Surface Plasmon Resonance (SPR) to Magneto-Optic SPR
Abstract
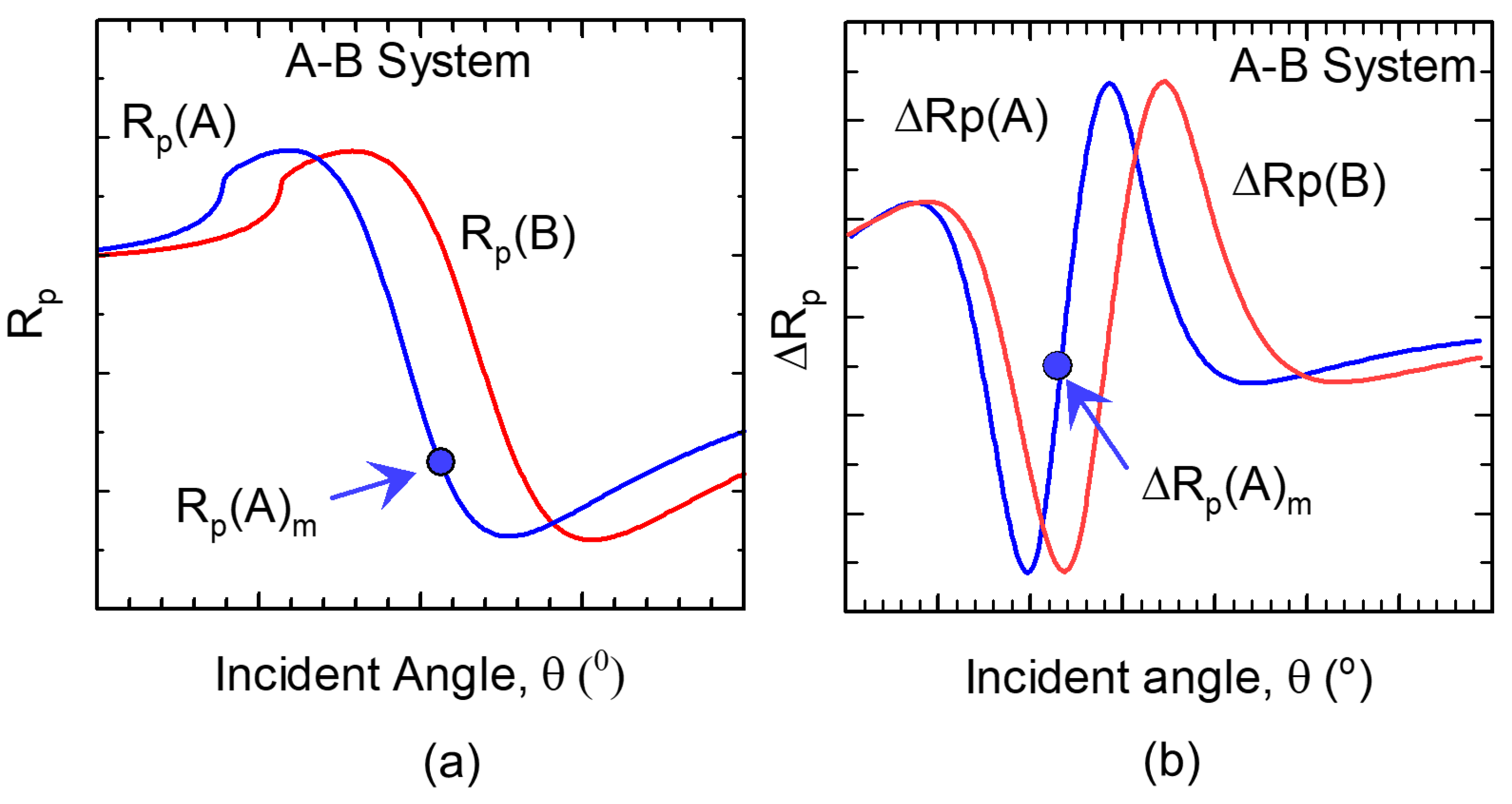
1. Background
2. Sensitivity Metrics
3. Potential Applications
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Wood, R.W. On a remarkable case of uneven distribution of light in a diffraction grating spectrum. Lond. Edinburgh Dublin Philosoph. Mag. J. Sci. 1902, 4, 396–402. [Google Scholar] [CrossRef]
- Rayleigh, H.L. On the dynamical theory of gratings. Proc. R. Soc. Lond. Ser. A 1907, 79, 399–416. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Radiative decay of non radiative surface plasmons excited by light. Zeitschrift für Naturforschung A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Zeitschrift für Physik A Hadrons and nuclei 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Säfsten, P.; Klakamp, S.L.; Drake, A.W.; Karlsson, R.; Myszka, D.G. Screening antibody–antigen interactions in parallel using Biacore A100. Anal. Biochem. 2006, 353, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Owen, V. Real-time optical immunosensors–A commercial reality. Biosensor. Bioelectron. 1997, 12, 1–2. [Google Scholar] [CrossRef]
- Piliarik, M.; Homola, J. Surface plasmon resonance (SPR) sensors: approaching their limits? Opt. Exp. 2009, 17, 6505–16517. [Google Scholar] [CrossRef]
- Stockman, M.I. Nanoplasmonics: past, present, and glimpse into future. Opt. Exp. 2011, 19, 22029–22106. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 3, 205. [Google Scholar] [CrossRef]
- Brolo, A.G. Plasmonics for future biosensors. Nat. Photon. 2012, 6, 709–713. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Monteiro, J.P.; de Oliveira, J.H.; Radovanovic, E.; Brolo, A.G.; Girotto, E.M. Microfluidic plasmonic biosensor for breast cancer antigen detection. Plasmonics 2016, 11, 45–51. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Zvezdin, A.K.; Kotov, V.A. Modern Magnetooptics and Magnetooptical Materials; Coey, M., Ed.; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- González-Díaz, J.; Sepúlveda, B.; García-Martín, A.; Armelles, G. Cobalt dependence of the magneto-optical response in magnetoplasmonic nanodisks. Appl. Phys. Lett. 2010, 97, 043114. [Google Scholar]
