Abstract
We present an experimental apparatus to investigate electron transport and thermalization in cryogenic para-hydrogen crystal matrices. This paper describes the techniques used to grow and characterize the cryogenic para-hydrogen crystals, the optical system employed to photoextract electrons, and the charge collection system used to study the behavior of electrons within the solid matrix. By probing the fundamental charge transport and energy loss processes in a quantum solid, such as para-hydrogen, this study paves the way for future precision experiments that leverage the unique properties of cryogenic crystal matrices.
1. Introduction
Understanding electron behavior in quantum solid systems is of great interest for various applications, such as the design of bolometers for particle detectors [1,2] and precision measurements with molecules embedded in cryogenic matrices [3,4].
In this work, we present an experimental setup specifically designed to investigate electron transport and thermalization in cryogenic para-hydrogen (p-H2) crystal matrices. Our setup enables the growth of high-purity p-H2 crystals and allows for the direct injection of electrons into the solid matrix. An external electric field then extracts and collects these electrons to measure their thermalization length and other transport properties within the crystal. With this experimental apparatus, we have performed the first measurements, to the best of our knowledge, of the thermalization length of electrons in solid p-H2 by injection of quasi-free electrons [5].
This study represents a preliminary step toward measuring the electric dipole moment of the electron (eEDM) using polar molecules and the matrix isolation technique. Previous proposals have suggested using rare-gas crystals doped with diatomic radicals [3,4]. In our proposal [6], we explored the possibility of leveraging the unique properties of cryogenic p-H2 as a host matrix to embed barium monofluoride (BaF) molecules. A p-H2 crystal can host large molecules like BaF without producing many defects, while its low temperature and absence of permanent electric moments minimize unwanted interactions with the guest molecules [7].
In our approach, BaF molecules are embedded in the matrix as positively charged ions, as they are produced by an ion-beam source in which solid BaF2 is vaporized, dissociated, and ionized by an electron beam. The ions are accelerated to 1 keV, mass-selected with a Wien filter, and decelerated to approximatively 5 eV before being merged with the para-hydrogen gas flow during matrix growth. The neutralization of the BaF+ ions is a critical step in our procedure, which we aim to achieve by injecting electrons photoextracted from a gold cathode using short UV laser pulses into the p-H2 matrix.
The behavior of electrons in solid p-H2 is primarily influenced by electron–phonon interactions, which drive energy dissipation and thermalization. A key parameter for our eEDM measurement is the thermalization length, which represents the average distance electrons travel before dissipating their excess energy and reaching thermal equilibrium within the solid. Shorter thermalization lengths are preferred, as they enable a more efficient neutralization of the embedded BaF+ ions.
In Section 2, we provide a detailed description of the experimental apparatus. In Section 3, we present the measurements we performed to characterize the cryogenic crystals and study the charge extraction process in vacuum and solid p-H2. Finally, Section 4 outlines our conclusions and discusses future work related to studying electron dynamics in these systems.
2. Materials and Methods
The experimental setup we used for this study has three main components: a cryogenic system to grow the crystal matrix, an optical system consisting of a UV laser to photoextract electrons and an infrared spectroscopy system to characterize the p-H2 crystals, and a charge collection system to study the electron behavior within the matrix. These components are described in detail below.
2.1. Cryogenic System
The cryogenic system, shown in Figure 1, is used to grow p-H2 crystal matrices following the approach and techniques described in [8,9]. Hydrogen gas with an impurity concentration less than 1 ppm (N6.0 grade) is further purified by a cryogenic trap at liquid nitrogen temperature. It then flows through a copper line containing hydrous ferric oxide, a catalyst that facilitates the conversion to the p-H2 state [10]. This gas line is wound around a copper block connected to the cold head of a pulse tube refrigerator (Leybold-Heraeus RG210, Leybold GmbH, Cologne, Germany) operating at approximately 20 K. The copper block is designed to maximize thermal contact with the hydrogen line, and indium is used for efficient heat transfer between the two parts.
