Abstract
A new Center for Radiopharmaceutical Cancer Research was established at the Helmholtz-Zentrum Dresden-Rossendorf in 2017 to centralize radionuclide and radiopharmaceutical production, as well as enable chemical and biochemical research. Routine production of several radionuclides was put into operation in recent years. We report on the production methods of radiopharmaceutical radionuclides, in particular 11C, 18F, and radio metals like 61Cu, 64Cu, 67Cu, 67Ga, 131Ba, and 133La that are used regularly. In the discussion, we report typical irradiation parameters and achieved saturation yields.
1. Introduction
The Center for Radiopharmaceutical Tumor Research at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) combines the production of radionuclides, the preparation of radiopharmaceuticals and radiochemical and biochemical research facilities in a unique structure [1]. The radiopharmacy and research activities strongly depend on a reliable supply of radionuclides. In-house production of several radionuclides is carried out with the TR-Flex cyclotron. This cyclotron is equipped with solid-state, liquid and gas target systems. The energy ranges from 18 MeV up to 30 MeV while featuring proton currents of up to 200 µA per extraction port, thus enabling the production of diagnostic radioisotopes for SPECT and PET and also therapeutic radionuclides for research and clinical applications. We present here a report on the status of routine production with the cyclotron TR-Flex and the process to establish new radionuclides, carried out over the last few years.
2. Materials and Methods
2.1. Equipment
The cyclotron TR-Flex, shown in Figure 1, from Advanced Cyclotron Systems Inc. (Richmond, BC, Canada) [2] was put into operation in 2017. The cyclotron presents two extraction ports. Both extraction foils are radially movable to adjust the energy of the extracted proton beam in the range of 18 MeV up to 30 MeV.

Figure 1.
The TR-Flex installed at the HZDR with target selector 1B at the front.
Two beamlines are connected behind a switching magnet on extraction port 1. The energy at the beamline extraction port can be reduced to 14 MeV. A decrease in the energy results in a change in the beam shape and a beam loss of about 15% within the beamline. Two 4-port target selectors are installed at one beamline and the second extraction port. The cyclotron and the targetry are characterized by the following key parameters:
- Acceleration of H− and extraction of H+ ions;
- External multi-cusp ion source, ion current up to 300 μA;
- Adjustable energy in the range of 18 MeV (14 MeV) up to 30 MeV;
- Dual-beam operation with split ratios 1:100 to 50:50;
- Two [18F]F− water targets and one [18F]F2 gas target;
- One [11C]CO2 target;
- One 30° and one 90° solid-state target holder.
Radionuclide characterization is carried out by high-resolution gamma spectroscopy using an energy- and efficiency-calibrated Mirion Technologies (Canberra, Australia) CryoPulse 5 HPGe detector. Activities between 100 kBq and 800 kBq are measured shortly after the end of purification (EOP), while larger activities are saved to decay (100 MBq to 1 GBq, depending on the radionuclide) to perform later radionuclide purity analysis. The measurements are carried out for 10 min to 1 h, ensuring a dead-time below 5%, using 200 µL of the solution in a vial with calibrated geometry. Activities are automatically calculated with the software Genie2000 (V. 3.4.1).
ICP-MS measurements were performed by VKTA e.V. (Radiation Protection, Analytics & Disposal—Dresden, Germany) with a ThermoFischer Element 2.
2.2. Materials
The solutions used consisted of ultrapure 30% hydrochloric acid (Merck KGaA, Darmstadt, Germany), ultrapure 95% sulfuric acid (Roth GmbH), ultrapure 69% nitric acid (Roth GmbH, Karlsruhe, Germany) and ultrapure 20% aqueous ammonia (Roth GmbH, Karlsruhe, Germany) in addition to deionized milli-Q® water.
The following chromatographic resins and ion exchangers were acquired: 1 mL pre-packed TK201 cartridge (Triskem, Bruz, France), 1 mL pre-packed TK400 cartridge (Triskem, Bruz, France), normal dyglicolamyde–DGA resin (Triskem, Bruz, France), CU-Resin (Triskem, Bruz, France), SR-Resin (Triskem, Bruz, France), 2 mL pre-packed branched DGA resin (Triskem, Bruz, France) and 2 mL Poly-Prep (pre-packed) anion exchanger AG-1x8 (Bio-Rad, Hercules, USA).
The following enriched target materials are used: 62Ni 99.36% (metallic ingots, Isoflex, San Francisco, CA, USA) for 61Cu production, 64Ni 99.63% (metallic ingots, Isoflex, San Francisco, USA) for 64Cu production, 68Zn 98.2% (metallic ingots, Isoflex, San Francisco, USA) for 67Ga production, 70Zn 97.5% (metallic powder, ECP Rosatom, Zelenogorsk, Russia) for 67Cu production and 134Ba 88.10% (as [134Ba]BaCO3 powder production, Isoflex, USA). Anhydrous natural CsCl (99.999% purity, Sigma-Aldrich, St. Louis, MO, USA) is used for 131Ba production.
3. Results
3.1. Routine Production of PET Radionuclides
3.1.1. [18F]F− Production with a Water Target
Fluorine-18 is the most widely used radioisotope in positron emission tomography (PET). Its applications are numerous in the fields of oncology, neurology and cardiology. It is produced in a standard ACSI water target with a 3.8 mL volume made from aluminum and niobium by using the 18O(p,n)18F nuclear reaction. An amount of 20 GBq up to 300 GBq of [18F]fluoride can be produced in a daily routine with irradiation times from 20 min to 60 min and ion currents in the range of 80 µA up to 110 µA at an energy of 18 MeV. The [18F]fluoride is unloaded through a PTFE tube line to a central activimeter from where it can be distributed to several destinations for further processing.
The [18F]F− target is generally maintained once per year after an accumulated charge of about 15 mAh. The vacuum, target foils and all gaskets are replaced. The target body and the foils are evacuated and heated in a vacuum-drying oven. Furthermore, the unloading tube line is rinsed with 100 mL Ethanol, 100 mL Heptane, 100 mL Ethanol again and 250 mL water twice a year.
