526-6. [Google Scholar]
- Van Schöll, L.; Kuyper, T.W.; Smits, M.M.; Landeweert, R.; Hoffland, E.; Van Breemen, N. Rock-eating mycorrhizas: Their role in plant nutrition and biogeochemical cycles. Plant Soil 2008, 303, 35–47. [Google Scholar] [CrossRef]
- Leake, J.R.; Duran, A.L.; Hardy, K.E.; Johnson, I.; Beerling, D.J.; Banwart, S.A.; Smits, M.M. Biological weathering in soil: The role of symbiotic root-associated fungi biosensing minerals and directing photosynthate-energy into grain-scale mineral weathering. Mineral. Mag. 2008, 72, 85–89. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, W.; Adams, T.S.; Wei, X.; Li, L.; McCormack, M.L.; DeForest, J.L.; Koide, R.T.; Eissenstat, D.M. Mycorrhizal fungi and roots are complementary in foraging within nutrient patches. Ecology 2016, 97, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Jongmans, A.G.; Van Breemen, N.; Lundström, U.; Van Hees, P.A.W.; Finlay, R.D.; Srinivasan, M.; Unestam, T.; Giesler, R.; Melkerud, P.A.; Olsson, M. Rock-eating fungi. Nature 1997, 389, 682–683. [Google Scholar] [CrossRef]
- Van Breemen, N.; Finlay, R.; Lundström, U.; Jongmans, A.G.; Giesler, R.; Olsson, M. Mycorrhizal weathering: A true case of mineral plant nutrition? Biogeochemistry 2000, 49, 53–67. [Google Scholar] [CrossRef]
- Blum, J.D.; Klaue, A.; Nezat, C.A.; Driscoll, C.T.; Johnson, C.E.; Siccama, T.G.; Eagar, C.; Fahey, T.J.; Likens, G.E. Mycorrhizal weathering of apatite as an important calcium source in base-poor forest ecosystems. Nature 2002, 417, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Hoffland, E.; Giesler, R.; Jongmans, T.; Van Breemen, N. Increasing feldspar tunneling by fungi across a north Sweden podzol chronosequence. Ecosystems 2002, 5, 11–22. [Google Scholar] [CrossRef]
- Smits, M.M.; Hoffland, E.; Jongmans, A.G.; van Breemen, N. Contribution of mineral tunneling to total feldspar weathering. Geoderma 2005, 125, 59–69. [Google Scholar] [CrossRef]
- Calvaruso, C.; Turpault, M.P.; Leclerc, E.; Frey-Klett, P. Impact of ectomycorrhizosphere on the functional diversity of soil bacterial and fungal communities from a forest stand in relation to nutrient mobilization processes. Microb. Ecol. 2007, 54, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Frey-Klett, P.; Garbaye, J.A.; Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 2007, 176, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Balogh-Brunstad, Z.; Keller, C.K.; Gill, R.A.; Bormann, B.T.; Li, C.Y. The effect of bacteria and fungi on chemical weathering and chemical denudation fluxes in pine growth experiments. Biogeochemistry 2008, 88, 153–167. [Google Scholar] [CrossRef]
- Courty, P.E.; Buée, M.; Diedhiou, A.G.; Frey-Klett, P.; Le Tacon, F.; Rineau, F.; Turpault, M.P.; Uroz, S.; Garbaye, J. The role of ectomycorrhizal communities in forest ecosystem processes: New perspectives and emerging concepts. Soil Biol. Biochem. 2010, 42, 679–698. [Google Scholar] [CrossRef]
- Bonneville, S.; Smits, M.M.; Brown, A.; Harrington, J.; Leake, J.R.; Brydson, R.; Benning, L.G. Plant-driven fungal weathering: Early stages of mineral alteration at the nanometer scale. Geology 2009, 37, 615–618. [Google Scholar] [CrossRef]