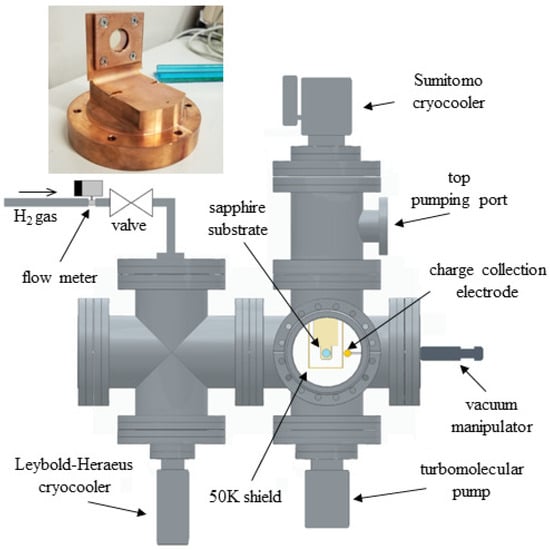
Figure 1.
Schematic illustration of the experimental setup used for crystal growth: Hydrogen gas flows through a copper line containing a hydrous ferric oxide catalyst. The gas line is in thermal contact with the cold head of a Leybold-Heraeus cryocooler operating at approximately 20 K. The substrate where the p-H2 gas is condensed is cooled to approximately 2.9 K by a Sumitomo cryocooler. The growth chamber is a six-way cross stainless-steel chamber pumped by two systems—one at the bottom and the other at the top to increase the pumping speed for hydrogen in the upper section of the vacuum chamber. Inset: the sapphire substrate holder is machined from an oxygen-free, high-thermal conductivity copper plate with a diameter of 90 mm.
The p-H2 gas flow is regulated to about 10 sccm, and a gas nozzle with a diameter of 3 mm is positioned 30 mm from a 1" sapphire substrate (clear aperture ≈ 15 mm), as shown in Figure 2a. The sapphire substrate is coated with a thin gold layer to enable electron photoemission when illuminated with UV light. The coating thickness is 10 nm to maintain a good transparency in the infrared range. The growth of the p-H2 crystal is achieved by condensing the gas on the gold-coated side of the sapphire substrate.
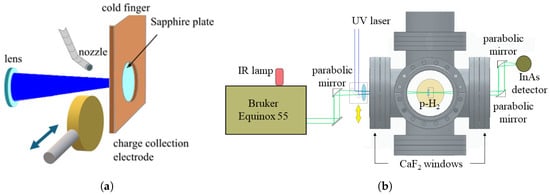
Figure 2.
(a) Crystal growth and charge collection system: p-H2 gas flows out of a nozzle near the surface of the sapphire substrate that is coated with a 10 nm gold layer. The p-H2 gas condenses on the gold-coated side of the sapphire substrate and creates a solid crystal. A 25 mm copper disk is placed at 1 cm from the sapphire substrate and connected to a high-voltage generator. During crystal growth, a vacuum manipulator moves the charge collection electrode away from the sapphire substrate. A lens outside the vacuum tank focuses the UV laser beam on the gold layer to photoextract electrons that are then collected by the electrode. (b) Optical layout for the measurement of the thickness of the crystal matrix. A Fourier transform infrared interferometer (Bruker Equinox 55) equipped with a near-infrared light source measures the absorption spectrum of the p-H2 layer. Gold-coated parabolic mirrors focus and collimate the infrared light as it passes through the sapphire substrate and the p-H2 crystal. The transmitted infrared light is then collected by an InAs detector for spectral analysis.
The substrate is mounted on a holder machined from a high-thermal conductivity oxygen-free copper plate (see the inset of Figure 1) cooled to approximately 2.9 K using a two-stage helium pulse tube cryocooler (Sumitomo RP62B, Sumitomo Heavy Industries, Ltd., Tokyo, Japan). A calibrated silicon diode temperature sensor monitors the copper plate temperature near the sapphire substrate. The cold head of the cryocooler is enclosed in a copper shield at 50 K and covered with multiple layers of Mylar foil to reduce radiation heating.
The cryostat chamber where the crystal is grown is a six-way cross stainless-steel chamber with DN 200CF flanges. A pumping system consisting of a turbomolecular pump and a backing pump is connected to the bottom flange of the vacuum tank. An extra turbomolecular pump is also installed at the top to increase the pumping speed for hydrogen in the upper section of the vacuum chamber. Before starting the growth process, the system is evacuated to a base pressure below mbar and, during crystal growth, the pressure is always maintained below mbar.
Two CaF2 windows are mounted on opposite sides of the growth chamber to provide optical access to the cryostat, as shown in Figure 2b. One window is used to illuminate the gold layer on the sapphire substrate with UV laser pulses and investigate the absorption of the p-H2 sample using broadband infrared light. The second optical window, on the other side of the growth chamber, allows the collection of infrared light transmitted through the p-H2 crystal by an InAs detector.