- Temnov, V.V. Ultrafast acousto-magneto-plasmonics. Nat. Photon. 2012, 11, 728–736. [Google Scholar] [CrossRef]
- Belotelov, V.I.; Akimov, I.A.; Pohl, M.; Kotov, V.A.; Kasture, S.; Vengurlekar, A.S.; Gopal, A.V.; Yakovlev, D.R.; Zvezdin, A.K.; Bayer, M. Enhanced magneto-optical effects in magnetoplasmonic crystals. Nat. Nanotechnol. 2011, 6, 370. [Google Scholar] [CrossRef]
- Chin, J.Y.; Steinle, T.; Wehlus, T.; Dregely, D.; Weiss, T.; Belotelov, V.I.; Stritzker, B.; Giessen, H. Nonreciprocal plasmonics enables giant enhancement of thin-film Faraday rotation. Nat. Commun. 2013, 4, 1599. [Google Scholar] [CrossRef]
- Bonanni, V.; Bonetti, S.; Pakizeh, T.; Pirzadeh, Z.; Chen, J.; Noguacs, J.; Vavassori, P.; Hillenbrand, R.; Akerman, J.; Dmitriev, A. Designer magnetoplasmonics with nickel nanoferromagnets. Nano Lett. 2011, 11, 5333–5338. [Google Scholar] [CrossRef]
- Maksymov, I.S. Magneto-plasmonics and resonant interaction of light with dynamic magnetisation in metallic and all-magneto-dielectric nanostructures. Nanomaterials 2015, 5, 577–613. [Google Scholar] [CrossRef]
- Pellegrini, G.; Mattei, G. High-performance magneto-optic surface plasmon resonance sensor design—An optimization approach. Plasmonics 2014, 9, 1457–1462. [Google Scholar] [CrossRef]
- Regatos, D.; Sepúlveda, B.; Fariña, D.; Carrascosa, L.G.; Lechuga, L.M. Suitable combination of noble/ferromagnetic metal multilayers for enhanced magneto-plasmonic biosensing. Opt. Expr. 2011, 19, 8336–8346. [Google Scholar] [CrossRef]
- Wang, L.; Clavero, C.; Huba, Z.; Carroll, K.J.; Carpenter, E.E.; Gu, D.; Lukaszew, R.A. Plasmonics and enhanced magneto-optics in core-shell Co-Ag nanoparticles. Nano Lett. 2011, 11, 1237–1240. [Google Scholar] [CrossRef]
- Rizal, C.; Niraula, B.; Lee, H.H.W. Biomagnetoplasmonics, emerging biomedical technologies and beyond. J. Nanomed. Res. 2016, 3, 00059–00065. [Google Scholar] [CrossRef]
- Szunerits, S.; Maalouli, N.; Wijaya, E.; Vilcot, J.-P.; Boukherroub, R. Recent advances in the development of graphene-based surface plasmon resonance (SPR) interfaces. Anal. Bioanal. Chem. 2013, 405, 1435. [Google Scholar] [CrossRef]
- Rizal, C.; Pisana, S.; Hrvoic, I. Improved magneto-optic surface plasmon resonance biosensors. Photonics 2018, 5, 15. [Google Scholar] [CrossRef]