- Bonneville, S.; Morgan, D.J.; Schmalenberger, A.; Bray, A.; Brown, A.; Banwart, S.A.; Benning, L.G. Tree-mycorrhiza symbiosis accelerate mineral weathering: Evidences from nanometerscale elemental fluxes at the hypha–mineral interface. Geochim. Cosmochim. Acta 2011, 75, 6988–7005. [Google Scholar] [CrossRef]
- McMaster, T.J. Atomic Force Microscopy of the fungi–mineral interface: Applications in mineral dissolution, weathering and biogeochemistry. Curr. Opin. Biotechnol. 2012, 23, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Saccone, L.; Gazzè, S.A.; Duran, A.L.; Leake, J.R.; Banwart, S.A.; Ragnarsdóttir, K.V.; Smits, M.M.; McMaster, T.J. High resolution characterization of ectomycorrhizal fungal-mineral interactions in axenic microcosm experiments. Biogeochemistry 2012, 111, 411–425. [Google Scholar] [CrossRef]
- Gazzè, S.A.; Saccone, L.; Vala Ragnarsdottir, K.; Smits, M.M.; Duran, A.L.; Leake, J.R.; Banwart, S.A.; McMaster, T.J. Nanoscale channels on ectomycorrhizal-colonized chlorite: Evidence for plant-driven fungal dissolution. J. Geophys. Res. Biogeosci. 2012, 117, G00N09. [Google Scholar] [CrossRef]
- Sverdrup, H. Chemical weathering of soil minerals and the role of biological processes. Fungal Biol. Rev. 2009, 23, 94–100. [Google Scholar] [CrossRef]
- Ward, M.B.; Kapitulčinová, D.; Brown, A.P.; Heard, P.J.; Cherns, D.; Cockell, C.S.; Hallam, K.R.; Ragnarsdóttir, K.V. Investigating the role of microbes in mineral weathering: Nanometre-scale characterisation of the cell–mineral interface using FIB and TEM. Micron 2013, 47, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Johansson, L.; Wallander, H. Soil fungi appear to have a retarding rather than a stimulating role on soil apatite weathering. Plant Soil 2014, 385, 217–228. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Chen, J.; Teng, H.H. Cellular dissolution at hypha-and spore-mineral interfaces revealing unrecognized mechanisms and scales of fungal weathering. Geology 2016, 44, 319–322. [Google Scholar] [CrossRef]
- Rosenstock, N.P.; Berner, C.; Smits, M.M.; Krám, P.; Wallander, H. The role of phosphorus, magnesium and potassium availability in soil fungal exploration of mineral nutrient sources in Norway spruce forests. New Phytol. 2016, 211, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Bonneville, S.; Haward, S.; Leake, J.R. Ectomycorrhizal weathering, a matter of scale? Mineral. Mag. 2008, 72, 131–134. [Google Scholar] [CrossRef]
- Smits, M.M.; Herrmann, A.M.; Duane, M.; Duckworth, O.W.; Bonneville, S.; Benning, L.G.; Lundström, U. The fungal–mineral interface: Challenges and considerations of micro-analytical developments. Fungal Biol. Rev. 2009, 23, 122–131. [Google Scholar] [CrossRef]
- Augustin, F.; Houle, D.; Gagnon, C.; Courchesne, F. Long-term base cation weathering rates in forested catchments of the Canadian Shield. Geoderma 2015, 247, 12–23. [Google Scholar] [CrossRef]
- Balogh-Brunstad, Z.; Keller, C.K.; Bormann, B.T.; O’Brien, R.; Wang, D.; Hawley, G. Chemical weathering and chemical denudation dynamics through ecosystem development and disturbance. Glob. Biogeochem. Cycles 2008, 22, GB1007. [Google Scholar] [CrossRef]