2.2. Optical System
We use UV laser pulses at a wavelength of 266 nm to extract electrons from the gold layer on the sapphire substrate. These UV pulses are generated through fourth-harmonic generation of a 1064 nm Nd:YAG laser. This laser produces 10 ns long pulses at a repetition rate of 10 Hz, with an adjustable energy per pulse ranging from 50 J to 1 mJ. A dichroic mirror separates the 266 nm beam from the fundamental 1064 nm and second harmonic 532 nm components. A telescope consisting of two lenses with UV anti-reflection coating and 1 m focal length transports the laser beam from the cleanroom, where the laser is located, to the entrance of the vacuum chamber. The UV beam was focused to a diameter of approximately 8 mm to ensure uniform illumination across the p-H2 surface while avoiding clipping at the substrate edges and preventing unwanted illumination of the surrounding copper holder.
In addition to the UV laser system for photoelectron extraction, we use a Fourier transform interferometer (Bruker model Equinox 55, Bruker Corporation, Billerica, MA, USA) with a near-infrared light source (Thorlabs model SLS202L/M, Thorlabs, Inc., Newton, NJ, USA) to measure the infrared absorption spectrum of the p-H2 matrix [5]. Two gold-coated parabolic mirrors at the entrance and exit of the vacuum chamber focus and collimate the infrared beam transmitted through the sapphire substrate and p-H2 crystal, as shown in Figure 2a. After the collimating paraboloid, the beam is focused by another mirror and collected by an infrared detector (Hamamatsu model P7163, Hamamatsu Photonics K.K., Hamamatsu City, Japan).
2.3. Charge Collection System
The photoextracted electrons drift within the p-H2 crystal matrix under the effect of an external electric field, traverse the solid–vacuum interface, and are then collected by an electrode. The charge collection electrode, shown in Figure 2a, is a 25 mm-diameter copper disk placed at 1 cm from the p-H2 surface. This is connected to a high-voltage supply via a vacuum feedthrough to apply the electric field. DC voltages up to 700 V can be applied to the charge collection electrode to control and accelerate the electron drift through the solid–vacuum interface. A low-noise charge amplifier with a gain of 0.27 mV/fC and a time constant of approximately 400 s integrates the current due to the drifting electrons within the crystal. The output of the charge amplifier is connected to a digital oscilloscope for signal acquisition, where 2000 individual charge collection signals are acquired for each applied voltage setting to improve the signal-to-noise ratio. The signals are then downloaded to a computer and averaged in an offline analysis.
During crystal growth, the vacuum manipulator is used to move the charge collection electrode away from the sapphire substrate, allowing the p-H2 crystal to grow without any obstruction from the electrode. Then, the electrode is placed again in front of the sapphire substrate for the charge collection measurements.
3. Results and Discussion
3.1. Para-Hydrogen Thickness
We periodically monitored the thickness and ortho-hydrogen fraction of the p-H2 crystal during growth via infrared absorption spectroscopy. Figure 3a illustrates a representative absorption spectrum after roughly 2.5 h of p-H2 growth.
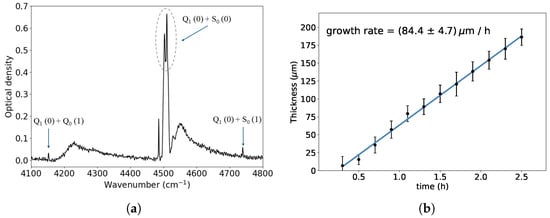
Figure 3.
(a) Optical density spectrum of a solid p-H2 crystal after 2.5 h of growth. The absorption range used for calculating the crystal thickness is indicated by the dashed oval shape. The other two absorption features, indicated by arrows, are used to determine the ortho-hydrogen fraction. The notation used to indicate the different transitions is explained in [9]. (b) Measured crystal thickness as a function of time: the growth process is linear and begins with a delay of about 20 min following the start of the p-H2 gas flux at t = 0.
Following the methodology described in [11,12], the crystal thickness and its ortho-hydrogen fraction are calculated from the optical density OD = , where I) and ) are, respectively, the transmitted intensities with and without the p-H2 crystal.
The measured crystal growth rate was approximately 80 m/h (Figure 3b, while the ortho-hydrogen fraction was about 2–3%. A delay of about 20 min was observed between the start of the p-H2 gas flow and the beginning of crystal growth. This behavior is consistent with previous observations [9], where this delay was attributed to the adsorption of hydrogen molecules onto the surface of the hydrous ferric oxide catalyst. Only after this surface becomes saturated, p-H2 begins to flow to the cold substrate in sufficient quantity to initiate the crystal growth.
