Exploring the Ultrafast Charge-Transfer and Redox Dynamics in Layered Transition Metal Oxides
Abstract
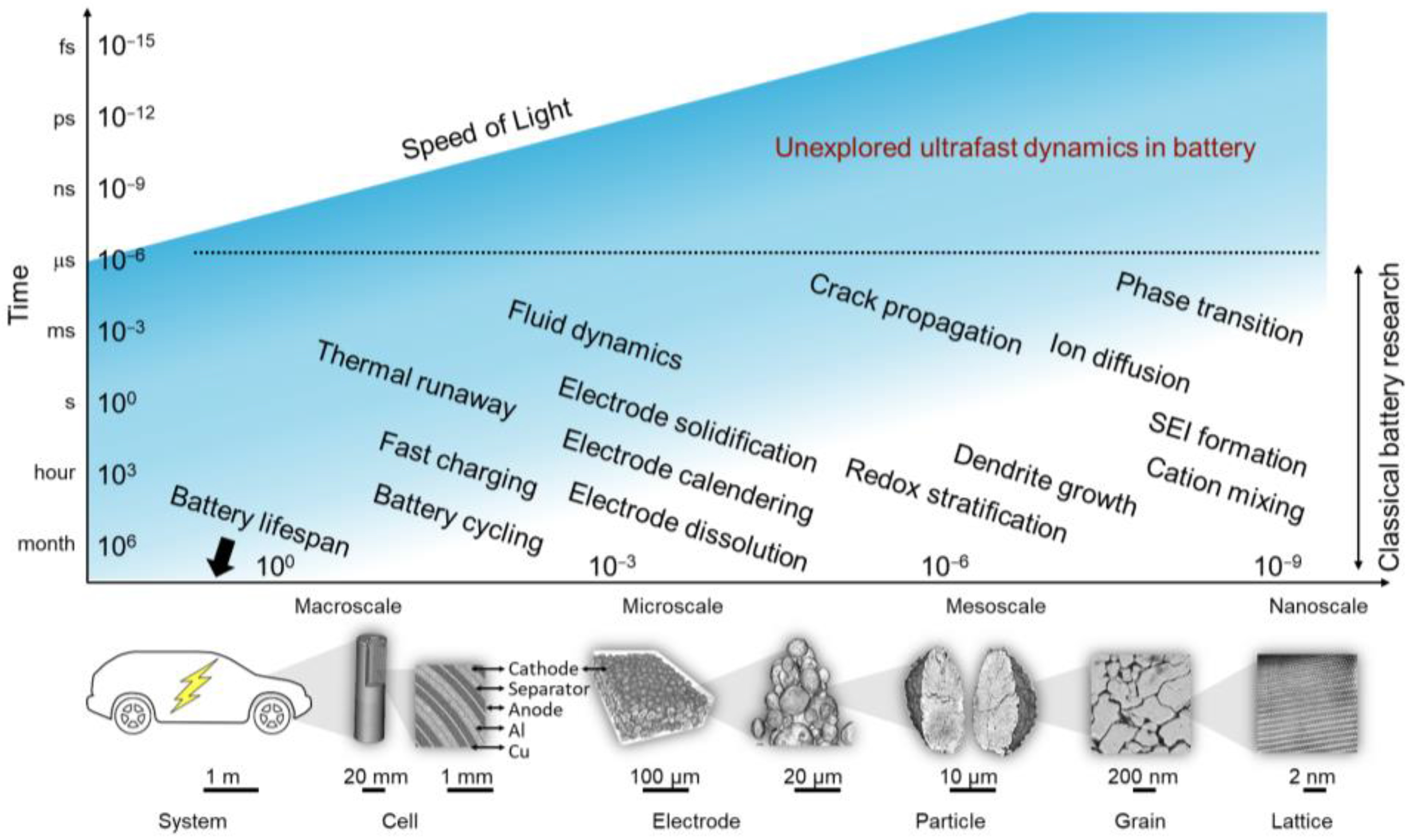
1. Introduction
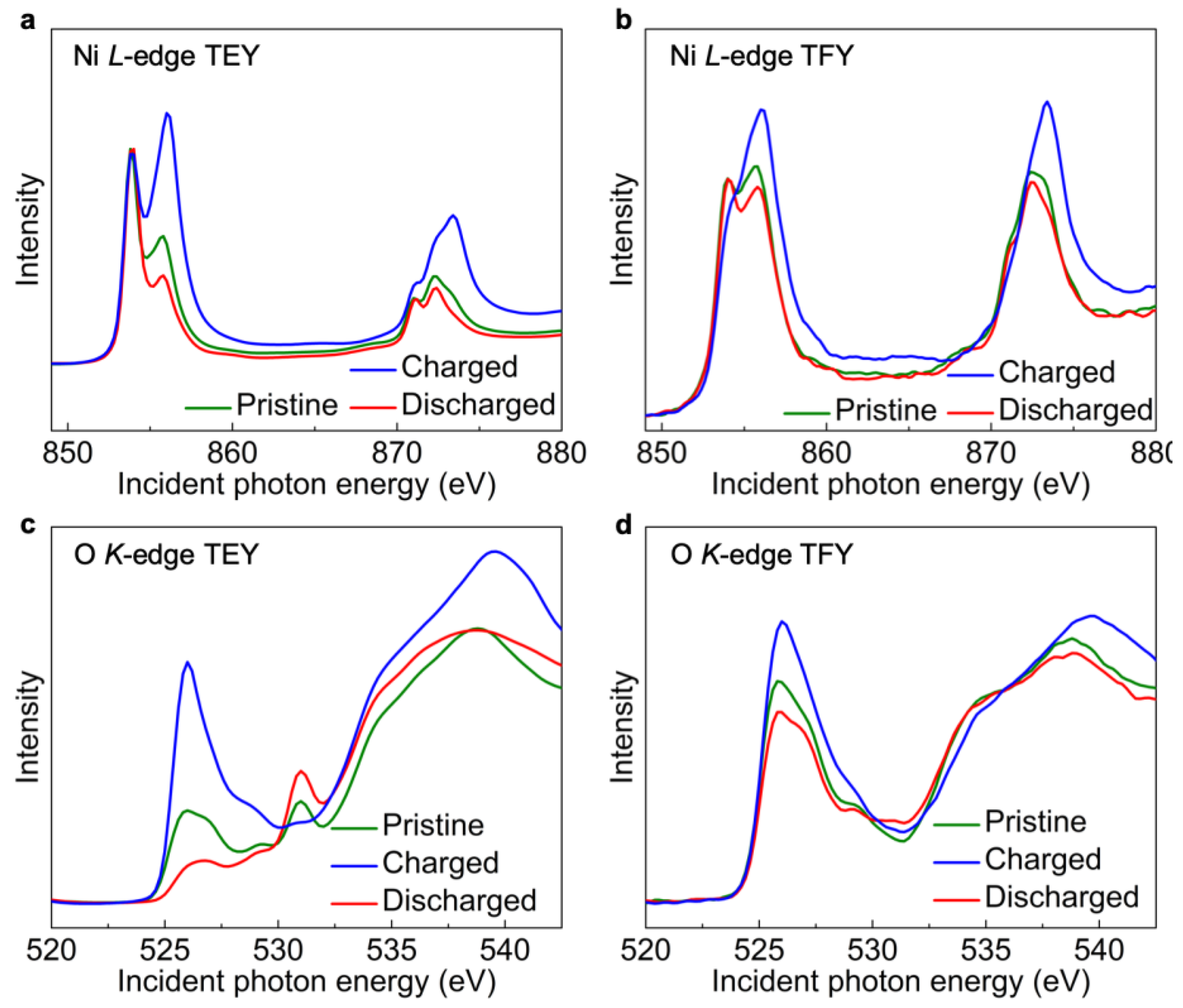
2. X-ray Spectroscopy for Probing the Co-Existing Redox Centers in Battery Cathodes
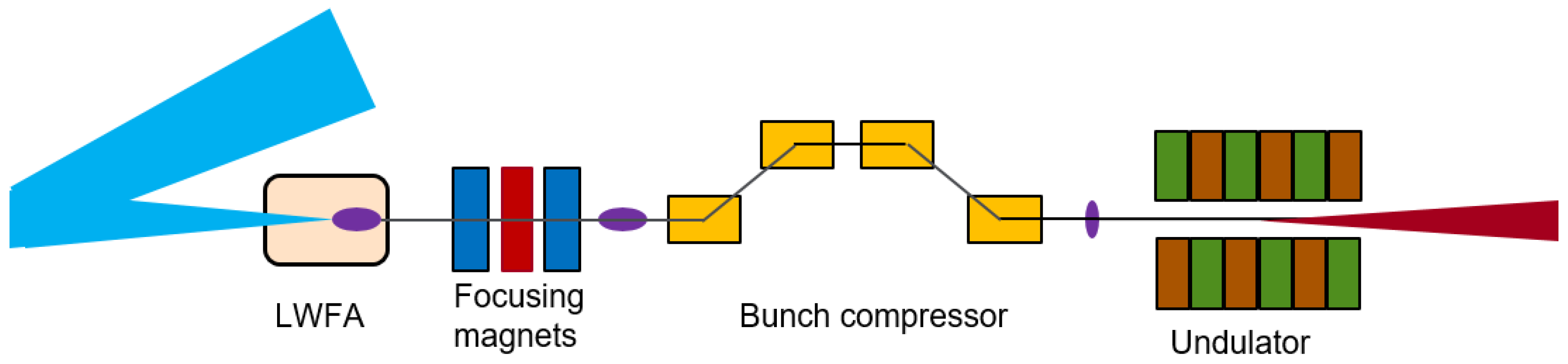
3. The Opportunities Enabled by Plasma-Acceleration-Based FEL
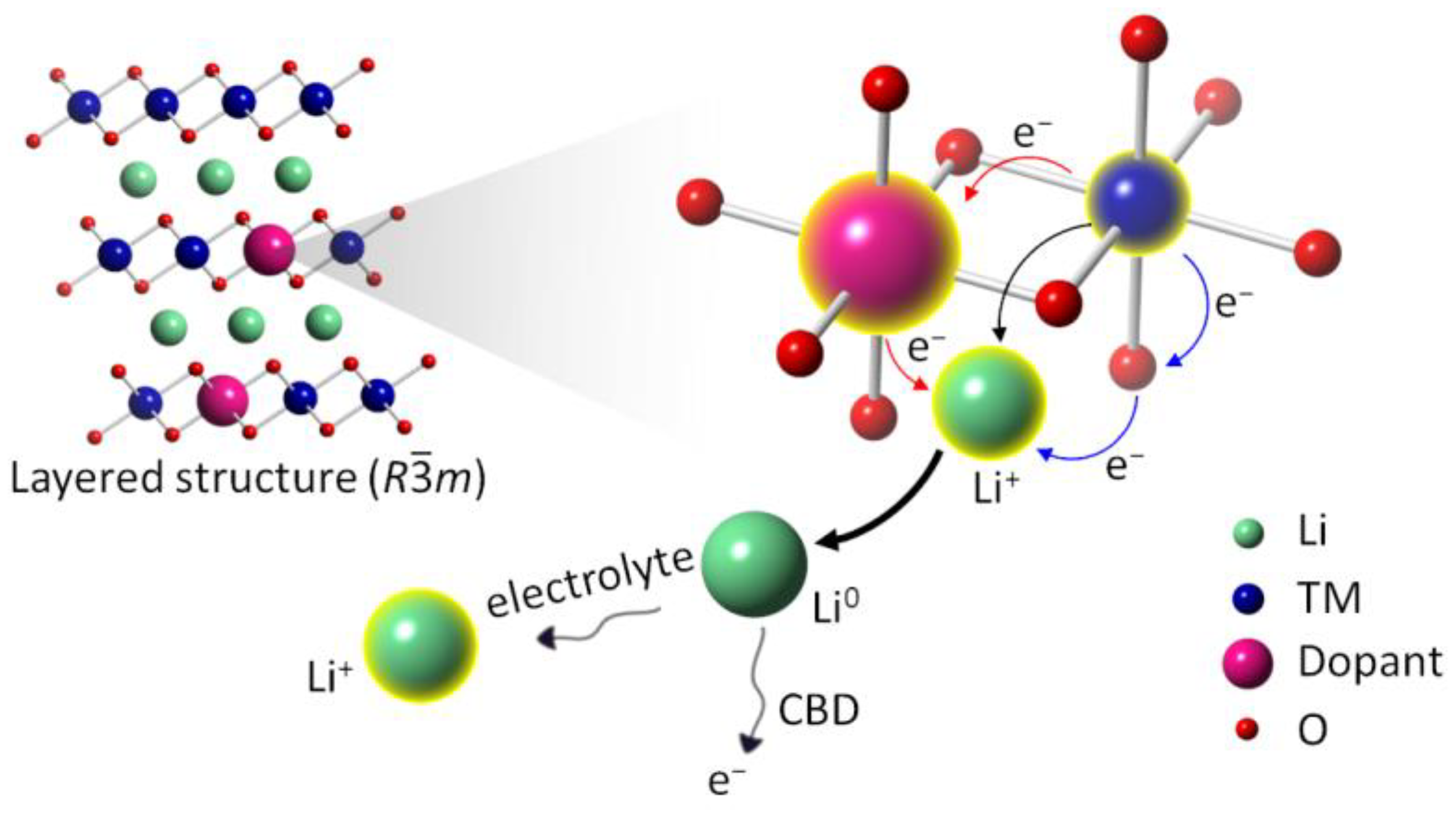
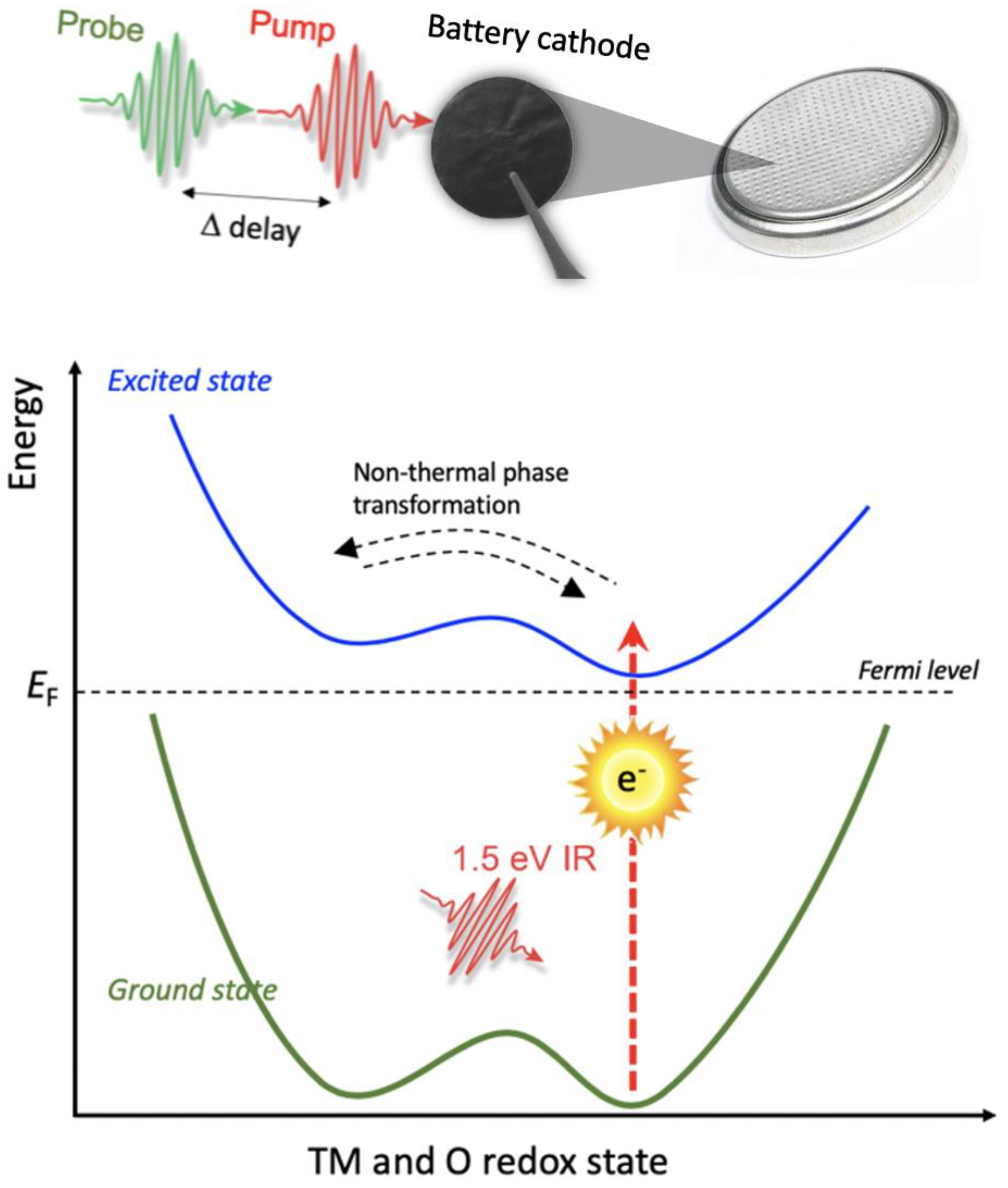
4. The Hypothesis: Ultrafast Charge Transfer among Different Redox-Active Elements
5. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lin, F. Oxygen Redox Chemistry in Rechargeable Li-Ion and Na-Ion Batteries. Matter 2021, 4, 490–527. [Google Scholar] [CrossRef]
- Lin, F.; Liu, Y.; Yu, X.; Cheng, L.; Singer, A.; Shpyrko, O.G.; Xin, H.L.; Tamura, N.; Tian, C.; Weng, T.-C.; et al. Synchrotron X-ray Analytical Techniques for Studying Materials Electrochemistry in Rechargeable Batteries. Chem. Rev. 2017, 117, 13123–13186. [Google Scholar] [CrossRef]
- Ament, L.J.P.; van Veenendaal, M.; Devereaux, T.P.; Hill, J.P.; van den Brink, J. Resonant inelastic x-ray scattering studies of elementary excitations. Rev. Mod. Phys. 2011, 83, 705–767. [Google Scholar] [CrossRef]
- Qian, G.; Wang, J.; Li, H.; Ma, Z.-F.; Pianetta, P.; Li, L.; Yu, X.; Liu, Y. Structural and chemical evolution in layered oxide cathodes of lithium-ion batteries revealed by synchrotron techniques. Natl. Sci. Rev. 2021, 9, nwab146. [Google Scholar] [CrossRef]