- Taylor, L.L.; Leake, J.R.; Quirk, J.; Hardy, K.; Banwart, S.A.; Beerling, D.J. Biological weathering and the long-term carbon cycle: Integrating mycorrhizal evolution and function into the current paradigm. Geobiology 2009, 7, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Hasenmueller, E.A.; Gu, X.; Weitzman, J.N.; Adams, T.S.; Stinchcomb, G.E.; Eissenstat, D.M.; Drohan, P.J.; Brantley, S.L.; Kaye, J.P. Weathering of rock to regolith: The activity of deep roots in bedrock fractures. Geoderma 2017, 300, 11–31. [Google Scholar] [CrossRef]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, B. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Balogh-Brunstad, Z.; Grant, M.; Harsh, J.; Gill, R.; Thomashow, L.; Dohnalkova, A.; Stacks, D.; Letourneau, M.; Keller, C.K. Cation uptake and allocation by red pine seedlings under cation-nutrient stress in a column growth experiment. Plant Soil 2014, 378, 83–98. [Google Scholar] [CrossRef]
- Balogh, Z. Chemical Hydrology of Vascular Plant Growth: Role of Root-Fungus Associations. Doctoral Dissertation, Washington State University, Pullman, WA, USA, 2006. [Google Scholar]
- Wallander, H.; Wickman, T. Biotite and microcline as potassium sources in ectomycorrhizal and non-mycorrhizal Pinus sylvestris seedlings. Mycorrhiza 1999, 9, 25–32. [Google Scholar] [CrossRef]
- Glowa, K.R.; Arocena, J.M.; Massicotte, H.B. Extraction of potassium and/or magnesium from selected soil minerals by Piloderma. Geomicrobiol. J. 2003, 20, 99–111. [Google Scholar] [CrossRef]
- Van Schöll, L.; Hoffland, E.; Van Breemen, N. Organic anion exudation by ectomycorrhizal fungi and Pinus sylvestris in response to nutrient deficiencies. New Phytol. 2006, 170, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Van Schöll, L.; Smits, M.M.; Hoffland, E. Ectomycorrhizal weathering of the soil minerals muscovite and hornblende. New Phytol. 2006, 171, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.H. The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenetic infection. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 1969, 59, 159–163. [Google Scholar]
- Johnson, D.M.; Hooper, P.R.; Conrey, R.M. XRF analysis of rocks and minerals for major and trace elements on a single low dilution Li-tetraborate fused bead. Adv. X-Ray Anal. 1999, 41, 843–867. [Google Scholar]
- Deer, W.A.; Howie, R.A.; Zussman, J. An Introduction to the Rock-Forming Minerals, 2nd ed.; Longman: London, UK, 1992. [Google Scholar]
- Peterson, R.L.; Massicotte, H.B.; Melville, L.H. Mycorrhizas: Anatomy and Cell Biology; NRC Research Press: Ottawa, ON, Canada, 2004. [Google Scholar]
- SW-846 EPA Method 3050B, Acid digestion of sediments, sludges, and soils. In Test Methods for Evaluating Solid Waste, 3rd ed.; U.S. EPA: Washington, DC, USA, 1995.
- McIntosh, J.L. Bray and Morgan soil test extractants modified for testing acid soils from different parent materials. Agron. J. 1969, 61, 259–265. [Google Scholar] [CrossRef]
- Bray, A.W.; Oelkers, E.H.; Bonneville, S.; Wolff-Boenisch, D.; Potts, N.J.; Fones, G.; Benning, L.G. The effect of pH, grain size, and organic ligands on biotite weathering rates. Geochim. Cosmochim. Acta 2015, 164, 127–145. [Google Scholar] [CrossRef]
- Hodson, M.E. Searching for the perfect surface area normalizing term—A comparison of BET surface area, geometric surface area-and mass-normalized dissolution rates of anorthite and biotite. J. Geochem. Explor. 2006, 88, 288–291. [Google Scholar] [CrossRef]
