High-Energy X-Ray Compton Scattering Imaging of 18650-Type Lithium-Ion Battery Cell
Abstract
1. Introduction
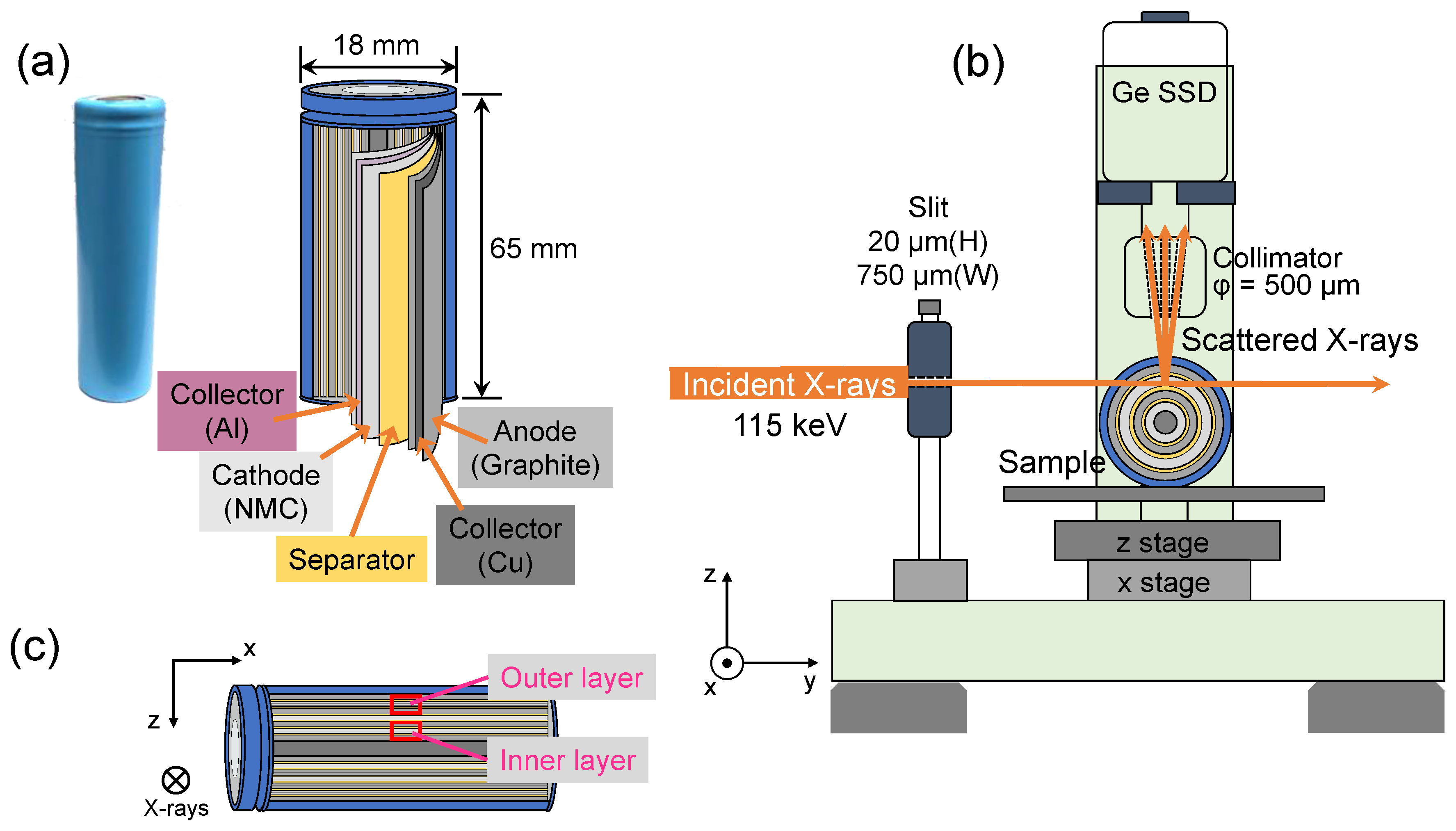
2. Experimental
2.1. Commercial 18650-Type Lithium-Ion Cells