The purity of p-H2 was limited by the temperature reached by the Leybold-Heraeus cryocooler used in the p-H2 converter. We plan to upgrade our system with a more powerful cryocooler to obtain lower conversion temperatures and improve the purity of the crystals. The crystal growth was stopped once the thickness reached about 500–600 m.
3.2. Charge Extraction in Vacuum
As an initial test, we characterized the field-assisted photoemission process in vacuum without any deposited crystal. The charge collected as a function of the applied electric field is shown in Figure 4a for a UV laser pulse energy of 20 J. The charge signal increases with the electric field and reaches saturation at ≈25 V/cm, where most of the electrons produced by the laser pulse are collected.
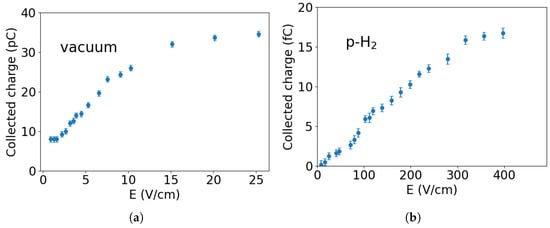
Figure 4.
Electric field dependence of the collected charge in vacuum and in p-H2. (a) In vacuum, the collected charge reaches saturation at approximately E ≈ 25 V/cm. (b) In the p-H2 matrix (thickness ≈ 500 m), the amount of collected charge is significantly lower compared to the vacuum case, and saturation occurs at higher electric fields, greater than 400 V/cm. The decrease in electron penetration can be attributed to various factors, including quantum reflection and scattering processes within the p-H2 crystal.
From these measurements, we find that the quantum efficiency of photoemission in our experimental setup is approximately . This is slightly lower than the quantum efficiency of about observed in [13] using a thicker gold cathode in a reflective system. The reduced quantum efficiency in our case can be attributed to the different optical arrangement and gold layer thickness that we employed.
3.3. Charge Extraction in Para-Hydrogen
Figure 4b shows the collected charge as a function of the applied electric field for a p-H2 crystal approximately 500 m thick. In this configuration, the laser pulse energy was reduced to approximately 10 J, which was sufficient to extract a detectable signal while minimizing the risk of heating or perturbing the matrix. Photoemission occurs at the interface between the gold layer and the p-H2 crystal, and the extracted electrons drift through the matrix before being collected at the electrode.
The amount of collected charge in p-H2 is significantly lower than in vacuum, despite the efficient electron drift through the crystal. A key reason for this is the substantial energy barrier for electron injection into the solid. Theoretical calculations suggest that electrons encounter a potential barrier on the order of 1–2 eV due to repulsive interactions and dielectric polarization effects in the hydrogen medium [14]. As a result, the effective work function is higher than that of the gold cathode in vacuum, significantly reducing the number of injected electrons. A more detailed discussion of the associated transport processes is available in our previous work [5].
We performed several measurements at different laser pulse energies and observed that the amount of collected charge is, as expected, directly proportional to the energy of the laser pulses, as shown in Figure 5. The electric field dependence, instead, remained the same and always reached a saturation point for field strengths greater than 400 V/cm.
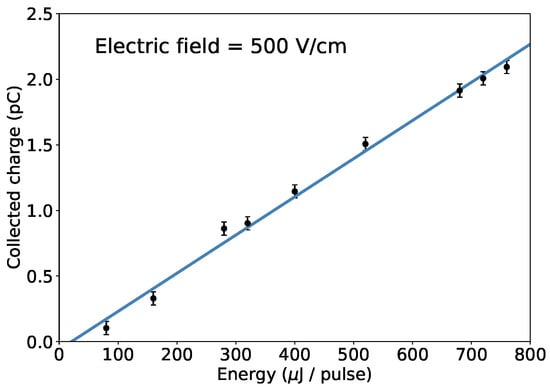
Figure 5.
The charge collected at a fixed electric field in function of the energy per pulse of the UV laser. An electric field strength of 500 V/cm is chosen to ensure full charge extraction.
From the analysis of the individual charge collection signals in p-H2, we did not observe any evidence of charge accumulation in the crystal, which otherwise would have determined a progressive decrease in the collected charge. Our measurements indicate that the electrons move effectively through the crystal and are collected at the electrode without creating a significant space charge buildup.