The production of radiopharmaceuticals is carried out in a clean room area according to GMP guidelines by the application of automated systems for radiolabeling and dispensing. Among the variety of diagnostic radiotracers based on [18F]fluoride, [18F]FDG ([18F]fluoro-2-desoxy-D-glucose), [18F]PSMA-1007, sodium [18F]fluoride for injection, [18F]FMISO ([18F]Fluoromisonidazole) and [18F]Flutemetamol (Vizamyl©) are the periodically used ones. Since at least one or two radiopharmaceuticals are produced and released by quality control per day, a number of about 300 batches per year is added up in the Center for Radiopharmaceutical Tumor Research. Figure 2 gives an overview of the production of radiopharmaceuticals from 2018 to 2022.
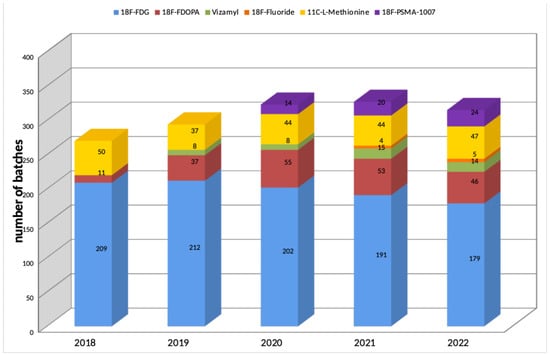
Figure 2.
Produced radiopharmaceuticals from 2018 to 2022.
3.1.2. [18F]F2 Production with a Gas Target
[18F]F2 gas, an essential prerequisite for the electrophilic radiosynthesis of 6-L-[18F]FDOPA (3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine), is produced in the TR-Flex cyclotron in a standard ACSI conical aluminum body gas target with a volume of 58 mL, covered by a HAVAR target foil. The HAVAR foil was originally sealed by an indium wire; however, for an easier maintenance process, we replaced the indium wire with a lead gasket. The [18F]F2 is produced in a three-step irradiation process. First, the gas target is filled with 20 bar of Argon containing 0.2% [19F]F2, irradiated with 18 MeV protons at a current of 35 µA for 5 min and unloaded into the production hot cell via a stainless steel tube line. This procedure is repeated two times for passivation of the target surface and the transport lines. After that, the target is evacuated below 0.1 mbar for more than 30 min to remove any traces of moisture and oxygen. In the next step, the target is loaded with 20 bar [18O]O2 gas and irradiated with 18 MeV protons at a current of 35 µA for 60 min. Part of the produced Fluorine-18 is deposited on the surface of the target body. After the irradiation is finished, the [18O]O2 gas is recovered by cryogenic cooling. The [18F]F2 is extracted by loading the target with 20 bar Argon containing 0.2% [19F]F2 and a 15 min irradiation with 18 MeV protons at a current of 30 µA. During the third irradiation, a 19F/18F gas-phase exchange takes place on the target walls. Generally, 20 GBq of [18F]F2 can be produced within this irradiation regime, which serves as the starting activity for the electrophilic radiosynthesis of 6-L-[18F]FDOPA using an N-acetyl-protected 6-trimethylstannyleted precursor. The mean radiochemical yield is 16.2%, which corresponds to 2.6 ± 0.7 GBq of [18F]FDOPA. As visible in Figure 2, between 37 and 55 batches of [18F]FDOPA are produced per year.
The [18F]F2 gas target is maintained once a year; all gaskets and the vacuum and target foils are replaced. The target body is mechanically polished and cleaned in an ultrasonic bath with Ethanol and water. Furthermore, all parts are evacuated and heated in a vacuum-drying oven. The produced amount of [18F]F2 slightly decreases over the year because the target surface is roughened by the Fluorine. Polishing the target increases the target yield again [3,4,5].
3.1.3. [11C]CO2 Production with a Gas Target
[11C]CO2 is produced by the 14N(p,α)11C nuclear reaction, also in the standard ACSI conical aluminum gas target with a volume of 58 mL. The target is likewise covered by a HAVAR target foil sealed by a lead gasket due to an easier maintenance process. The target is filled with a 0.5% O2/N2 gas mixture at 20 bar. By 35 min of irradiation with 18 MeV protons at a current of 45 µA, about 120 GBq [11C]CO2 can be produced. The yield is quite stable over a year. If, in routine use, a slight decrease in the yield is observed, the target is evacuated to a rough vacuum in the range of 0.1 mbar for 24 h to remove impurities from the target. For maintenance, once a year, the target foils and seals are changed and the target is cleaned with water in an ultrasonic bath and heated in a vacuum-drying oven.
The so-produced [11C]CO2 is transferred directly to a hot cell via a stainless steel tube line where it is trapped, reduced to [11C]CH4 and further reacted to form [11C]CH3I by an automated synthesizer. By reaction with the L-homocysteine thiolactone hydrochloride precursor, L-[11C]methionine is formed in a 28–41% radiochemical yield [6,7]. The number of L-[11C]methionine batches produced at the Center of Radiopharmaceutical Tumor Research is 44–50 per year; see Figure 2.
3.1.4. [64Cu]CuCl2 Production with a Solid Target System
Copper-64 is produced via the 64Ni(p,n)64Cu nuclear reaction. The beam energy should be below 14 MeV to minimize the co-production of (stable) 63Cu. We designed and installed an energy degrader in front of the 90° solid-state target holder. The vacuum foil of the used standard ACSI solid target holder was replaced by a water-cooled 0.7 mm thick aluminum window, as shown in Figure 3. The protons are decelerated within the aluminum window in the range of 4.5 MeV to 2.5 MeV, depending on the incidence energy of the protons in the range of 18 MeV up to 30 MeV. The energy degrader was tested up to a beam current of 80 µA and had to be replaced after an accumulated charge of about 5000 µAh.