2.2. Compton Scattering Experiment and Data Analysis
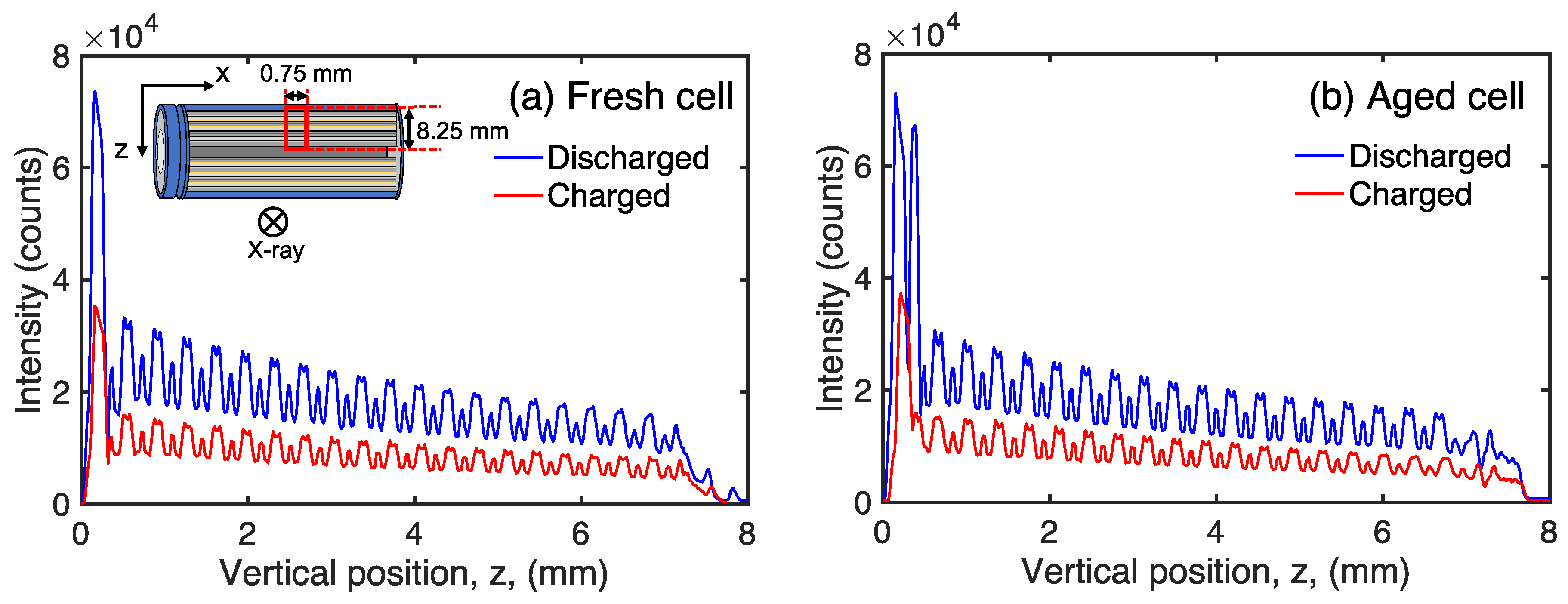
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- XCOM. Available online: https://physics.nist.gov/PhysRefData/Xcom/html/xcom1.html (accessed on 9 April 2019).