The absence of charge accumulation can be attributed to the low density of defects and impurities of the p-H2 crystal but also to the electron injection methodology used. In particular, our UV excitation technique might have facilitated the release of electrons from potential trapping sites, as observed in previous studies [15,16]. Those works noted that protonated molecules in solid p-H2, created by electron bombardment during deposition, experienced a decrease in infrared absorption attributed to their neutralization by residual solvated electrons. These observations suggest that the neutralization process could be significantly accelerated and enhanced by irradiating the sample with UV light.
Efficient neutralization is critical in our eEDM experiment because trapped charges could interfere with the embedding of molecules within the matrix and negatively affect the precision of the measurement. The results we obtained support the proposed approach to neutralize BaF+ ions in p-H2 using UV pulses.
3.4. Thermalization Length
The measurement of the thermalization length is discussed in detail in [5]. Here, we briefly summarize the key aspects of our analysis.
Electrons at the interface between the photocathode and the crystal are subject to two opposing forces: the image potential, which draws electrons back toward the photocathode, and the externally applied electric field, which accelerates them away from the surface. By varying the external electric field strength and observing how the collected charge changes with the position of the potential maximum, we determine the electron thermalization length to be z0 = (26.1 ± 1.0) nm.
This short thermalization length indicates that electrons rapidly dissipate their kinetic energy via interactions with phonons, thermalizing very close to the crystal surface. Such quick thermalization is essential for our neutralization approach, as it ensures that electrons are immediately available to neutralize the embedded molecular ions.
4. Conclusions
We have successfully developed an experimental apparatus capable of growing high-purity p-H2 crystal matrices on sapphire substrates at temperatures around 2.9 K. Additionally, we have established a reliable method for injecting electrons directly into the p-H2 matrix through UV photoemission in gold.
With this setup, we have measured the thermalization length of electrons in solid p-H2 and demonstrated that these low-energy electrons rapidly thermalize within the bulk of the matrix, where they would become available for the neutralization of embedded molecular ions.
We also investigated the extraction of these thermalized electrons across the crystal interface under an applied electric field. Our results indicate that a field of approximately 400 V/cm is sufficient to extract most of the photoemitted electrons from the matrix.
This experimental work provides valuable insights into the charge transport and energy loss processes in quantum solid systems such as p-H2. The ability to inject and study the behavior of electrons within the p-H2 crystal matrix represents a significant advancement in demonstrating the neutralization technique required for our eEDM experiment and for future precision measurements using diatomic polar molecules in cryogenic solid matrices.
Future efforts will focus on refining the neutralization procedure during crystal growth. We also plan to enhance our experimental setup by implementing a 1 cm3 p-H2 cell to enable direct measurements of electron mobility within the matrix. The growth of large-volume p-H2 crystals presents additional challenges related to maintaining structural quality and minimizing defects. Addressing these issues will require careful optimization of deposition parameters, including thermal management and gas flow control. These improvements are currently under investigation as part of ongoing system development.
Author Contributions
Conceptualization, A.F.B. and G.C.; data curation, A.F.B. and G.M.; formal analysis, A.F.B.; funding acquisition, R.C., G.C., J.P. and M.Z.; investigation, A.F.B., G.M. and J.P.; methodology, A.F.B. and G.C.; project administration, G.C.; resources, P.A., M.B., G.C., U.G., F.G., A.L. (Augusto Lombardi), E.M. and L.T.; software, A.F.B.; supervision, G.C.; validation, A.F.B. and G.M.; visualization, G.M.; writing—original draft, G.M.; writing—review and editing, A.F.B., C.B., F.C., M.G., A.K., A.L. (Alessandro Lippi), M.M.M., G.M., J.P. and G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the PNRR MUR project PE0000023-NQSTI, financed by the European Union—Next Generation EU. This work was also supported in part by the Italian MUR “Departments of Excellence grant 2023—2027—Quantum Frontiers” and by the “Progetti di Ricerca di Rilevante Interesse Nazionale” (PRIN grant 20227F5W4N) of the Italian Ministry of University and Research. G. Messineo acknowledges the financial support of the European Union’s Horizon 2020 Research and Innovation Programme under Marie Skłodowska-Curie grant no. 754496.
Data Availability Statement
Data will be made available by the corresponding author upon request.