- Ignatyeva, D.O.; Kapralov, P.O.; Knyazev, G.A.; Sekatskii, S.K.; Dietler, G.; Nur-E-Alam, M.; Vasiliev, M.; Alameh, K.; Belotelov, V.I. High-Q surface modes in photonic crystal/iron garnet film heterostructures for sensor applications. JETP Lett. 2016, 104, 679. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Y.; Liang, X.; Liu, C.; Wang, C.; Kang, T.; Lu, H.; Zhang, L.; Zhou, P.; Wang, X. Ultrahigh Figure-of-Merit in Metal-Insulator-Metal Magnetoplasmonic Sensors Using Low Loss Magneto-optical Oxide Thin Films. ACS Photon. 2017, 4, 1403. [Google Scholar] [CrossRef]
- Konopsky, V.; Karakouz, T.; Alieva, E.V.; Vicario, C.; Sekatskii, S.; Dietler, G. Photonic crystal biosensor based on optical surface waves. Sensors 2013, 13, 2566–2578. [Google Scholar] [CrossRef]
- Ignatyeva, D.O.; Knyazev, G.A.; Kapralov, P.O.; Dietler, G.; Sekatskii, S.K.; Belotelov, V.I. Magneto-optical plasmonic heterostructure with ultranarrow resonance for sensing applications. Sci. Rep. 2016, 6, 28077. [Google Scholar] [CrossRef]
- Otipka, P.; Vlceka, J.; Lesnak, M.; Sobotac, J. Design of MO-SPR sensor element with photonic crystal. Photon. Nanostruct. Fundam. Appl. 2018, 31, 77. [Google Scholar] [CrossRef]
- Tomitaka, A.; Arami, H.; Raymond, A.; Yndart, A.; Kaushik, A.; Jayant, R.D.; Takemura, Y.; Cai, Y.; Toborek, M.; Nair, M. Development of magneto-plasmonic nanoparticles for multimodal image-guided therapy to the brain. Nanoscale 2017, 9, 764–773. [Google Scholar] [CrossRef]
- Rizal, C.; Moa, B.; Brolo, A.G. Recent progress in ferromagnetic multilayer-based magnetoplasmonic devices for potential biomedical applications. Can. Med. Biol. Soc. Ser. 2014, 4, 1–4. [Google Scholar]
- Menezes, J.W.; Ferreira, J.; Santos, M.J.L.; Cescato, L.; Brolo, A.G. Large area fabrication of periodic arrays of nanoholes in metal films and their application in biosensing and plasmonic enhanced photovoltaics. Adv. Funct. Mater. 2010, 20, 3918–3924. [Google Scholar] [CrossRef]
- Berini, P.; de Leon, I. Surface plasmon–polariton amplifiers and lasers. Nat. Photon. 2012, 6, 16. [Google Scholar] [CrossRef]
- Osterfeld, S.J.; Yu, H.; Gaster, R.S.; Caramuta, S.; Xu, L.; Han, S.J.; Hall, D.A.; Wilson, R.J.; Sun, S.; White, R.L.; et al. Multiplex protein assays based on real-time magnetic nanotag sensing. PNAS 2008, 105, 20637–20640. [Google Scholar] [CrossRef]