- Schülke, W. The theory of Compton scattering. In X-ray Compton Scattering, 1st ed.; Cooper, M.J., Mijnarends, P.E., Shiotani, N., Sakai, N., Bansil, A., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 22–81. [Google Scholar]
- Barbiellini, B. A natural orbital method for the electron momentum distribution in matter. J. Phys. Chem. Solids 2000, 61, 341–344. [Google Scholar] [CrossRef]
- Barbiellini, B.; Bansil, A. Treatment of correlation effects in electron momentum density: Density functional theory and beyond. J. Phys. Chem. Solids 2001, 62, 2181–2189. [Google Scholar] [CrossRef]
- Suzuki, K.; Barbiellini, B.; Orikasa, Y.; Go, N.; Sakurai, H.; Kaprzyk, S.; Itou, M.; Yamamoto, K.; Uchimoto, Y.; Wang, Y.J.; et al. Extracting the Redox Orbitals in Li Battery Materials with High-Resolution X-ray Compton Scattering Spectroscopy. Phys. Rev. Lett. 2015, 114, 087401. [Google Scholar] [CrossRef] [PubMed]
- Barbiellini, B.; Suzuki, K.; Orikasa, Y.; Kaprzyk, S.; Itou, M.; Yamamoto, K.; Wang, Y.J.; Hafiz, H.; Yamada, R.; Uchimoto, Y.; et al. Identifying a descriptor for d-orbital delocalization in cathodes of Li batteries based on X-ray Compton scattering. J. Appl. Phys. 2016, 109, 073102. [Google Scholar] [CrossRef]
- Hafiz, H.; Suzuki, K.; Barbiellini, B.; Orikasa, Y.; Callewaert, V.; Kaprzyk, S.; Itou, M.; Yamamoto, K.; Yamada, R.; Uchimoto, Y.; et al. Visualizing redox orbitals and their potentials in advanced lithium-ion battery materials using high-resolution X-ray Compton scattering. Sci. Adv. 2017, 3, e1700971. [Google Scholar] [CrossRef] [PubMed]