- Lin, F.; Nordlund, D.; Markus, I.M.; Weng, T.-C.; Xin, H.L.; Doeff, M.M. Profiling the nanoscale gradient in stoichiometric layered cathode particles for lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3077–3085. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Li, L.; Zhang, R.; Xu, J.; Cheng, Z.; Xie, S.; Wang, H.; Rao, Q.; He, Y.; et al. Single-crystal nickel-rich layered-oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 2020, 27, 140–149. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhou, Y.; Du, C.; Zuo, P.; Cheng, X.; Han, L.; Nordlund, D.; Gao, Y.; Yin, G.; Xin, H.L.; et al. A New Anion Receptor for Improving the Interface between Lithium- and Manganese-Rich Layered Oxide Cathode and the Electrolyte. Chem. Mater. 2017, 29, 2141–2149. [Google Scholar] [CrossRef]
- Li, S.; Lee, S.-J.; Wang, X.; Yang, W.; Huang, H.; Swetz, D.S.; Doriese, W.B.; O’Neil, G.C.; Ullom, J.N.; Titus, C.J.; et al. Surface-to-Bulk Redox Coupling through Thermally Driven Li Redistribution in Li- and Mn-Rich Layered Cathode Materials. J. Am. Chem. Soc. 2019, 141, 12079–12086. [Google Scholar] [CrossRef]
- Emma, C.; Xu, X.; Fisher, A.; Robles, R.; MacArthur, J.P.; Cryan, J.; Hogan, M.J.; Musumeci, P.; White, G.; Marinelli, A. Terawatt attosecond x-ray source driven by a plasma accelerator. APL Photonics 2021, 6, 076107. [Google Scholar] [CrossRef]