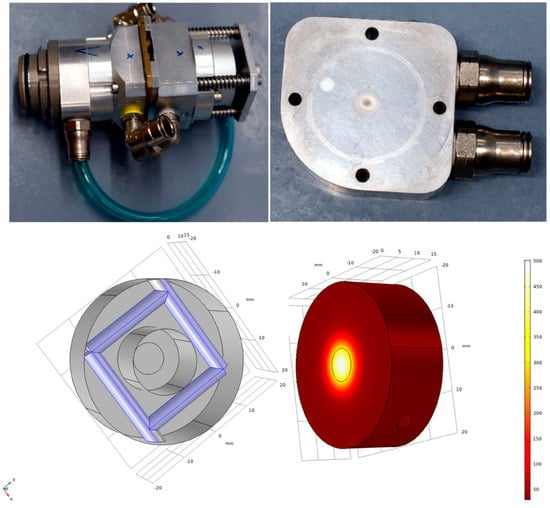
Figure 3.
The solid target holder including the energy degrader (right side after irradiation with about 5000 µAh) is shown in the upper part of the picture. The energy degrader is integrated into the cooling circuit of the solid target holder. In the lower part, a scheme of the energy degrader including the cooling channels is shown on the left side. The temperature of the energy degrader is simulated to be below 400 °C with ion currents up to 80 µA using the COMSOL 5.5 software.
64Ni targets are electrodeposited onto gold circular backings from [64Ni][Ni(NH3)6]SO4 solutions, similar to what we already described for 62Ni [8]. We decided to use gold as the target backing due to its high thermal conductivity, which allows proper target cooling, in combination with a rather low activation. This last factor has even more importance considering our weekly 64Cu production, which requires re-using the target backing at least six times a year. In particular, either fresh metallic 64Ni (ca. 100 mg) dissolved in 8 M HCl or recycled [64Ni]NiCl2 are evaporated to dryness and re-dissolved in 1 mL 47.5% (w/w) H2SO4 followed by the addition of 10% (w/w) NH3 to obtain a deep-blue-colored solution. This solution is loaded onto the electroplating device, where the gold substrate acts as the cathode (99.999%, 23 mm diameter, 2 mm thickness, 10 mm diameter and 0.5 mm depth centered deepening). A constant voltage of 2.7 V (ca. 26 mA) is applied to the electrodeposition cell overnight, with a graphite electrode as the anode. The electrodeposition takes place on a 7 mm circle, obtaining 64Ni masses in the 40 mg to 110 mg range. A typical 64Ni target is presented in Figure 4.

Figure 4.
Electroplated 64Ni target on a gold backing used for routine 64Cu production.
Such targets are irradiated with (13.5 ± 0.2) MeV or (13.0 ± 0.2) MeV protons, depending on target mass, at a 70 µA current for times between 90 and 180 min, subject to the activity demand. Activities between 10 GBq (40 mg 64Ni, 2 h irradiation) and 55 GBq (85 mg 64Ni, 3 h irradiation) at EOB are usually produced. The corresponding saturation yields are between 1.35 GBq/µA for a 40 mg 64Ni target and 5.00 GBq/µA for targets with masses greater than 85 mg. The theoretical saturation yields for such targets are 3.62 GBq/µA and 7.67 GBq/µA.
Radiochemical purification of 64Cu is performed with an in-house-assembled module immediately after EOB (15 to 30 min), where the liquids are pumped with a peristaltic pump at a 0.25 mL/min rate. The irradiated 64Ni targets are first purged with 3 mL of 6.5 M HCl at room temperature to reduce the content of stable metal impurities, followed by target dissolution in 3 mL of 6.5 M HCl at 90 °C. This raw target solution is loaded onto a preconditioned (1 × 2 mL H2O, 2 × 3 mL 6.5 M HCl) 2 mL AG-1x8 column and washed with 4 mL of 6.5 M HCl to recover the 64Ni target material quantitively. The anion exchanger column is then washed with 2 mL of 4 M HCl and 1 mL of H2O to remove the radiocobalt followed by 64Cu elution with 1.5 mL of H2O. This fraction is evaporated to dryness and re-dissolved in 700 µL of H2O to obtain a ready-to-label [64Cu]CuCl2 solution. On average, separation efficiencies of over 70% are obtained.
Upon use, no radionuclide impurity can be quantified through gamma spectroscopy as a consequence of the high 64Cu content. Due to the high enrichment of the target material and considering that there is a 3.5 h gap between EOB and EOP, no other radiocopper isotope can be quantified since they are all shorter-lived than 64Cu. What is more, longer-lived 57Co and 58Co are detected first after at least five 64Cu half-lives.
Moreover, test radiolabeling following the method established by our group [8] proved apparent molar activities (AMA) of up to 2 TBq/µmol for the macrocyclic chelator TETA (1,4,8,11-tetraazacyclotetradecane-1,4,8,11-tetraacetic acid). The obtained yields and AMA are in accordance with results reported from other groups [9,10].
3.2. Targetry and Process Development
3.2.1. [61Cu]CuCl2 Production
Another interesting radiocopper isotope is the positron emitter 61Cu, with a 3.339 h half-life and a higher β+-emission intensity and energy than 64Cu (average energy 500 keV with 61% intensity and 278 keV with 17.5%, respectively) [11]. Moreover, while 64Cu is interesting for the radiolabeling of molecules with slower pharmacokinetics, e.g., antibodies, 61Cu is attractive for tracking faster pharmacokinetics in connection with hypoxia tracers and small proteins [12,13].
In contrast to other most common production routes [14,15,16], we produce copper-61 via the 62Ni(p,2n)61Cu nuclear reaction. One main advantage of the nickel target is its straightforward radiochemical purification [17]. Enriched 62Ni electrodeposition onto gold backings is performed analogously to that of 64Ni, obtaining similar target masses. Proton irradiation at a 70 µA current for 1 to 2 h is performed with the 90° solid-state target configuration. The incident proton energy is modified depending on the target mass not to exceed 16.5 MeV as the exiting energy, in order to reduce the activation of the gold backing. In particular, the incident energy range lies between (18.6 ± 0.1) MeV and (20.8 ± 0.1) MeV. A saturation yield in the range 800–1500 MBq/µA at EOB, for targets with 62Ni masses 50 to 100 mg, has been obtained. It is remarkable that in this case, only 25% of the theoretical yield is achieved [8]. Moreover, activities between 10 GBq and 20 GBq at EOB could be reached [8].