- Itou, M.; Orikasa, Y.; Gogyo, Y.; Suzuki, K.; Sakurai, H.; Uchimoto, Y.; Sakurai, Y. Compton scattering imaging of a working battery using synchrotron haigh-energy X-rays. J. Synchrotron Radiat. 2015, 22, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Barbiellini, B.; Orikasa, Y.; Kaprzyk, S.; Itou, M.; Yamamoto, K.; Wang, Y.J.; Hafiz, H.; Uchimoto, Y.; Bansil, A.; et al. Non-destructive measurement of in-operando lithium concentration in batteries via X-ray Compton scattering. J. Appl. Phys. 2016, 119, 025103. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, A.; Ishikawa, T.; Itou, M.; Yamashige, H.; Orikasa, Y.; Uchimoto, Y.; Sakurai, Y.; Sakurai, H. In-operando quantitation of Li concentration for commercial Li-ion rechargeable battery using high-energy X-ray Compton scattering. J. Synchrotron Radiat. 2017, 24, 1006. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kanai, R.; Tsuji, N.; Yamashige, H.; Orikasa, Y.; Uchimoto, Y.; Sakurai, Y.; Sakurai, H. Dependency of the charge-discharge rate on lithium reaction distributions for a commercial lithium coin cell visualized by Compton scattering imaging. Condens. Matter. 2018, 3, 27. [Google Scholar] [CrossRef]
- Harks, P.P.R.K.L.; Mulder, F.M.; Notten, P.H.L. In situ methods for Li-ion battery research: A review of recent development. J. Power Sources 2015, 288, 92–105. [Google Scholar] [CrossRef]
- He, H.; Liu, B.; Abouimrane, A.; Ren, Y.; Liu, Y.; Liu, Q.; Chao, Z.S. Dynamic lithium intercalation/deintercalation in 18650 lithium ion battery by time-resolved high energy synchrotron X-ray diffraction. J. Electrochem. Soc. 2015, 162, 2195–2200. [Google Scholar] [CrossRef]
- Yao, K.P.C.; Okasinski, J.S.; Kalaga, K.; Shkrob, I.A.; Abraham, D.P. Quantifying lithium concentration gradients in the graphite electrode of Li-ion cells using operando energy dispersive X-ray diffraction. Energy Environ. Sci. 2019, 12, 656. [Google Scholar] [CrossRef]