- Emma, P.; Akre, R.; Arthur, J.; Bionta, R.; Bostedt, C.; Bozek, J.; Brachmann, A.; Bucksbaum, P.; Coffee, R.; Decker, F.J.; et al. First lasing and operation of an ångstrom-wavelength free-electron laser. Nat. Photonics 2010, 4, 641–647. [Google Scholar] [CrossRef]
- Bonifacio, R.; Pellegrini, C.; Narducci, L.M. Collective instabilities and high-gain regime in a free electron laser. Opt. Commun. 1984, 50, 373–378. [Google Scholar] [CrossRef]
- Ding, Y.; Behrens, C.; Coffee, R.; Decker, F.J.; Emma, P.; Field, C.; Helml, W.; Huang, Z.; Krejcik, P.; Krzywinski, J.; et al. Generating femtosecond X-ray pulses using an emittance-spoiling foil in free-electron lasers. Appl. Phys. Lett. 2015, 107, 191104. [Google Scholar] [CrossRef]
- Huang, S.; Ding, Y.; Feng, Y.; Hemsing, E.; Huang, Z.; Krzywinski, J.; Lutman, A.A.; Marinelli, A.; Maxwell, T.J.; Zhu, D. Generating Single-Spike Hard X-Ray Pulses with Nonlinear Bunch Compression in Free-Electron Lasers. Phys. Rev. Lett. 2017, 119, 154801. [Google Scholar] [CrossRef]
- Marinelli, A.; MacArthur, J.; Emma, P.; Guetg, M.; Field, C.; Kharakh, D.; Lutman, A.A.; Ding, Y.; Huang, Z. Experimental demonstration of a single-spike hard-X-ray free-electron laser starting from noise. Appl. Phys. Lett. 2017, 111, 151101. [Google Scholar] [CrossRef]