After irradiation, a 1 h decay time is waited to reduce the 62Cu content produced by the 62Ni(p,n)62Cu nuclear reaction. On the other hand, due to the higher radiocobalt production, slight variations of the radiochemical purification method were introduced to that of 64Cu. In particular, two different columns can be used, either the same 2 mL anion exchanger AG-1x8 or a 1 mL TrisKem TK201 resin. A scheme of the radiochemical separation is shown in Figure 5. After purification, between 4 GBq and 8 GBq of a [61Cu]CuCl2 solution are achieved, accounting for 65% to 80% of the 61Cu activity.
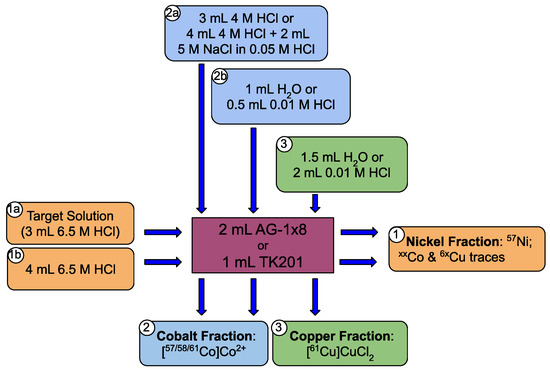
Figure 5.
Radiochemical separation methods used for 61Cu purification from 62Ni targets [8].
The obtained [61Cu]CuCl2 has proven a radionuclidic purity (RNP) of over 99.6% at EOP, i.e., 99.8% at EOB, with 64Cu as the main radio impurity (0.35% at EOP) and minor radiocobalt impurities, 57Co and 58(m)Co (lower than 0.05%) [8]. The detected radiocobalt impurities are comparable to those obtained by other nickel-based methods previously described [12,15].
Moreover, molar activities of up to 600 GBq/µmol at EOP (1280 GBq/µmol at EOB) have been determined by measuring the stable copper content in the product fraction by ICP-MS means and quantifying the activity. In addition, test radiolabeling with the macrocycle chelator TETA has also provided comparable results for the AMA, with values of up to 260 GBq/µmol at EOP. What is more, higher AMAs have been determined in batches containing higher 61Cu activity.
3.2.2. [67Cu]CuCl2 Production
Matched pairs of radionuclides have aroused interest in the last couple of years following the theranostic approach, and in particular, copper radioisotopes show huge potential [18,19,20]. On the one hand, the β+-emitters 61Cu and 64Cu, PET nuclides, are produced with high yields at our institute as described in the previous sections. On the other hand, the β−-emitter 67Cu stands as the therapeutic counterpart. However, due to the low availability of the latter, these truly matched radionuclides cannot be fully exploited.
Starting with some test irradiations in 2021 and following them through 2022, by November of that year, satisfactory activity yields were reached, with a semi-monthly [67Cu]CuCl2 production established. In particular, copper-67 is produced via the 70Zn(p,α)67Cu nuclear reaction; the cross-section of this reaction as well as the energy degraded in the target are shown in Figure 6 (cross-section data from [11]). Proton irradiation is carried out with the 30° solid-state target configuration of the TR-Flex, a 16.8 MeV energy of the proton beam, a 60 µA current and up to 20 h irradiation time (2 days: 7 h and 13 h, decay-corrected effective 17 h). Target production consists of enriched 70Zn electrodeposition from [70Zn]ZnSO4 solutions, pH 2, with (NH4)2SO4 as the electrolyte, onto a rectangular silver substrate. The obtained target masses lie between 115 and 160 mg, i.e., area densities from 100 to 140 mg/cm2. Silver was selected due to its excellent heat conductivity, which ensures a great target cooling, in combination with its acceptable activation. Activity yields of up to 1100 MBq at EOB, i.e., 820 MBq at EOP, were achieved, which corresponds to a saturation yield of 90 MBq/µA for a 140 mg 70Zn target, while the theoretical lies at 165 MBq/µA.
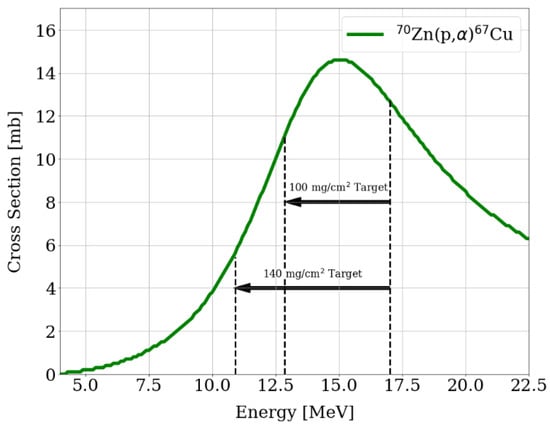
Figure 6.
Cross-section of the 70Zn(p,α)67Cu. Dotted lines: the energy degraded for two different targets’ thicknesses [12].
Work-up of the target is performed 8 to 16 h after EOB to reduce the activity of short-lived radionuclides, e.g., 68Ga. Radiochemical purification is based on a three-step chromatographic separation; First, a 1 mL CU resin is used for the bulk target separation, followed by a 1 mL TK400 resin for 66/67Ga retention, where the radiocopper runs through the column with 8 M HCl. The last step involves a 1 mL TK201 resin, where 70Zn traces are retained and a ready-to-label [67Cu]CuCl2 fraction is obtained, accounting for roughly 85% of the activity of the raw solution (decay-corrected) [21].
The radionuclide content of the produced [67Cu]CuCl2 solution is characterized by gamma spectroscopy, quantifying a RNP of 99.5%, with radionuclide impurities consisting of 64Cu (0.3%), 61Cu (0.03%), 67Ga (0.1%) and 66Ga (0.05%), comparable to previously published results [22]. It is worthwhile mentioning that the 67Ga content is estimated from the detected 66Ga activity, since both 67Cu and 67Ga share the same gamma lines only with different intensities, making it nearly impossible to directly quantify the gallium-67 impurities. Moreover, gamma spectroscopy results of the target raw solution as well as the product fraction are shown in Figure 7.