Acknowledgments
We express our sincere gratitude to A. C. Vutha for contributing to insightful discussions that greatly contributed to this work. We also thank E. Berto, F. Calaon, M. Tessaro, M. Rebeschini, and M. Zago for their invaluable technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| p-H2 | Para-hydrogen |
| eEDM | Electric dipole moment of the electron |
| BaF | Barium monoflouride |
References
- Baudis, L. Direct dark matter detection: The next decade. Phys. Dark Universe 2012, 1, 94–108. [Google Scholar] [CrossRef]
- Guarise, M.; Braggio, C.; Calabrese, R.; Carugno, G.; Dainelli, A.; Khanbekyan, A.; Luppi, E.; Mariotti, E.; Poggi, M.; Tomassetti, L. Experimental setup for the growth of solid crystals of inert gases for particle detection. Rev. Sci. Instruments 2017, 88, 113303. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.G.; Derevianko, A. Proposal for a Sensitive Search for the Electric Dipole Moment of the Electron with Matrix-Isolated Radicals. Phys. Rev. Lett. 2006, 97, 063001. [Google Scholar] [CrossRef] [PubMed]
- Vutha, A.C.; Horbatsch, M.; Hessels, E.A. Orientation-dependent hyperfine structure of polar molecules in a rare-gas matrix: A scheme for measuring the electron electric dipole moment. Phys. Rev. A 2018, 98, 032513. [Google Scholar] [CrossRef]
- Borghesani, A.F.; Carugno, G.; Messineo, G.; Pazzini, J. Electron thermalization length in solid para-hydrogen at low-temperature. J. Chem. Phys. 2023, 159, 104501. [Google Scholar] [CrossRef] [PubMed]
- Messineo, G.; Antonini, P.; Benettoni, M.; Borghesani, A.; Braggio, C.; Calabrese, R.; Carugno, G.; Chiossi, F.; Dainelli, A.; Gasparini, U.; et al. Measuring the electric dipole moment of the electron using polar molecules in a parahydrogen matrix. Nucl. Instruments Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2024, 1069, 169951. [Google Scholar] [CrossRef]
- Upadhyay, S.; Kanagin, A.N.; Hartzell, C.; Christy, T.; Arnott, W.P.; Momose, T.; Patterson, D.; Weinstein, J.D. Longitudinal Spin Relaxation of Optically Pumped Rubidium Atoms in Solid Parahydrogen. Phys. Rev. Lett. 2016, 117, 175301. [Google Scholar] [CrossRef] [PubMed]
- Tom, B.A.; Bhasker, S.; Miyamoto, Y.; Momose, T.; McCall, B.J. Producing and quantifying enriched para-H2. Rev. Sci. Instruments 2009, 80, 016108. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Rollings, A.P.; Ratto, L.; Weinstein, J.D. High-purity solid parahydrogen. Rev. Sci. Instruments 2021, 92, 073202. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, D.H.; Loebenstein, W.V.; Draper, J.W.; Park, O.E. Ortho-para catalysis in liquid-hydrogen production. J. Res. Natl. Bur. Stand. 1958, 60, 221–227. [Google Scholar] [CrossRef]
- Fajardo, M.E. Physics and Chemistry at Low Temperatures, 1 ed.; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2011; Chapter Matrix Isolation Spectroscopy in Solid Parahydrogen: A Primer; pp. 167–202. [Google Scholar]
- Fajardo, M.E. Solid Parahydrogen Thickness Revisited. Appl. Spectrosc. 2019, 73, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Rao-Srinivasan, T. UV Photoemission Studies of Metal Photocathodes for Particle Accelerators; Tech. Rep. Brookhaven National Lab.: Upton, NY, USA, 1988. [Google Scholar]
- Miyakawa, T.; Dexter, D.L. Stability of Electronic Bubbles in Liquid Neon and Hydrogen. Phys. Rev. 1969, 184, 166–172. [Google Scholar] [CrossRef]
- Bahou, M.; Wu, Y.J.; Lee, Y.P. Infrared Spectra of Protonated Pyrene and Its Neutral Counterpart in Solid para-Hydrogen. J. Phys. Chem. Lett. 2013, 4, 1989–1993. [Google Scholar] [CrossRef] [PubMed]
- Leicht, D.; Rittgers, B.M.; Douberly, G.E.; Wagner, J.P.; McDonald, D.C., II; Mauney, D.T.; Tsuge, M.; Lee, Y.P.; Duncan, M.A. Infrared spectroscopy of H+(CO)2 in the gas phase and in para-hydrogen matrices. J. Chem. Phys. 2020, 153, 084305. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).