- Duris, J.; Li, S.; Driver, T.; Champenois, E.G.; MacArthur, J.P.; Lutman, A.A.; Zhang, Z.; Rosenberger, P.; Aldrich, J.W.; Coffee, R.; et al. Tunable isolated attosecond X-ray pulses with gigawatt peak power from a free-electron laser. Nat. Photonics 2020, 14, 30–36. [Google Scholar] [CrossRef]
- McPherson, A.; Gibson, G.; Jara, H.; Johann, U.; Luk, T.S.; McIntyre, I.A.; Boyer, K.; Rhodes, C.K. Studies of multiphoton production of vacuum-ultraviolet radiation in the rare gases. J. Opt. Soc. Am. B 1987, 4, 595–601. [Google Scholar] [CrossRef]
- Ferray, M.; Huillier, A.L.; Li, X.F.; Lompre, L.A.; Mainfray, G.; Manus, C. Multiple-harmonic conversion of 1064 nm radiation in rare gases. J. Phys. B At. Mol. Opt. Phys. 1988, 21, L31. [Google Scholar] [CrossRef]
- Li, X.F.; L’Huillier, A.; Ferray, M.; Lompré, L.A.; Mainfray, G. Multiple-harmonic generation in rare gases at high laser intensity. Phys. Rev. A 1989, 39, 5751–5761. [Google Scholar] [CrossRef]
- Teichmann, S.M.; Silva, F.; Cousin, S.L.; Hemmer, M.; Biegert, J. 0.5-keV Soft X-ray attosecond continua. Nat. Commun. 2016, 7, 11493. [Google Scholar] [CrossRef]
- Gaumnitz, T.; Jain, A.; Pertot, Y.; Huppert, M.; Jordan, I.; Ardana-Lamas, F.; Wörner, H.J. Streaking of 43-attosecond soft-X-ray pulses generated by a passively CEP-stable mid-infrared driver. Opt. Express 2017, 25, 27506–27518. [Google Scholar] [CrossRef]