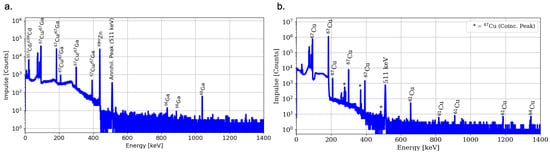
Figure 7.
Gamma spectroscopy of the raw target solution (a) and [67Cu]CuCl2 product fraction (b) [21].
In addition, the molar activity of the product fraction was determined to be up to 250 GBq/µmol by means of ICP-MS and gamma spectroscopy, while an AMA of up to 200 GBq/µmol was quantified for the TETA chelator in the same manner as described for 61Cu and 64Cu.
Due to the high target material costs, the recovery of the 70Zn is of extreme importance, and therefore, [70Zn]Zn(OH)2 precipitation is performed for target recycling, with a recovery yield of over 92%.
Standardization of longer irradiation times and automation of the radiochemical purification are planned to be performed, in order to lead to higher [67Cu]CuCl2 activities and reduce the absorbed dose while performing the work-up of the target.
3.2.3. [67Ga]GaCl3 Production
The theranostic pair 68Ga/177Lu is the most widely used matched set of metallic radionuclides currently [23]. Due to the chemical similarities of both 3+ cations, the PET radionuclide 68Ga has been used as a diagnostic counterpart to the therapeutic β−-emitter 177Lu [24]; however, due to its short half-life (68 min), other alternatives have also been considered [25,26]. In particular 67Ga, with a half-life of 3.26 days, is the longest-lived of the radiogallium isotopes and has γ-lines that can be used for SPECT (93.3 keV—38.8%, 184 keV—21.4%), which makes it possible to perform experiments for longer times. One possible production route is the 68Zn(p,2n)67Ga nuclear reaction, which leads to 68Ga co-production. Taking advantage of the zinc targetry and target chemistry developed for 67Cu production at HZDR, monthly [67Ga]GaCl3 production has also been established.
Target irradiation is performed with 19 MeV protons at a 35 µA current using the 30° solid-state target configuration. These optimized parameters are calculated analogously to the ones for 67Cu production [21]. Target production follows the method described for 67Cu targets, but replaces the enriched 70Zn with enriched 68Zn. In this case, target masses range from 65 to 80 mg (i.e., 55 to 70 mg/cm2). Furthermore, gold backings instead of silver are selected in this case due to the former’s lower activation, since higher proton energies are required than the ones used for 67Cu production. What is more, due to the huge cross-section of the 68Zn(p,2n)67Ga reaction (ca. 700 mb at 19 MeV), the target is not exposed to extreme currents that could damage it. A saturation yield of approximately 3.2 GBq/µA for a 70 mg 68Zn target has been quantified, leading to activities of up to 2 GBq at EOB for a 2 h proton irradiation. This yield accounts for about 70% of the theoretical one calculated, which corresponds with the results obtained for other radionuclides produced at our cyclotron [21,27,28].
After the decay of co-produced 68Ga (12 to 16 h), the target is dissolved in 0.6 mL 6 M HCl. Radiochemical separation is based on a previously described method with some modifications [29]. Basically, after the addition of 1 mL of 8 M HCl to the target raw solution, this is loaded onto a 1 mL TK400 cartridge. Washing of the column is performed with 10.5 mL of 6 M HCl and the first 2.5 mL are collected for 68Zn recovery. The 67Ga is eluted with 4 mL 1.5 M HCl, which is then mixed with 0.6 mL 8 M HCl and loaded onto a 0.3 mL normal DGA column. The column is then washed with 2 mL of 2 M HCl and the elution is performed with 3 × 0.25 mL 0.05 M HCl. An overall purification yield of over 80% has been quantified, with the radioactivity contained in the second and third fractions (0.5 mL). A scheme of this separation method is shown in Figure 8.
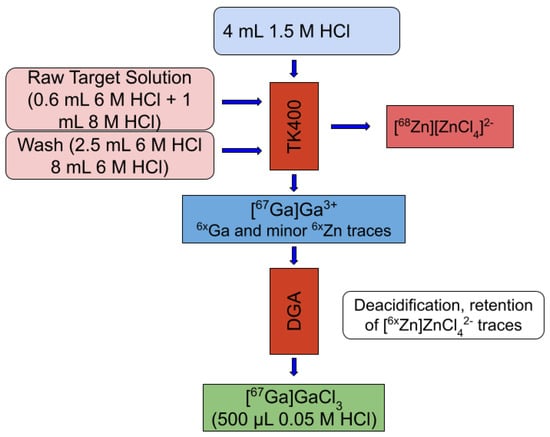
Figure 8.
Radiochemical separation method scheme for 67Ga purification.
Activities of up to 1.3 GBq [67Ga]GaCl3 in 0.5 mL of 0.05 M HCl at EOP are produced. The first 67Ga gamma spectroscopy results showed an RNP of over 98.7% at EOP, with 66Ga as the main and only quantified radioimpurity (<1.3%).
3.2.4. [133La]LaCl3 Production
Targeted Alpha Therapy (TAT) is a research field of highest interest in specialized radionuclide therapy. In particular, the radionuclide 225Ac has piqued the highest interest for TAT since it provides all necessary physical and chemical properties for successful clinical application [30,31]. Although the macropa chelator has shown beneficial properties regarding labeling and stability in vivo as compared with DOTA, the former lacks an imaging counterpart to 225Ac [32]. On the other hand, lanthanum is a perfect surrogate for actinium and, in particular, 133La is an attractive candidate as a diagnostic matched pair to 225Ac due to its imaging properties [28,33,34].