- Lamb, R.J.; Richards, B.N. Effect of mycorrhizal fungi on the growth and nutrient status of slash and radiata pine seedlings. Aust. For. 1971, 35, 1–7. [Google Scholar] [CrossRef]
- Leake, J.; Johnson, D.; Donnelly, D.; Muckle, G.; Boddy, L.; Read, D. Networks of power and influence: The role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can. J. Bot. 2004, 82, 1016–1045. [Google Scholar] [CrossRef]
- Wallander, H.; Nylund, J.E. Effects of excess nitrogen on carbohydrate concentration and mycorrhizal development of Pinus sylvestris L. seedlings. New Phytol. 1991, 119, 405–411. [Google Scholar] [CrossRef]
- Wallander, H.; Hagerberg, D. Do ectomycorrhizal fungi have a significant role in weathering of minerals in forest soil? Symbiosis 2004, 37, 249–257. [Google Scholar]
- Calvaruso, C.; Mareschal, L.; Turpault, M.P.; Leclerc, E. Rapid clay weathering in the rhizosphere of Norway spruce and oak in an acid forest ecosystem. Soil Sci. Soc. Am. J. 2009, 73, 331–338. [Google Scholar] [CrossRef]
- Pinzari, F.; Cuadros, J.; Napoli, R.; Canfora, L.; Bardají, D.B. Routes of phlogopite weathering by three fungal strains. Fungal Biol. 2016, 120, 1582–1599. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Li, S.; Li, X.; Lu, J. Effects of citrate on the dissolution and transformation of biotite, analyzed by chemical and atomic force microscopy. Appl. Geochem. 2014, 51, 101–108. [Google Scholar] [CrossRef]
- Haward, S.J.; Smits, M.M.; Ragnarsdóttir, K.V.; Leake, J.R.; Banwart, S.A.; McMaster, T.J. In situ atomic force microscopy measurements of biotite basal plane reactivity in the presence of oxalic acid. Geochim. Cosmochim. Acta 2011, 75, 6870–6881. [Google Scholar] [CrossRef]
- Munkholm, L.J.; Heck, R.J.; Deen, B. Long-term rotation and tillage effects on soil structure and crop yield. Soil Tillage Res. 2013, 127, 85–91. [Google Scholar] [CrossRef]
- Keller, C.K.; O’brien, R.; Havig, J.R.; Smith, J.L.; Bormann, B.T.; Wang, D. Tree harvest in an experimental sand ecosystem: Plant effects on nutrient dynamics and solute generation. Ecosystems 2006, 9, 634–646. [Google Scholar] [CrossRef]
- Phillips, R.P.; Ibanez, I.; D’Orangeville, L.; Hanson, P.J.; Ryan, M.G.; McDowell, N.G. A belowground perspective on the drought sensitivity of forests: Towards improved understanding and simulation. For. Ecol. Manag. 2016, 380, 309–320. [Google Scholar] [CrossRef]
- Schmalenberger, A.; Duran, A.L.; Bray, A.W.; Bridge, J.; Bonneville, S.; Benning, L.G.; Romero-Gonzalez, M.E.; Leake, J.R.; Banwart, S.A. Oxalate secretion by ectomycorrhizal Paxillus involutus is mineral-specific and controls calcium weathering from minerals. Sci. Rep. 2015, 5, 12187. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.P.; Fahey, T.J. Fertilization effects on fineroot biomass, rhizosphere microbes and respiratory fluxes in hardwood forest soils. New Phytol. 2007, 176, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Gazzè, S.A.; Saccone, L.; Smits, M.M.; Duran, A.L.; Leake, J.R.; Banwart, S.A.; Ragnarsdottir, K.V.; McMaster, T.J. Nanoscale observations of extracellular polymeric substances deposition on phyllosilicates by an ectomycorrhizal fungus. Geomicrobiol. J. 2013, 30, 721–730. [Google Scholar] [CrossRef]