- Rosenzweig, J.B.; Cline, D.B.; Cole, B.; Figueroa, H.; Gai, W.; Konecny, R.; Norem, J.; Schoessow, P.; Simpson, J. Experimental Observation of Plasma Wake-Field Acceleration. Phys. Rev. Lett. 1988, 61, 98–101. [Google Scholar] [CrossRef]
- Rosenzweig, J.B.; Breizman, B.; Katsouleas, T.; Su, J.J. Acceleration and focusing of electrons in two-dimensional nonlinear plasma wake fields. Phys. Rev. A 1991, 44, R6189–R6192. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.J.; Assmann, R.; Decker, F.J.; Iverson, R.; Raimondi, P.; Rokni, S.; Siemann, R.H.; Walz, D.; Whittum, D.; Blue, B.; et al. E-157: A 1.4-m-long plasma wake field acceleration experiment using a 30 GeV electron beam from the Stanford Linear Accelerator Center Linac. Phys. Plasmas 2000, 7, 2241–2248. [Google Scholar] [CrossRef]
- Hogan, M.J.; Barnes, C.D.; Clayton, C.E.; Decker, F.J.; Deng, S.; Emma, P.; Huang, C.; Iverson, R.H.; Johnson, D.K.; Joshi, C.; et al. Multi-GeV Energy Gain in a Plasma-Wakefield Accelerator. Phys. Rev. Lett. 2005, 95, 054802. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Dawson, J.M. Laser Electron Accelerator. Phys. Rev. Lett. 1979, 43, 267–270. [Google Scholar] [CrossRef]
- Pukhov, A.; Meyer-ter-Vehn, J. Laser wake field acceleration: The highly non-linear broken-wave regime. Appl. Phys. B 2002, 74, 355–361. [Google Scholar] [CrossRef]
- Leemans, W.P.; Nagler, B.; Gonsalves, A.J.; Tóth, C.; Nakamura, K.; Geddes, C.G.R.; Esarey, E.; Schroeder, C.B.; Hooker, S.M. GeV electron beams from a centimetre-scale accelerator. Nat. Phys. 2006, 2, 696–699. [Google Scholar] [CrossRef]