- Ding, Y.; Judy, J.H.; Wang, J.P. Magneto-resistive read sensor with perpendicular magnetic anisotropy. IEEE Trans. Magnet. 2005, 41, 707–712. [Google Scholar] [CrossRef]
- Rizal, C. Microstructure, magnetism, surface plasmon resonance, and magneto-optic surface plasmon resonance biosensors. IEEE Trans. Magnet. 2018, 55, 530030. [Google Scholar]
- Rizal, C.; Pisana, S.; Hrvoic, I.; Fullerton, E. Microstructure and magneto-optical surface plasmon resonance of Co/Au multilayers. J. Phys. Commun. 2018, 2, 055010. [Google Scholar] [CrossRef]
- Rizal, C.; Moa, B.; Niraula, B.B. Ferromagnetic multilayers: Magnetoresistance, magnetic anisotropy, and beyond. Magnetochemistry 2016, 2, 22. [Google Scholar] [CrossRef]
- Lu, D.; Kan, J.J.; Fullerton, E.E.; Liu, Z. Enhancing spontaneous emission rates of molecules using nanopatterned multilayer hyperbolic metamaterials. Nat. Nanotechnol. 2014, 9, 48–53. [Google Scholar] [CrossRef]
- Visnovsky, S.; Nyvlt, M.; Prosser, V.; Ferr, J.; Penissard, G.; Renard, D.; Sczigel, G. Magneto-optical effects in Au/Co/Au ultrathin film sandwiches. J. Magnet. Magnet. Mater. 1993, 128, 179. [Google Scholar] [CrossRef]
- Schubert, M.; Tiwald, T.; Woollam, J. Explicit solutions for the optical properties of arbitrary magneto-optic materials in generalized ellipsometry. Appl. Opt. 1999, 38, 177. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizal, C.; Belotelov, V.; Ignatyeva, D.; Zvezdin, A.K.; Pisana, S. Surface Plasmon Resonance (SPR) to Magneto-Optic SPR. Condens. Matter 2019, 4, 50. https://doi.org/10.3390/condmat4020050
Rizal C, Belotelov V, Ignatyeva D, Zvezdin AK, Pisana S. Surface Plasmon Resonance (SPR) to Magneto-Optic SPR. Condensed Matter. 2019; 4(2):50. https://doi.org/10.3390/condmat4020050
Chicago/Turabian StyleRizal, Conrad, Vladimir Belotelov, Daria Ignatyeva, Anatoly K. Zvezdin, and Simone Pisana. 2019. "Surface Plasmon Resonance (SPR) to Magneto-Optic SPR" Condensed Matter 4, no. 2: 50. https://doi.org/10.3390/condmat4020050
APA StyleRizal, C., Belotelov, V., Ignatyeva, D., Zvezdin, A. K., & Pisana, S. (2019). Surface Plasmon Resonance (SPR) to Magneto-Optic SPR. Condensed Matter, 4(2), 50. https://doi.org/10.3390/condmat4020050