We started the production of 133La in July 2022 following the 134Ba(p,2n)133La nuclear reaction. The target consists of 25 mg of [134Ba]BaCO3 powder contained in a circular silver backing (2 mm thick, 22 mm diameter with a 0.3 mm depth and a 9 mm diameter centered deepening) and is caped with a 10 µm platinum or 100 µm aluminum foil [28]. We irradiate the target in an energy window of (17.9 ± 0.1) MeV up to (18.6 ± 0.1) MeV to minimize the production of further lanthanum isotopes; however, the energy window of (20.4 ± 0.1) MeV up to (21.0 ± 0.1) MeV has also been studied with similar yields and without co-produced 132La detected. The studied energy windows are presented in Figure 9, along with the cross-section of the nuclear reactions that lead to lanthanum nuclides [35]. All the (p,xn) nuclear reactions are weighted for the enriched [134Ba]BaCO3, with the following abundances: 130Ba (0.01%), 132Ba (0.01%), 134Ba (88.10%), 135Ba (5.36%), 136Ba (1.21%), 137Ba (1.07%) and 138Ba (4.26%). Proton currents of up to 35 µA for 15 to 45 min are used, with saturation yields in the (600 ± 100) MBq/µA range leading to activities of ca. 1 GBq at EOB for a 15 min irradiation. What is more, the theoretical saturation yield for such a target is about 1000 MBq/µA.
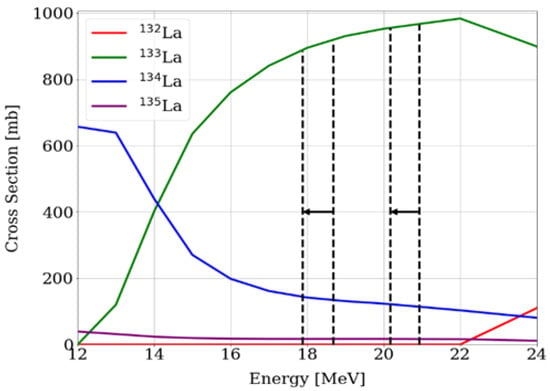
Figure 9.
Cross-section of relevant nuclear reactions leading to lanthanum radionuclides weighted for the [134Ba]BaCO3-enriched target [35]. The energy windows studied are shown with the dotted lines.
Work-up of the target is performed at least 30 min after EOB and takes about another 30 min. Radiochemical separation consists of a single-step chromatographic column with a 2 mL branched diglycolamide (bDGA) cartridge, although alternative chromatographic resins are under investigation. After irradiation, the [134Ba]BaCO3 is dissolved in 3 mL of 1 M HNO3 and loaded onto the cartridge. While the barium educt is collected in the load (3 mL) and the first two washing fractions (10 mL) with 3 M HNO3, the 133La is first eluted with 5 × 1 mL of 0.05 M HCl. Remarkably, up to 85% of the activity is collected in the second fraction (1 mL), while over 95% is recovered when considering the 5 mL. The elution profile of this cartridge is shown in Figure 10.
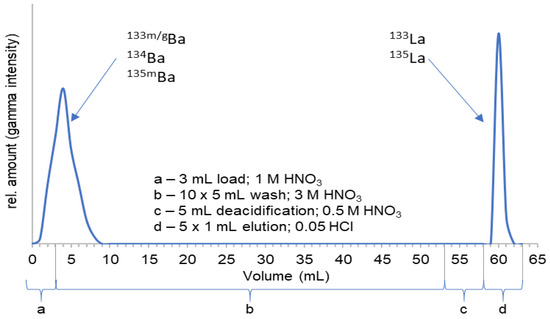
Figure 10.
Elution profile of the used 2 mL bDGA cartridge for the 133La purification [28].
From gamma spectroscopy analysis, an RNP of 99.5% is quantified, with 135La (0.4%), 135mBa (0.03%), 133mBa (0.01%) and 133Ba (0.0004%) impurities calculated at EOB. Additionally, radiolabeling with macropa-functionalized PSMA radioconjugates, previously published by our group, has been performed [28], with an AMA of up to 200 GBq/µmol.
Testing of other chromatographic columns, as well as optimization of the target design, proton current and irradiation time, is being performed to scale up the [133La]LaCl3 production to the 10 GBq range.
3.2.5. [131Ba]Ba(NO3)2 Production
The γ-emitter 131Ba (t½ = 11.5 d) decays via 131Cs (t½ = 9.7 d) to stable 131Xe by electron capture. In particular, the γ-energy 123.8 keV (30%) of the first decay is suitable for SPECT imaging. Moreover, due to the similar chemistry of barium and radium, and since there is no reported true diagnostic counterpart of the therapeutic α-emitters 223Ra and 224Ra, 131Ba is particularly interesting to be used as a surrogate [34,36,37].
Barium-131 is produced by proton irradiation of natural monoisotopically occurring cesium-133 as CsCl via the 133Cs(p,3n)131Ba nuclear reaction. As the target backing, a 2 mm thick and 22 mm diameter platinum disk is used with a 0.2 × 12 mm2 centered circular deepening filled with 80 mg of water-free cesium chloride and covered with a 10 µm thick platinum foil. This target is irradiated with 28 MeV protons at a 20 µA current for 2 to 4 h, depending on radionuclide demand.
The irradiated target is stored overnight after EOB before opening and removal of the cesium salt to avoid high gamma doses from several co-produced short-lived radionuclides in the platinum backing and the chloride. The previously white powder turns black after irradiation and is separated from the backing and foil for processing. The described target shows a saturation yield of (1.2 ± 0.2) GBq/µA, leading to 131Ba activities of 130 to 260 MBq for the described irradiation parameters. The obtained activities are in accordance with the theoretical saturation yield of roughly 2 GBq/µA.
Dissolution of the darkened dry CsCl is performed with 0.5 mL of 3 M HNO3 and loaded onto a pre-conditioned (7 mL H2O, 5 mL 3 M HNO3) SR resin column (ca. 600 mg and 2 mL). Radiochemical separation follows the one-step method previously described by our group [27]. Basically, after loading the dissolved CsCl, the column is washed with 7 mL of 3 M HNO3 to further remove the (radio)cesium. The 131Ba is eluted with 7 × 1 mL of 0.05 M HNO3, with the highest [131Ba]Ba(NO3)2 concentration in the third and fourth fractions. The recovery yield obtained from this separation is about 80% and the produced [131Ba]Ba(NO3)2 can be directly used for radiolabeling [27]. HPGe gamma spectroscopy proved an RNP of over 95% at EOP with 133mBa as the main impurity (4.5%). Due to the longer half-life of 133Ba (10.5 years), some concerns about its co-production may arise. However, after a decay time of 3 months, only 1 kBq of 133Ba is detected for each 7 MBq of 131Ba.