- Grüner, F.; Becker, S.; Schramm, U.; Eichner, T.; Fuchs, M.; Weingartner, R.; Habs, D.; Meyer-ter-Vehn, J.; Geissler, M.; Ferrario, M.; et al. Design considerations for table-top, laser-based VUV and X-ray free electron lasers. Appl. Phys. B 2007, 86, 431–435. [Google Scholar] [CrossRef]
- Nakajima, K. Towards a table-top free-electron laser. Nat. Phys. 2008, 4, 92–93. [Google Scholar] [CrossRef]
- Couprie, M.E.; Loulergue, A.; Labat, M.; Lehe, R.; Malka, V. Towards a free electron laser based on laser plasma accelerators. J. Phys. B At. Mol. Opt. Phys. 2014, 47, 234001. [Google Scholar] [CrossRef]
- van Tilborg, J.; Barber, S.K.; Isono, F.; Schroeder, C.B.; Esarey, E.; Leemans, W.P. Free-electron lasers driven by laser plasma accelerators. AIP Conf. Proc. 2017, 1812, 020002. [Google Scholar] [CrossRef]
- Esarey, E.; Schroeder, C.B.; Leemans, W.P. Physics of laser-driven plasma-based electron accelerators. Rev. Mod. Phys. 2009, 81, 1229–1285. [Google Scholar] [CrossRef]
- Blaj, G.; Carini, G.; Carron, S.; Haller, G.; Hart, P.; Hasi, J.; Herrmann, S.; Kenney, C.; Segal, J.; Stan, C. Detector Damage at X-ray free-electron laser sources. IEEE Trans. Nucl. Sci. 2016, 63, 1818–1826. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Yang, Y.; Mu, L.; Wei, C.; Yu, X.; Pianetta, P.; Zhao, K.; Cloetens, P.; Lin, F.; et al. Machine-learning-revealed statistics of the particle-carbon/binder detachment in lithium-ion battery cathodes. Nat. Commun. 2020, 11, 2310. [Google Scholar] [CrossRef]