- Finegan, D.O.; Tudisco, E.; Scheel, M.; Robinson, J.B.; Taiwo, O.O.; Eastwood, D.S.; Lee, P.D.; Michiel, M.D.; Bay, B.; Hall, S.A.; et al. Quantifying bulk electrode strain and material displacement within lithium batteries via high-speed operando tomography and digital volume correlation. Adv. Sci. 2016, 3, 1500332. [Google Scholar] [CrossRef]
- Shiotani, S.; Naka, T.; Morishima, M.; Yonemura, M.; Kamiyama, T.; Ishikawa, Y.; Ukyo, Y.; Uchimoto, Y.; Ogumi, Z. Degradation analysis of 18650-type lithium-ion cells by operando neutron diffraction. J. Power Sources 2016, 325, 404–409. [Google Scholar] [CrossRef]
- Paul, N.; Keil, J.; Kindermann, F.M.; Schebesta, S.; Dolotko, O.; Mühlbauer, M.J.; Kraft, L.; Erhard, S.V.; Jossen, A.; Gilles, R. Aging in 18650-type Li-ion cells examined with neutron diffraction, electrochemical analysis and physico-chemical modering. J. Energy Storage 2018, 17, 383–394. [Google Scholar] [CrossRef]
- Biggs, F.; Mendelson, L.B.; Mann, J.B. Hartree-Fock Compton profiles for the elements. At. Data Nucl. Data Tables 1975, 16, 201. [Google Scholar] [CrossRef]
- Cheng, J.; Li, X.; Wang, Z.; Guo, H. Mechanism for capacity fading of 18650 cylindrical lithium ion batteries. Trans. Nonferrous Met. Soc. China 2017, 27, 1602–1607. [Google Scholar] [CrossRef]
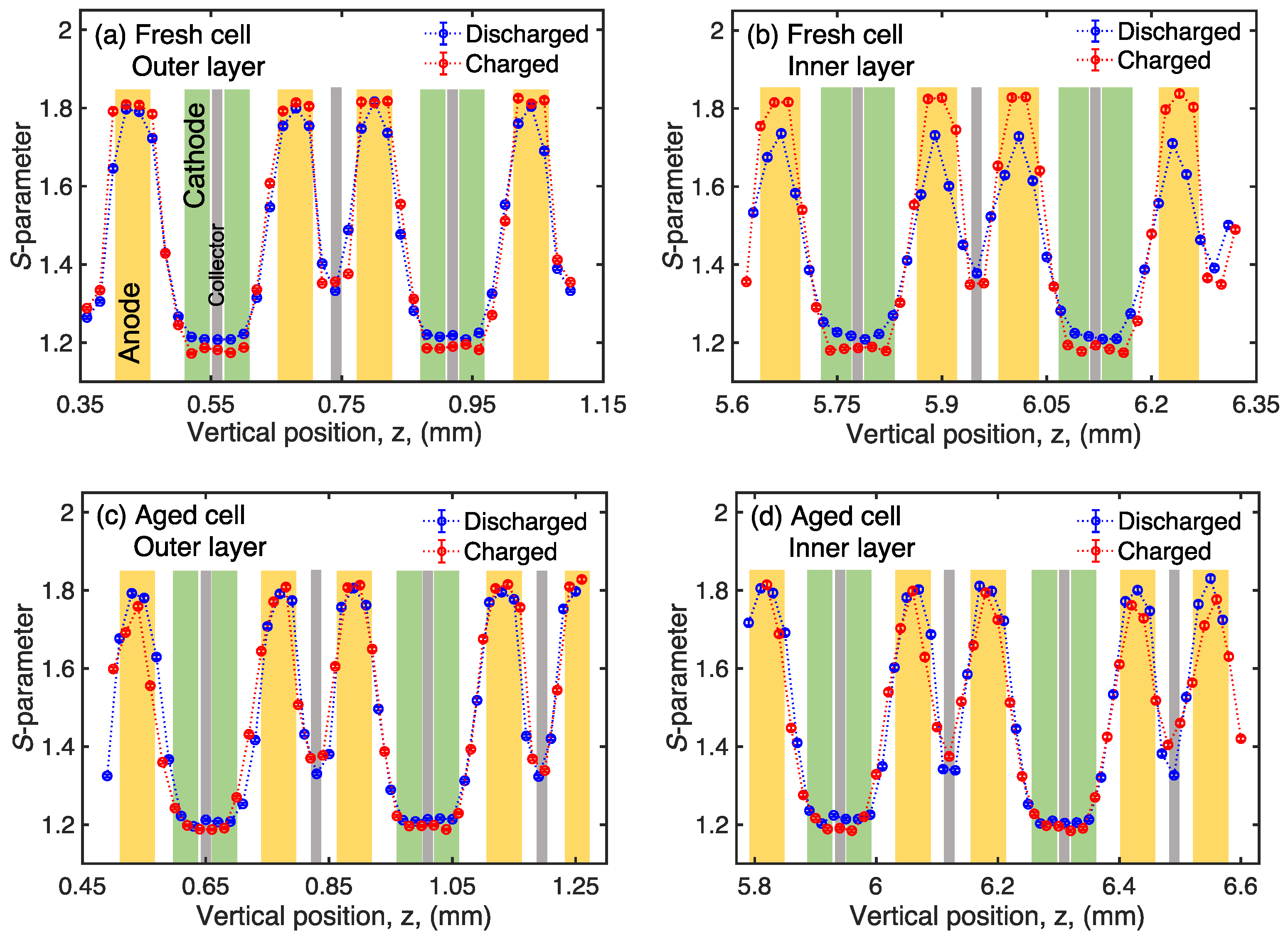
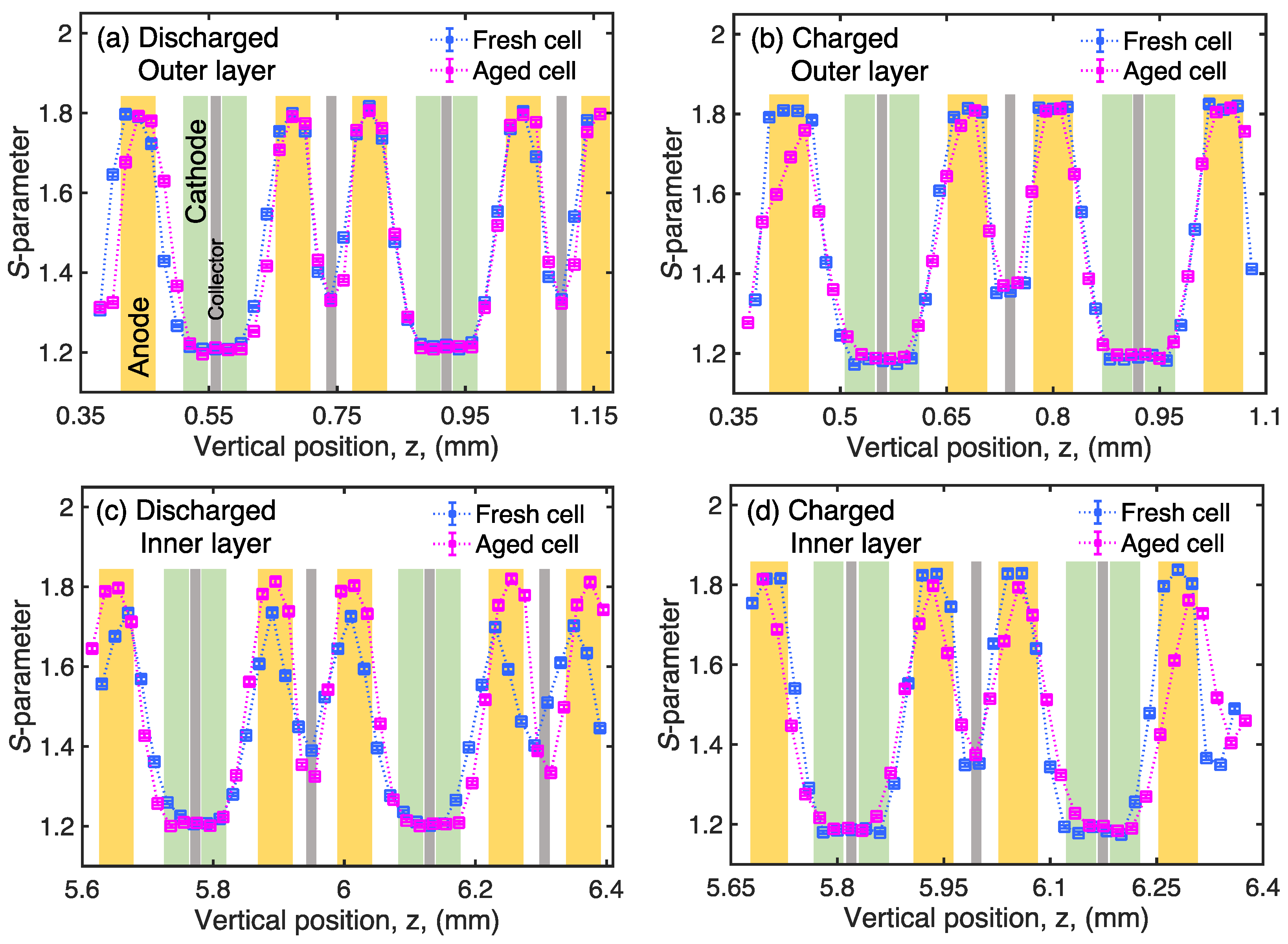
| Discharged | Charged | Relative Change | ||
|---|---|---|---|---|
| (a) Fresh cell Outer layer | Cathode | 1.219 ± 0.001 | 1.196 ± 0.001 | 1.9 % |
| Anode | 1.756 ± 0.002 | 1.809 ± 0.002 | 3.0 % | |