[131Ba]Ba(NO3)2 production takes place two to four times a year, and therefore, currently target work-up is performed manually. However, in the case of an increasing production frequency, automation of the process could be carried out.
4. Discussion
Regarding solid targets, at the HZDR, we are currently performing weekly [64Cu]CuCl2, semi-monthly [67Cu]CuCl2 and monthly [61Cu]CuCl2 productions. Moreover, [67Ga]GaCl3 is produced upon request, approximately once a month. The same applies to our [133La]LaCl3 production, which takes place ca. once every three weeks, and for [131Ba]Ba(NO3)2 twice to four times a year. All of these radionuclides are produced with high RNP and AMA and are used at our institute for the development of novel radiotracers and their characterization in in vitro and even in vivo experiments.
The radionuclides 18F and 11C are produced for the routine production of radiopharmaceuticals on a daily basis. Typical values of produced radionuclides can be found in Table 1.

Table 1.
Typical radionuclides produced at the HZDR with irradiation conditions, activities and saturation yields achieved.
Author Contributions
Conceptualization, M.K., S.A.B. and M.W.; methodology, M.K., S.A.B., T.K. and M.W.; validation, M.K., S.A.B., T.K. and M.W.; investigation, M.K., S.A.B., T.K. and M.W.; resources, M.K., T.K. and K.K.; data curation, M.K., S.A.B., T.K. and M.W.; visualization and writing—original draft preparation, M.K., S.A.B. and T.K.; writing—review and editing, K.K. and M.W.; supervision, M.K., T.K. and M.W.; project administration, M.K. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the TR-Flex Cyclotron technical team, Sandra Hübner, Marcus Lösel, Jens Reinhardt, Jan Roßig and Claudia Steglich. Moreover, we would like to acknowledge the assistance of Christian Jentschel and Jens Reinhardt in the radionuclide purification. Additionally, we would like to acknowledge Steffen Happel from TrisKem for the provision and the recent insights on the chromatographic resins. Furthermore, we would like to thank Uta Czeslik and Diana Walther from VKTA for the ICP-MS measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kreller, M.; Pietzsch, H.J.; Walther, M.; Tietze, H.; Kaever, P.; Knieß, T.; Füchtner, F.; Steinbach, J.; Preusche, S. Introduction of the New Center for Radiopharmaceutical Cancer Research at Helmholtz-Zentrum Dresden-Rossendorf. Instruments 2019, 3, 9. [Google Scholar] [CrossRef]
- Watt, R.; Gyles, W.; Zyuzin, A. Building on TR-24 success: Advanced Cyclotron Systems Inc. launches a new cyclotron model. J. Radioanal. Nucl. Chem. 2015, 305, 93–98. [Google Scholar] [CrossRef]
- Knieß, T.; Kreller, M.; Zessin, J.; Kopka, K. Elektrophile Synthese von 6-L-[18F]FDOPA mit [18F]F2: Ein Statusbericht über das Gastarget am Zyklotron TR-Flex. Nuklearmedizin 2023, 62, 150. [Google Scholar] [CrossRef]
- Antuganov, D.O.; Zykov, M.P.; Ryzhkova, D.V.; Zykova, T.A.; Vinal’ev, A.A.; Antuganova, Y.O.; Samburov, O.P. Synthesis of [18F]-L-DOPA radiopharmaceutical on a modified GE TracerLAB Fx FE platform. Radiochemistry 2016, 58, 649–653. [Google Scholar] [CrossRef]
- Andersen, V.L.; Soerensen, M.A.; Dam, J.H.; Langkjaer, N.; Petersen, H.; Bender, D.A.; Fugloe, D.; Huynh, T.H.V. GMP production of 6-[18F]Fluoro-L-DOPA for PET/CT imaging by different synthetic routes: A three center experience. EJNMMI Radiopharm. Chem. 2021, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Mäding, P.; Zessin, J.; Kreller, M.; Kopka, K.; Knieß, T. Actions for increased yields and easier maintenance at the Tracerlab FXC-pro system in the synthesis of L-[11C]methionine. EJNMMI Radiopharm. Chem. 2023, 8, 14. [Google Scholar]
- Pichler, V.; Vraka, C.; Berroterán-Infante, N.; Krcal, A.; Eidherr, H.; Traub-Weidinger, T.; Hacker, M.; Mitterhauser, M.; Wadsak, W. L-[S-methyl-11C]methionine—An example of radiosynthetic optimization. Appl. Rad. Isot. 2018, 141, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Brühlmann, S.A.; Walther, M.; Kopka, K.; Kreller, M. Production of the PET radionuclide 61Cu via the 62Ni(p,2n)61Cu nuclear reaction. EJNMMI Radiopharm. Chem. 2024, 9, 3. [Google Scholar] [CrossRef]
- Avila-Rodriguez, M.A.; Nye, J.A.; Nickles, R.J. Simultaneous production of high specific activity 64Cu and 61Co with 11.4MeV protons on enriched 64Ni nuclei. Appl. Radiat. Isot. 2007, 65, 1115–1120. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient Production of High Specific Activity 64Cu Using a Biomedical Cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef]
- IAEA. Nuclear Data Services. 2021. Available online: https://www-nds.iaea.org/ (accessed on 1 December 2023).