- Dohnalkova, A.C.; Tfaily, M.M.; Smith, A.P.; Chu, R.; Crump, A.; Brislawn, C.J.; Varga, T.; Shi, Z.; Thomashow, L.S.; Harsh, J.B.; et al. Molecular and Microscopic Insights into the Persistence of Soil Organic Matter in a Red Pine Rhizosphere. Soils 2017, 1, 4. [Google Scholar] [CrossRef]
| Experiment | Treatment Codes | Description |
|---|---|---|
| KU | KU_A | Abiotic (A) unplanted treatment |
| SP_UI | Scots pine (SP) uninoculated (UI) | |
| SP_SB | Scots pine inoculated with Suillus bovinus (SB) | |
| SP_PF | Scots pine inoculated with Piloderma fallax (PF) | |
| SP_PI | Scots pine inoculated with Paxillus involutus (PI) | |
| ZS [49] | ZS_A | Abiotic unplanted treatment |
| RP_UI | Red pine (RP) uninoculated (UI) | |
| RP_SS | Red pine inoculated with soil slurry (SS) | |
| BB [30] | BB_A | Abiotic unplanted treatment, both antibiotics and fungicides are used to prevent growth the bacteria and fungi. |
| RP_E | Red pine inoculated with Ewingella americana (E), which was isolated from the Suillus tomentosus sporocarps, and fungal growth was prevented by fungicides. | |
| RP_BP | Red pine inoculated with Bacillus megaterium (B) and Pantoea agglomerans (P), which were isolated from the Pisolithus tinctorius sporocarps, and fungal growth was prevented by fungicides. | |
| RP_ST_E | Red pine inoculated with Suillus tomentosus (ST) and its associated bacterium, Ewingella americana | |
| RP_PT_BP | Red pine inoculated with Pisoluthus tinctorius (PT) and its associated bacteria, Bacillus megaterium and Pantoea agglomerans |
| Experiment | Treatment Codes | Above-Ground Biomass (mg) | Below-Ground Biomass (mg) | Root:Shoot Ratio | % of Mycorrhizal Root Tips |
|---|---|---|---|---|---|
| KU | SP_SB | 20.3 (±2.4) a | 7.13 (±2.8) a | 0.37 (±0.2) a | 54 (±17) a |
| SP_PF | 27.0 (±4.4) a | 13.1 (±4.9) a | 0.48 (±0.1) a | 67 (±15) a | |
| SP_PI | 28.8 (±6.3) a | 24.0 (±11) b | 0.82 (±0.3) a | 53 (±19) a | |
| ZS [49] | RP_UI | 209 (±120) b | 73.0 (±46) c | 0.35 (±0.1) a | 0.0 (±0) b |
| RP_SS | 242 (±170) b | 61.2 (±18) c | 0.35 (±0.2) a | 2.1 (±2) b | |
| BB [30,50] | RP_E | 116 (±30) a | 288 (±80) d | 2.49 (±0.36) b | 0.0 (±0) b |
| RP_BP | 106 (±34) a | 280 (±75) d | 2.74 (±0.63) b | 0.0 (±0) b | |
| RP_ST_E | 92.6 (±23) a | 218 (±39) d | 2.39 (±0.35) b | 22 (±5) c | |
| RP_PT_BP | 105 (±16) a | 276 (±65) d | 2.62 (±0.36) b | 5.3 (±0.4) d |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogh-Brunstad, Z.; Keller, C.K.; Shi, Z.; Wallander, H.; Stipp, S.L.S. Ectomycorrhizal Fungi and Mineral Interactions in the Rhizosphere of Scots and Red Pine Seedlings. Soils 2017, 1, 5. https://doi.org/10.3390/soils1010005
Balogh-Brunstad Z, Keller CK, Shi Z, Wallander H, Stipp SLS. Ectomycorrhizal Fungi and Mineral Interactions in the Rhizosphere of Scots and Red Pine Seedlings. Soils. 2017; 1(1):5. https://doi.org/10.3390/soils1010005
Chicago/Turabian StyleBalogh-Brunstad, Zsuzsanna, C. Kent Keller, Zhenqing Shi, Håkan Wallander, and Susan L. S. Stipp. 2017. "Ectomycorrhizal Fungi and Mineral Interactions in the Rhizosphere of Scots and Red Pine Seedlings" Soils 1, no. 1: 5. https://doi.org/10.3390/soils1010005
APA StyleBalogh-Brunstad, Z., Keller, C. K., Shi, Z., Wallander, H., & Stipp, S. L. S. (2017). Ectomycorrhizal Fungi and Mineral Interactions in the Rhizosphere of Scots and Red Pine Seedlings. Soils, 1(1), 5. https://doi.org/10.3390/soils1010005