- Qian, G.; Monaco, F.; Meng, D.; Lee, S.-J.; Zan, G.; Li, J.; Karpov, D.; Gul, S.; Vine, D.; Stripe, B.; et al. The role of structural defects in commercial lithium-ion batteries. Cell Rep. Phys. Sci. 2021, 2, 100554. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, G.; Huang, X.; Lee, J.-S.; Pianetta, P.; Liu, Y. Exploring the Ultrafast Charge-Transfer and Redox Dynamics in Layered Transition Metal Oxides. Condens. Matter 2023, 8, 25. https://doi.org/10.3390/condmat8010025
Qian G, Huang X, Lee J-S, Pianetta P, Liu Y. Exploring the Ultrafast Charge-Transfer and Redox Dynamics in Layered Transition Metal Oxides. Condensed Matter. 2023; 8(1):25. https://doi.org/10.3390/condmat8010025
Chicago/Turabian StyleQian, Guannan, Xiaobiao Huang, Jun-Sik Lee, Piero Pianetta, and Yijin Liu. 2023. "Exploring the Ultrafast Charge-Transfer and Redox Dynamics in Layered Transition Metal Oxides" Condensed Matter 8, no. 1: 25. https://doi.org/10.3390/condmat8010025
APA StyleQian, G., Huang, X., Lee, J.-S., Pianetta, P., & Liu, Y. (2023). Exploring the Ultrafast Charge-Transfer and Redox Dynamics in Layered Transition Metal Oxides. Condensed Matter, 8(1), 25. https://doi.org/10.3390/condmat8010025