| (b) Fresh cell Inner layer | Cathode | 1.224 ± 0.001 | 1.184 ± 0.001 | 3.3 % |
| Anode | 1.709 ± 0.003 | 1.809 ± 0.002 | 5.9 % | |
| (c) Aged cell Outer layer | Cathode | 1.211 ± 0.001 | 1.203 ± 0.001 | 0.7 % |
| Anode | 1.772 ± 0.002 | 1.791 ± 0.002 | 1.1 % | |
| (d) Aged cell Inner layer | Cathode | 1.213 ± 0.001 | 1.200 ± 0.001 | 1.1 % |
| Anode | 1.788 ± 0.002 | 1.779 ± 0.003 | 0.5 % |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Honkanen, A.-P.; Tsuji, N.; Jalkanen, K.; Koskinen, J.; Morimoto, H.; Hiramoto, D.; Terasaka, A.; Hafiz, H.; Sakurai, Y.; et al. High-Energy X-Ray Compton Scattering Imaging of 18650-Type Lithium-Ion Battery Cell. Condens. Matter 2019, 4, 66. https://doi.org/10.3390/condmat4030066
Suzuki K, Honkanen A-P, Tsuji N, Jalkanen K, Koskinen J, Morimoto H, Hiramoto D, Terasaka A, Hafiz H, Sakurai Y, et al. High-Energy X-Ray Compton Scattering Imaging of 18650-Type Lithium-Ion Battery Cell. Condensed Matter. 2019; 4(3):66. https://doi.org/10.3390/condmat4030066
Chicago/Turabian StyleSuzuki, Kosuke, Ari-Pekka Honkanen, Naruki Tsuji, Kirsi Jalkanen, Jari Koskinen, Hideyuki Morimoto, Daisuke Hiramoto, Ayumu Terasaka, Hasnain Hafiz, Yoshiharu Sakurai, and et al. 2019. "High-Energy X-Ray Compton Scattering Imaging of 18650-Type Lithium-Ion Battery Cell" Condensed Matter 4, no. 3: 66. https://doi.org/10.3390/condmat4030066
APA StyleSuzuki, K., Honkanen, A.-P., Tsuji, N., Jalkanen, K., Koskinen, J., Morimoto, H., Hiramoto, D., Terasaka, A., Hafiz, H., Sakurai, Y., Kanninen, M., Huotari, S., Bansil, A., Sakurai, H., & Barbiellini, B. (2019). High-Energy X-Ray Compton Scattering Imaging of 18650-Type Lithium-Ion Battery Cell. Condensed Matter, 4(3), 66. https://doi.org/10.3390/condmat4030066