- McCarthy, D.W.; Bass, L.A.; Cutler, P.; Shefer, R.E.; Klinkowstein, R.E.; Herrero, P.; Lewis, J.S.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. High purity production and potential applications of copper-60 and copper-61. Nucl. Med. Biol. 1999, 26, 351–358. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Niu, G.; Valdovinos, H.F.; Orbay, H.; Nayak, T.R.; Chen, X.; Barnhart, T.E.; Cai, W. Positron Emission Tomography Imaging of Vascular Endothelial Growth Factor Receptor Expression with 61Cu-Labeled Lysine-Tagged VEGF121. Mol. Pharm. 2012, 9, 3586–3594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dellepiane, G.; Casolaro, P.; Mateu, I.; Scampoli, P.; Voeten, N.; Braccini, S. Cross-section measurement for an optimized 61Cu production at an 18 MeV medical cyclotron from natural Zn and enriched 64Zn solid targets. Appl. Radiat. Isot. 2022, 190, 110466. [Google Scholar] [CrossRef]
- Svedjehed, J.; Kutyreff, C.J.; Engle, J.W.; Gagnon, K. Automated, cassette-based isolation and formulation of high-purity [61Cu]CuCl2 from solid Ni targets. EJNMMI Radiopharm. Chem. 2020, 5, 21. [Google Scholar] [CrossRef]
- Alves, V.H.; Carmo, S.J.C.D.; Alves, F.; Abrunhosa, A.J. Automated Purification of Radiometals Produced by Liquid Targets. Instruments 2018, 2, 17. [Google Scholar] [CrossRef]
- Fonseca, A.I.; Carmo, S.J.C.D.; Hrynchak, I.; Alves, V.; Alves, F.; Abrunhosa, A.J. Purification of Copper Radioisotopes for Medical Applications: Chromatographic Methods and Challenges. Sep. Purif. Rev. 2023, 1–22. [Google Scholar] [CrossRef]
- Hussain, M.; Qaim, S.M.; Spahn, I.; Aslam, M.N.; Neumaier, B. Copper radionuclides for theranostic applications: Towards standardisation of their nuclear data. A mini-review. Front. Chem. 2023, 11, 1270351. [Google Scholar] [CrossRef]
- Mou, L.; Martini, P.; Pupillo, G.; Cieszykowska, I.; Cutler, C.S.; Mikołajczak, R. 67Cu Production Capabilities: A Mini Review. Molecules 2022, 27, 1501. [Google Scholar] [CrossRef]
- IAEA. Copper-64 Radiopharmaceuticals: Production, Quality Control and Clinical Applications; IAEA: Vienna, Austria, 2023. [Google Scholar]
- Brühlmann, S.A.; Walther, M.; Kreller, M.; Reissig, F.; Pietzsch, H.-J.; Kniess, T.; Kopka, K. Cyclotron-Based Production of 67Cu for Radionuclide Theranostics via the 70Zn(p,α)67Cu Reaction. Pharmaceuticals 2023, 16, 314. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chae, J.H.; Hur, M.G.; Yang, S.D.; Kong, Y.B.; Lee, J.; Ju, J.S.; Choi, P.S.; Park, J.H. Theragnostic 64Cu/67Cu Radioisotopes Production With RFT-30 Cyclotron. Front. Med. 2022, 9, 889640. [Google Scholar] [CrossRef]
- Morgan, K.A.; Rudd, S.E.; Noor, A.; Donnelly, P.S. Theranostic Nuclear Medicine with Gallium-68, Lutetium-177, Copper-64/67, Actinium-225, and Lead-212/203 Radionuclides. Chem. Rev. 2023, 123, 12004–12035. [Google Scholar] [CrossRef] [PubMed]
- Engle, J.; Lopez-Rodriguez, V.; Gaspar-Carcamo, R.; Valdovinos, H.; Valle-Gonzalez, M.; Trejo-Ballado, F.; Severin, G.; Barnhart, T.; Nickles, R.; Avila-Rodriguez, M. Very high specific activity 66/68Ga from zinc targets for PET. Appl. Radiat. Isot. 2012, 70, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Price, E.W.; Zeglis, B.M.; Cawthray, J.F.; Ramogida, C.F.; Ramos, N.; Lewis, J.S.; Adam, M.J.; Orvig, C. H4octapa-trastuzumab: Versatile acyclic chelate system for 111In and 177Lu imaging and therapy. J. Am. Chem. Soc. 2013, 135, 12707–12721. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jaraquemada-Peláez, M.d.G.; Kuo, H.-T.; Merkens, H.; Choudhary, N.; Gitschtaler, K.; Jermilova, U.; Colpo, N.; Uribe-Munoz, C.; Radchenko, V.; et al. Functionally versatile and highly stable chelator for 111In and 177Lu: Proof-of-principle prostate-specific membrane antigen targeting. Bioconjugate Chem. 2019, 30, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Reissig, F.; Bauer, D.; Ullrich, M.; Kreller, M.; Pietzsch, J.; Mamat, C.; Kopka, K.; Pietzsch, H.-J.; Walther, M. Recent insights in barium-131 as a diagnostic match for radium-223: Cyclotron production, separation, radiolabeling, and imaging. Pharmaceuticals 2020, 13, 272. [Google Scholar] [CrossRef]
- Brühlmann, S.; Kreller, M.; Pietzsch, H.-J.; Kopka, K.; Mamat, C.; Walther, M.; Reissig, F. Efficient Production of the PET Radionuclide 133La for Theranostic Purposes in Targeted Alpha Therapy Using the 134Ba(p,2n)133La Reaction. Pharmaceuticals 2022, 15, 1167. [Google Scholar] [CrossRef]
- Svedjehed, J.; Pärnaste, M.; Gagnon, K. Demystifying solid targets: Simple and rapid distribution-scale production of [68Ga] GaCl3 and [68Ga] Ga-PSMA-11. Nucl. Med. Biol. 2022, 104, 1–10. [Google Scholar] [CrossRef]
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted Alpha Therapy: Current Clinical Applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49. [Google Scholar] [CrossRef]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 14712–14717. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. High yield cyclotron production of a novel 133/135La theranostic pair for nuclear medicine. Sci. Rep. 2020, 10, 22203. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications. Pharmaceuticals 2023, 16, 1622. [Google Scholar] [CrossRef] [PubMed]
- Proton Sub-Library-TENDL-2019. TENDL. (1 November 2019). Available online: https://tendl.web.psi.ch/tendl_2019/proton_html/Ba/ProtonBa.html (accessed on 30 July 2022).
- Spencer, R.P.; Lange, R.C.; Treves, S. Use of Ba-135m and Ba-131 as Bone-Scanning Agents. J. Nucl. Med. 1971, 12, 216–221. [Google Scholar] [PubMed]
- Salutsky, M.L.; Kirby, H.W. The Radiochemistry of Radium; National Bureau of Standards, U.S. Department of Commerce: Springfield, VA, USA, 1964; p. 210. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).